Harnessing and honing mesenchymal stem/stromal cells for the amelioration of graft-versus-host disease
Tang-Her Jaing, Tsung-Yen Chang, Chia-Chi Chiu
Tang-Her Jaing, Division of Hematology, Oncology, Department of Pediatrics, Chang Gung Children’s Hospital, Chang Gung University, Taoyuan 333, Taiwan
Tsung-Yen Chang, Department of Pediatrics, Chang Gung University College of Medicine,Taoyuan 333, Taiwan
Chia-Chi Chiu, Department of Nursing, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan
Abstract Allogeneic hematopoietic stem cell transplantation is a deterministic curative procedure for various hematologic disorders and congenital immunodeficiency.Despite its increased use, the mortality rate for patients undergoing this procedure remains high, mainly due to the perceived risk of exacerbating graft-versushost disease (GVHD). However, even with immunosuppressive agents, some patients still develop GVHD. Advanced mesenchymal stem/stromal cell (MSC)strategies have been proposed to achieve better therapeutic outcomes, given their immunosuppressive potential. However, the efficacy and trial designs have varied among the studies, and some research findings appear contradictory due to the challenges in characterizing the in vivo effects of MSCs. This review aims to provide real insights into this clinical entity, emphasizing diagnostic, and therapeutic considerations and generating pathophysiology hypotheses to identify research avenues. The indications and timing for the clinical application of MSCs are still subject to debate.
Key Words: Mesenchymal stem/stromal cells; Graft-versus-host disease; Immunomodulatory; Adaptive immunity; Exosomes
INTRODUCTION
Mesenchymal stem/stromal cells (MSCs) are multipotent cells with self -renewal abilities[1] that can be derived from different tissue sources. They attach to tissue culture dishes and express CD73, CD90, and CD105 but lack the expression of CD45, CD34, CD14, or CD11b, CD79α or CD19, and HLA-DR surface molecules. In vitro, MSCs can differentiate into osteoblasts, adipocytes, or chondroblasts[2,3]. MSCs can be effectively harvested without significant ethical concerns and have low immunogenicity. They have emerged as a promising cell source due to their regenerative and immunomodulatory potentials,limited ethical concerns, and low risk of tumor formation[4-6].
Malignancy relapse is a significant challenge in allogeneic hematopoietic stem cell transplantation(HSCT). Chronic graft-versus-host disease (GVHD) is associated with lower relapse rates, but the diagnosis, staging, and risk stratification of GVHD are challenging[7]. In this scoping review, we highlight recent evidence on different types of MSCs studied for GVHD, including bone marrow (BM),umbilical cord blood, placenta, adipose tissue, and others. MSCs have been found to inhibit immune cell proliferation and cytotoxic action, making them a potential treatment option for GVHD[8].
This review aims to provide a critical overview of the mechanisms by which MSC can treat GVHD,including immunomodulation, migration, homing, and clinical applications of MSC therapy. We searched peer-reviewed literature in PubMed and Embase to gather the latest information on this topic.
THERAPEUTIC STRATEGY OF GVHD
Immune pathways in GVHD
One of the most significant challenges in improving the prognosis for patients undergoing allogeneic HSCT is GVHD. This condition can be characterized as a rapid escalation in immune activation caused by massive target tissue apoptosis. The prevention of GVHD is primarily based on the use of calcineurin inhibitors and methotrexate, while the treatment of ongoing GVHD involves the use of corticosteroids.GVHD manifests as acute GVHD (aGVHD) in 53%-62.5% of the patients and chronic GVHD (cGVHD)in 20%-50.4% of patients[9,10], and the development of this complication may contribute to 6.3% of deaths following HSCT[9]. Although the administration of calcium inhibitors such as calcium sulphoaluminate can prevent the development of GVHD in some cases, about 19% of aGVHD II-IV cases are often resistant to all conventional therapy, resulting in a high mortality rate for these patients.Several potential second-line options have been proposed, including the use of MSCs. MSCs have attracted significant interest because they can actively undergo apoptosis by recipient cytotoxic cells[11]. Figure 1 illustrates the immune pathways involved in GVHD and the sites where therapy is used to block GVHD development.

Figure 1 Immune pathways in graft-versus-host disease and sites where therapy is used to block graft-versus-host disease development(red bars).
Danger signals in aGVHD development
In a typical case of aGVHD, which occurs following a triptych course, symptoms begin with the prodromal phase caused by the underlying disease and conditioning regimens that secrete proinflammatory cytokines, mainly tumor necrosis factor-α (TNF-α), interleukin-1B (IL-1B), and interleukin-6 (IL-6)[12]. Host conditioning facilitates donor cell grafting. Donor allograft T-cells are the primary effector cells for GVHD. However, tissue damage leads to the release of alarmins and the expression of pathogen-recognition receptors, triggering the next phase. This phase activates the innate immune system and, in turn, the adaptive immune system. Alarmins and exogenous pathogen-associated molecular patterns (PAMPs) elicit similar responses to relevant signals, and they belong to the group of damage-associated molecular patterns (DAMPs)[13]. DAMPs and PAMPs are potent stimulators for host and donor-derived antigen-presenting cells (APCs), which activate and enhance the responses of alloreactive donor T cells[14].
The immunosuppressive effects of MSCs are classified into soluble factor-mediated effects and cellcell contact-mediated effects. MSCs suppress the proliferation and survival of activated T lymphocytes and reduce the release of inflammatory factors such as IL-2, TNF-α, IL-1B, and IFN-γ. By the same means, MSCs also reduce the number of Th1/Th2 and Th17 cells. Through cell-to-cell contacts, MSCs can stimulate the expression of transcription complexes related to Runt 1 (RUNX1), RUNX3, and CBFB in Treg-specific regulatory regions to improve the stability of Foxp3[15]. MSCs have also been shown to be highly effective in inhibiting the cytotoxic effect, proliferation, and secretion of different cytokines of NK cells by directly contacting these cells and transforming their phenotype.
The effects of MSCs on B cells involve inhibiting their cell cycle progression by inducing G0/G1 cell cycle arrest and suppressing their proliferation. Additionally, the differentiation of B cells into IgM-,IgG-, and IgA-secreting cells is impaired by MSCs, thereby limiting their antibody production.Furthermore, MSCs can affect the chemotactic function of B cells[16].
GENETIC BASIS OF GVHD
Humanized mouse models
Most relevant models for studying human adaptive immune responses use immunocompromised mice whose immune system is reconstituted with human immune cells and immune system components. Leeet al[17] used a model of NSG mice reconstituted with human CD34 cells to evaluate the immunological safety of therapeutically compromising human MSCs. As major histocompatibility complex (MHC)molecules are the primary mediators of the allogeneic immune response, MHC expression levels are critical in the potential immunogenicity of cells. To investigate MSCs as a cellular therapy in GVHD,Tobinet al[18] treated NSG-PBMC humanized mice with human MSCs as a GVHD model. MSC treatment resulted in a reduction in liver and intestinal pathology and a significant increase in the survival of the GVHD NSG mouse.
In contrast to aGVHD, some MHC-mismatched animal models may mimic the features of cGVHD.However, due to the pathological resemblance between cGVHD and autoimmune diseases, there is a clear connection between the two entities, and the difference in cGVHD is primarily caused by the donor lymphoid graft[19]. These findings provide compelling evidence for the essential role of human leukocyte antigen (HLA) disparity in both aGVHD and cGVHD. The expression pattern of minor histocompatibility antigens (miHAs) determines the target organ involvement in aGVHD. The miHAs exhibit hierarchical immunodominance, which may contribute to the variability in GVHD variability[20].
Translation and clinical advances in GVHD
aGVHD:HLA mismatching is one of the most significant risk factors for aGVHD and cGVHD risk.HLA proteins are specifically encoded by MHC. In vitro studies have demonstrated that most T cells associated with GVHD are naïve T cells, whereas memory T cells mediate immunity against pathogens and the graft-versus-leukemia (GVL) effect[21]. Regardless of the graft source or conditioning intensity,the incidence of aGVHD is closely related to the number of HLA disparities. Although the impact of HLA disparity has been analyzed in the outcomes following allogeneic HSCT, relatively few studies have tried to correlate it with the incidence and severity of cGVHD. Some studies reported an association between HLA-A, -B, and -C disparity and aGVHD[22].
Although MHC antigens guarantee HLA matching, the donor and recipient may differ in various proteins presented in the form of HLA-peptide complexes to T cells that act as miHAs. The genomes include more than 107polymorphic sequences outside HLA, and the role of miHAs is supported by genome-wide analysis of single-nucleotide polymorphisms[7]. The disparity in a single immunodominant miHA is insufficient to cause aGVHD, although T cells primed against a single miHA may induce tissue damage in a human skin explant model[23]. It is unknown whether the number of miHAs triggering a GVL response in a given transplant is significant or whether a small number of antigens play a dominant role[24].
cGVHD:In contrast, cGVHD has been considered an autoimmune disease based on its clinical features[25]. Some experimental studies have shown that T cells from animals with cGVHD are specific for a public determinant of MHC class II molecules and are therefore considered autoreactive. These autoreactive cells of cGVHD are often associated with an injured thymus and adverse selection.
Recent clinical data has highlighted a significant link between immune responses against ubiquitous miHAs and cGVHD. Since cGVHD usually occurs after allogeneic HSCT, aGVHD is its related risk factor. Unlike syngeneic GVHD, which results from deficient thymic selection[26], cGVHD typically arises after allogeneic HSCT and is characterized by chronic T-cell activation due to continuous exposure to miHAs. This chronic stimulation can cause target organ damage that resembles autoimmune features, where the target is miHAs for cGVHD and non-polymorphic autoantigens for autoimmune diseases. A study on female-to-male HSCT demonstrated a good correlation between the presence of antibodies to the Y-chromosome-encoded gene and cGVHD[27]. A study in female-to-male HSCT demonstrated a good correlation between the presence of antibodies to Y-chromosome-encoded genes and cGVHD, suggesting that miHAs may indeed be the targets. However, it is not yet clear whether the miHAs targeted in cGVHD are the same as those targeted in aGVHD. A murine study had shown that the type and selection of immunodominant miHAs can determine the target and character of GVHD damage[28].
Epitope spreading and the failure of appropriate regulatory mechanisms in aGVHD may result in donor T cells recognizing both non-polymorphic and miHA epitopes, perpetuating cGVHD. In contrast,T cells directed against miHAs with hematopoietic restriction may also mediate a GVL response in the absence of GVHD[29]. However, the relevant immunogenic targets for cGVHD remain speculative and confidential.
Potent immunomodulatory role of MSCs-derived exosomes in preventing GVHD
The safety and effectiveness questions regarding using MSCs remain unresolved, and conflicting effects have been noted due to the heterogeneity observed among MSCs. MSCs-derived exosomes (MSCs-Exo),a subgroup of extracellular vesicles released by MSCs, have shown therapeutic benefits for inflammatory diseases and cancers due to their ability to transport proteins and nucleic acids from donor cells to recipient cells of the same or different tissues, making it a suitable candidate for cell-free therapy.MSCs-Exo have been found to reduce inflammation and fibrosis in the skin, lungs, and liver, and inhibit Th17 cells while inducing Treg cells, making it a potential alternative method for the treatment of cGVHD. The activation of CD4+ T cells and their infiltration into the inflamed mouse lung were reduced in MSCs-Exo-treated mice[30]. MSCs-Exo, extracted from healthy donors’ BM, suppress the expression of pro-inflammatory factors TNF-α and IL-1B but increase the level of anti-inflammatory factor TGF-B duringin vitroculture[31].
Typically, MSCs-Exo are characterized by endosomes that bud inward and package into multivesicular bodies (MVBs). These MVBs fuse with the plasma membrane and deliver the exosomes into the intracellular space. However, exosomes can enrich several molecules as cargo, such as proteins/cytokines, DNA, RNA, and other nucleic acids. Exosomes, as secretory components of MSCs, transport cytokines, and growth factors of immunoregulation, such as transforming growth factor beta-1 (TGF-B 1), IL-6, IL-10, hepatocyte growth factor, signaling lipids, mRNAs, and regulatory miRNAs, which exert biological effects on recipient cells, such as cell-to-cell communication, tissue regeneration, metabolism,immune modulation, and homing of immune cells[32,33]. Diverse immune cells establish complex interactions with each other. MSCs-Exo might represent a novel cell-free therapy with unique competitive advantages over parent MSCs, such as no apparent risk of tumor formation or lower immunogenicity.
IMMUNE CELL LANDSCAPE OF GVHD
Antigen-presenting cells
Antigen-presenting cells (APCs) play a critical role in inducing aGVHD, with dendritic cells (DCs) being one of the most formidable cells in this regard[26]. Innate immunity activation during acute inflammation leads to DCs maturation and subsequent T cell priming, which is central to the potential antitumor benefits of aGVHD. Experimental data suggest that modulating perceptible DC subsets can influence aGVHD[34]. For instance, the absence of RelB signaling in host DCs or enhancing host CD8+lymphoid DC subsets following HSCT significantly reduces aGVHD[35]. Other APCs, such as monocytes/macrophages, also play a crucial role in this phase. Some data suggest that the host B cells may reduce aGVHD in specific contexts. Although the precise mechanisms remain unclear when acting as APCs, MSCs from the donor, or host also reduce aGVHD.
Natural killer, γδ T, and natural killer T cells
Natural killer (NK) cells can directly kill tumor cells without specific immunization and also have a modulatory effect on aGVHD. In an allo-HSCT donor-to-F1 model, NK cells recognize the absence of donor class I on host APCs and eliminate them, resulting in a reduction of aGVHD reduction. Upon activation, NK cells may induce apoptosis of target cells through contact-dependent cytotoxicity primarilyviaperforin and granzyme[36]. Pro-apoptotic granzymes enter through perforin pores in the plasma membrane of target cells. Besides the cytotoxic activity, NK cell activation increases the secretion of various cytokines and chemokines, such as IFN-γ. However, the role of NK cells in GVHD remains controversial.
The infusion of donor γδ T cells may increase aGVHD, while the absence of host γδ T cells may reduce APC activation and aGVHD in an MHC-mismatched model. Conversely, in the absence of host γδ T cells, GVHD severity was not modified in an MHC-matched, miHA-disparate model of cGVHD.aGVHD could be more significant in patients with more considerable donor γδ T cells. The significance of γδ T cells in aGVHD and cGVHD is not fully understood and may reflect differences in immunobiology between the two or be solely a consequence of variation in the experimental models.
NKT cells, which are CD1d-reactive, are believed to play an immunoregulatory role in suppressing dysfunctional immune reactions, including GVHD[37]. The cumulative frequency of regulatory T cells(Tregs) is negatively correlated with GVHD development[38], and exogenous NKT cell infusion can reduce the degree of GVHD[39]. However, Treg populations have unstable Foxp3 expression, particularly those expanded in vitro. Because the expression Foxp3 is needed for the suppressive function,further research is necessary to determine if Foxp3 expression can be simplified, especially under proinflammatory conditions characteristic of the GVHD milieu[40].
T cells
The complex interactions between MSCs and T cells have been extensively studied, particularlyin vitroculture techniques. MSCs may facilitate activated T cells in the phase G0/G1 cell cycle, yet apoptosis is not applicable[41-43]. MSCs may suppress or downregulate the proliferation of both naïve and memory T cells through cell-cell contact or mitogenic stimuli. This suppression is generally not MHC-restricted.MSCs can further decrease IFN-γ producing T cells and contribute to the T-cell skewing toward Th2 cells producing IL-4. cGVHD is a Th2 cell dominant disease process[12].
Regulatory T cells
MSCs activateimmuneresponses that induce the expression of Tregs, which are a cluster of cells with a CD4+CD25+Foxp3+phenotype that regulate the body's immune response. Tregs highly and constitutively express CTLA-4, which binds to CD80, and CD86 on DCs, leading to impaired DC maturation and blocking CD80/CD86 to CD28 on conventional T cells, thereby preventing costimulation, and T-cell activation. Lower Tregs and deficient Foxp3 expression have been associated with cGVHD in peripheral blood and mucosal biopsies. However, levels of Foxp3 mRNA in the CD25+ T cell compartment do not predict the development of cGVHD, demonstrating that the presence, or absence of Tregs must be considered in the context of their impact on aGVHD and cGVHD. An intriguing possibility is that the negative impact of calcineurin inhibitors on Tregs could exacerbate cGVHD as a consequence of the suppression of the alloreactive donor cytopathic and Tregs.
B cells
Host B cells attenuate aGVHD in an IL-10-dependent manner. Recent data provide a rationale for the pathogenic role of donor B cells in cGVHD[12], including a robust correlation between cGVHD and (1)The effects of antibodies against Y-chromosome-encoded miHA; (2) higher numbers of B cells with altered TLR9 responses; (2) levels of a B-cell-activating factor, which enhances survival and differentiation of activated B cells; and (4) in animal models, levels of autoantibodies. Besides, emerging data from the depletion of B cells with rituximab further supports the theory of the pathogenic action of B cells in cGVHD[44]. However, whether B cells are the effectors or inducers of cGVHD remains unknown.
SEARCH STRATEGY, STUDY SCREENING, AND SELECTION
We systematically searched the electronic bibliographic databases MEDLINE, EMBASE, and Google Scholar for studies published before November 2022 using the keywords: “graft-versus-host disease”OR “acute GVHD” OR “chronic GVHD”AND “mesenchymal stem cells” AND “mesenchymal stromal cells” AND “treatment response” AND “outcome.” Publications were included if they met the following inclusion criteria: (1) Original research; (2) published in 2002 or later; and (3) specifically reporting on the use of MSCs in GVHD patients. Publications were excluded based on the following criteria: (1) Non-English literature; (2) small populations (n< 20 patients) or case studies; and (3) mixed population with non-GVHD patients. A meta-analysis was not performed for the limited number of published studies meeting the inclusion criteria. Pre-post design studies and case series were not included for lack of sustainability of the results. Additionally, reference lists of retrieved articles were cross-referenced for additional eligible articles.
RESULTS
This review provides an overview of clinical studies, animal models, and limited human patient trials regarding MSCs. MSCs have been widely studied and increasingly used in GVHD treatment since the first report of promising results by Le Blancet al[45] However, the studies have reported varying outcomes, which could be contributed to differences in cell concentration and MSC infusion dose. While MSC infusion has shown quite promising results following GVHD prophylaxis failure, some clinicians still prefer using methylprednisolone and calcineurin inhibitors before cell therapy with MSCs.
In addition to suppressing inflammation, MSCs have other beneficial effects, including increased angiogenesis, reduced apoptosis, and modified extracellular matrix dynamics. These cells mediate immune system components like macrophages and neutrophils, improving tissue microenvironments.After the injury, MSCs can either promote or suppress the immune system to guide the whole-tissue regeneration process[1]. Clinical responses to MSC infusion assessed as early as one week after treatment may predict patients' overall survival, indicating the potential of MSCs in treating GVHD[45].
Although the paracrine effects of MSCs are known to mediate the modulation of the immune response, the mechanisms underlying this modulation are not yet fully understood. However, it has been found that under conditions of chronic hypoxia or co-stimulation with IFN-γ, MSCs express proteins that have the immunosuppressive capacity, such as IDO, HLA-G, PGE2, and FasL, which can modulate the immune response[46]. While other cytokines play a crucial role in immunosuppression,blocking highly expressed proteins can result in the setback of the human immunosuppressed state,leading to the growth, and proliferation of immune cells. Moreover, MSCs do not trigger the activation of immune cells as they lack CD40, CD80, CD86, and HLA-DR-stimulating molecules. Given that GVHD occurs following the infusion of immune cells donated by the same donors, suppressing the immune activity can improve the patient’s prognosis. MSCs’ expression of paracrine effects can regulate these donor immune cells through various mechanisms (Table 1)[47-62].

Table 1 Immunosuppressive effect exerted by mesenchymal stem/stromal cells from different sources on immune cells
When MSCs are exposed to an insult, such as injury, or bacterial infection, MHC-II molecules facilitate the presentation of bacterial antigens, which induces further activation of T cells expressing IFN-γ. MHC-II is downregulated at high levels of IFN-γ, while B7-H1 is upregulated[45]. These presentation pathways are illustrated in Figure 2.
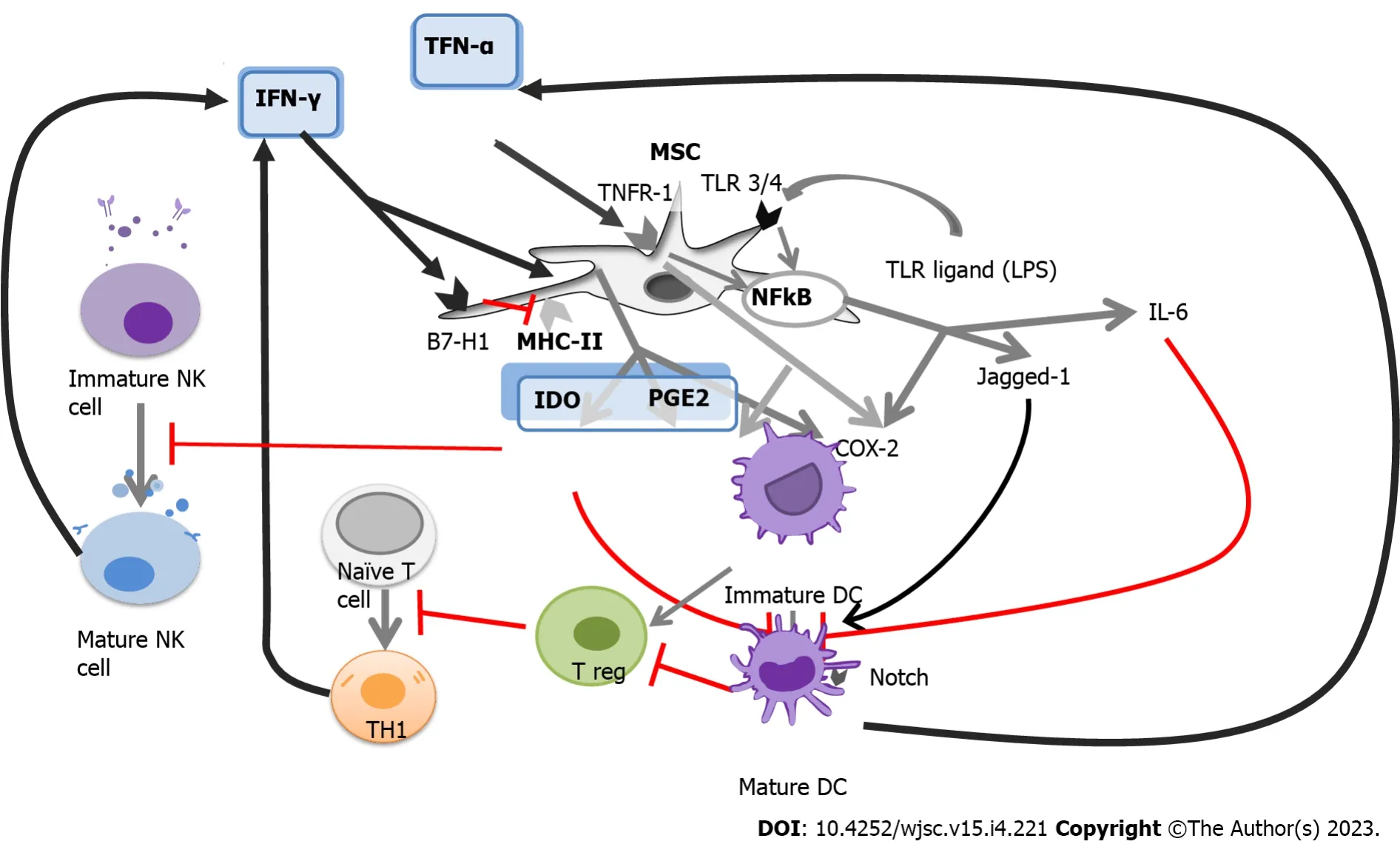
Figure 2 The complex network of antigen presentation and immunomodulation.
MSCs have been used to treat various conditions, including diabetes mellitus (DM), cardiovascular diseases, GVHD, and autoimmune diseases. Despite persistent questions, the immunomodulatory effects of MSCs make them a top choice for cell therapy. MSCs are early multipotent progenitors and non-hematopoietic cell populations that can be expanded ex-vivo to achieve large numbers necessary forin vivouse. Recently, adipose tissues, umbilical cord, placenta, and dental pulp have been recognized as multipotent sources of MSCs. MSCs can differentiate into a variety of cell types capable of osteogenic,chondrogenic, adipogenic, myogenic, and neurogenic differentiation. However, not all individual cells cultivated in tissue culture flasks result in the same degree of multipotency. Self-renewing progenitors can be identified in human BM, and it is currently unknown whether MSCs from other tissues exhibit this property. BM-MSCs are a critical source of multipotent stem cells and serve as a standard for comparing MSCs from different sources (Table 2)[49,63-85].
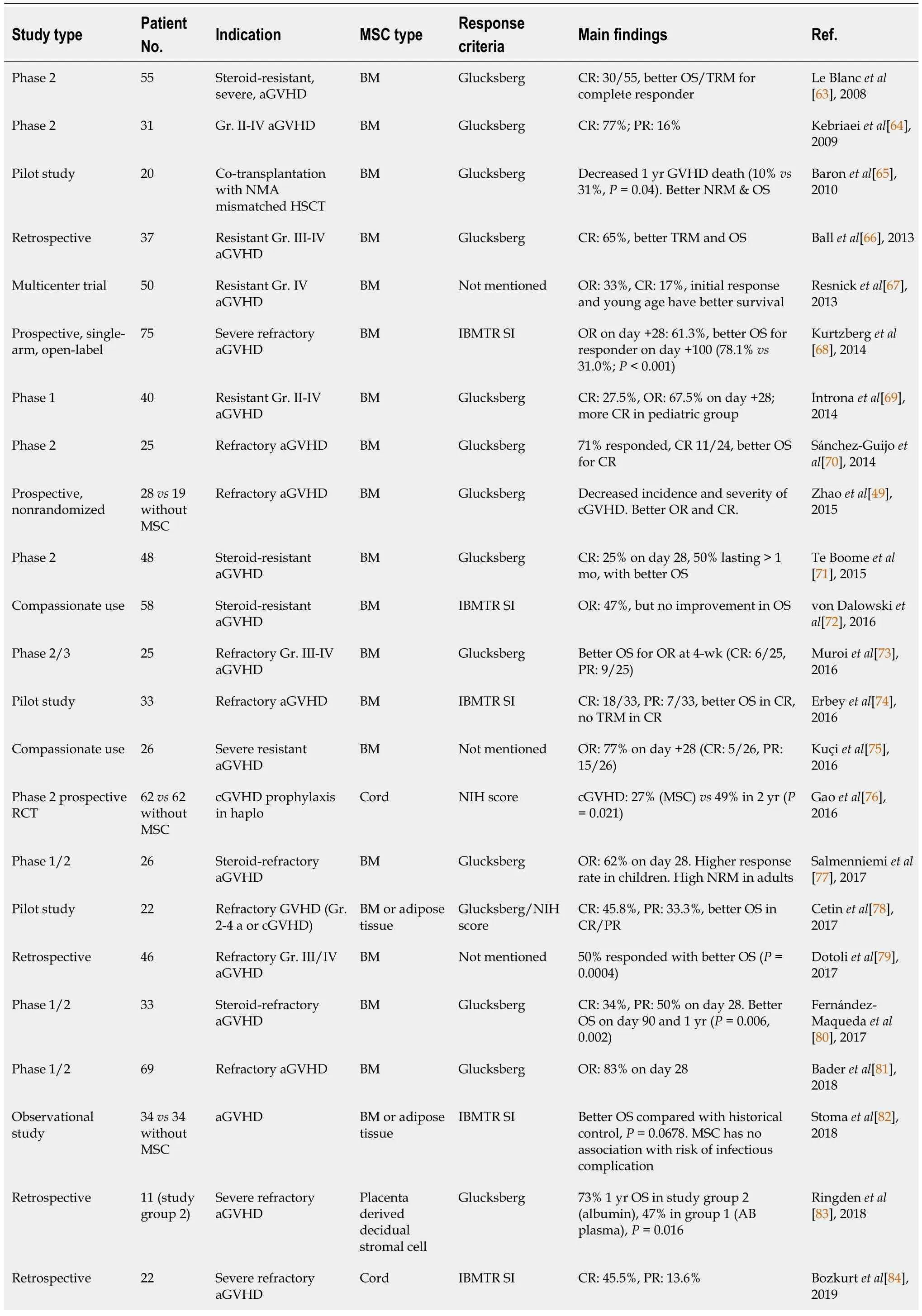
Table 2 Effects of different mesenchymal stem/stromal cells on refractory acute graft-versus-host disease
The term "mesenchymal stem cells" has been proposed as a more appropriate term than MSCs. These cells possess not only multipotency but also significant immunomodulatory and engraftmentpromoting properties. They create a specialized microenvironment for HSCs by promoting the secretion of various inflammatory cytokines, chemokines, growth factors, extracellular matrix, and extracellular vesicles that are crucial for HSC differentiation, proliferation, and maintenance[86-88]. Afterin vivobiological application, MSCs secrete a range of cytokines and regulatory molecules with anti-inflammatory, wound healing, and regenerative effects, promoting the repair of endogenous tissues or tissue replacement. Bereset al[40] demonstrated that even in otherwise immunocompetent humans, allogeneic MSCs may graft, and differentiate through significant histocompatible barriers.
Similar to hematopoietic stem cells, MSCs have multi-organ specificity, and plasticity. In 2006, the International Society for Cellular Therapy officially defined MSCs as plastic practitioners under standard growing conditions, expressing CD73, and CD90 surface molecules while lacking CD11b,CD14, CD19, CD34, CD45, CD79a, and HLA-DR[2]. In addition, MSCs can differentiate into various mesodermal lineages including osteoblast, adipocyte, and chondroblast, to different degrees.
MSCs are capable of modulating both innate and adaptive immunity through the release of various soluble factors, including indoleamine 2,3-dioxygenase[11], IL-10, prostaglandin 2, nitric oxide,transforming growth factor-B, HLA-G5, and anti-inflammatory molecule TNF-α-induced gene/protein 6[89]. These molecules are believed to play a key role in the immunomodulating effects of MSCs, which have been shown to be beneficial in certain immunopathological diseases, such as aGVHD, and type 1 DM. However, the precise mechanisms underlying this therapeutic potential are not yet fully understood. The literature suggests that the immunomodulating potential of MSCs involves interactions with both humoral and cellular components of the innate and adaptive immune systems. The literature refers to several fundamental cellular interactions. An integrated perspective on the utility of MSCs for GVHD has been strengthened by the recent findings that MSCs are induced to undergo necrosis/apoptosis by the recipient’s cytotoxic cells and that this process is assumed to elicit MSC-induced immunosuppression[90]. This finding made it possible to reconcile the dilemma between the effectiveness of MSC and its apparent lack of engraftment and highlighted the crucial role of the patient in the promotion and administration of immunosuppression of MSCs. Recent research has shed light on the role of the patient in promoting and administering immunosuppression of MSCs, with evidence suggesting that MSCs are induced to undergo necrosis/apoptosis by the recipient’s cytotoxic cells,leading to MSC-induced immunosuppression[90]. Table 2 provides an overview of recent studies on this topic, with 97 articles selected for full-text evaluation based on agreed-upon title and abstract criteria.
Innate immunity is primarily centered around the complementary system, with C3, and C5 being cleaved into anaphylatoxins C3a and C5a by convertases at the sites of inflammation. The labile C3convertases cleave C3 into C3a and C3b which can thereafter participate in forming distinct complexes and activate pathways for proliferation and protection against apoptosis through receptor binding.MSCs also secrete the factor H, which inhibits complement activation by limiting the activity of C3 and C5 convertases. In mice, MSCs promote pro-inflammatory repolarization and produce chemostatic cytokines, including IL-6, IL-8, GM-CSF, and macrophage inhibitory factors. IL-8, in particular, is a proinflammatory chemokine produced by multiple cell types that recruits leukocytes to sites of infection or tissue injury. Additionally, MSCs can inhibit mast cell degranulation and histamine release by binding allergens to allergen-specific IgEviaFcRε on mast cells, providing a potential therapeutic benefit for allergic reactions[91].
The molecular interaction between NK cells and MSCs is complex and depends on the immune microenvironment and NK cell activation status. MSC can inhibit cytokine proliferation and production and interfere with NK cell cytotoxicity. They also inhibit monocyte maturation and differentiation into DCs, which are the primary type of APC and play a key role in T lymphocyte activation through antigen presentation. Monocytes and macrophages are important for tissue development, homeostasis, and injury repair. Activated MSCs produce chemokines that attract circulating monocytes to sites of inflammation and injury[92].
MSCs can regulate the adaptive immune system through multiple redundant pathways. They suppress the proliferation of T cells, IFNγ production, CD4 T cell differentiation, and CD8 T-cell cytotoxicity. Di Nicolaet al[41] reported that MSCs can suppress T lymphocyte proliferationin vitrowith autologous and allogeneic MSCs, including T lymphocytes cultured with DCs or lymphocytes in mixed lymphocyte reactions. MSCs can express and secrete programmed death-ligand 1 and 2, which suppress T-cell proliferation in the presence of MSCs, secrete IL-2, induce apoptosis, and promote the induction of an irreversible hyporeactive state[93]. In vivo studies suggested that MSCs may restore the balance between T helper 1 and 2 cells in diseases associated with a shift to dominance of these T cell subpopulations[94].In vitromodels have shown that MSCs induce Tregs and maintain survival and suppressive phenotypes[95].
CONCLUSION
This article provides insights into the use of validated MSCs as a potential treatment strategy for GVHD in HSCT. Current prevention and treatment options involve immunosuppression, which can hinder immune recovery and limit the graft-versus-tumor effect. By using MSCs, clinicians can effectively treat GVHD, identify high-risk patients, and stratify patients based on disease severity. Therefore, MSCs can aid in promoting engraftment, ameliorating aGVHD, and preventing cGVHD, making them an attractive option for HSCT.
FOOTNOTES
Author contributions:Jaing TH and Chang TY designed the research study; Chang TY and Chiu CC performed the research; Jaing TH and Chiu CC analyzed the data and wrote the manuscript; all authors have read and approved the final manuscript.
Conflict-of-interest statement:The authors declare no competing interests.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Taiwan
ORCID number:Tang-Her Jaing 0000-0002-5407-1555; Tsung-Yen Chang 0000-0003-1123-4235.
S-Editor:Yan JP
L-Editor:A
P-Editor:Yan JP
 World Journal of Stem Cells2023年4期
World Journal of Stem Cells2023年4期
- World Journal of Stem Cells的其它文章
- Banking of perinatal mesenchymal stem/stromal cells for stem cellbased personalized medicine over lifetime: Matters arising
- Obesity and cancer stem cells: Roles in cancer initiation,progression and therapy resistance
- Clinical application prospects and transformation value of dental follicle stem cells in oral and neurological diseases
- Current status and prospects of basic research and clinical application of mesenchymal stem cells in acute respiratory distress syndrome
- Extracellular vesicles: Emerged as a promising strategy for regenerative medicine
- Human pluripotent stem cell-derived B cells: Truly immature islet B cells for type 1 diabetes therapy?
