Monkeypox and the perinatal period: what does maternal–fetal medicine need to know?
Kai Yan·Lu‑Kun Tang·Fei‑Fan Xiao·Peng Zhang·Chun‑Mei Lu,4·Li‑Yuan Hu·Lai‑Shuan Wang·Guo‑Qiang Cheng·Wen‑Hao Zhou,5
Abstract Background After the global elimination of smallpox,monkeypox has become the most threatening orthopoxvirus to human health.Very few studies have been reported on pregnant women and newborns.In the case of monkeypox infection,the virus can cause serious adverse pregnancy events in women,which can lead to fetal or neonatal death.Data sources We made a comprehensive review after an extensive literature search in the PubMed/Medline database and websites concerning smallpox and monkeypox.Results Two case reports reported a total of nine pregnant women,six of whom had fetal deaths.In the autopsy of a stillbirth,researchers found that the placenta was infected with monkeypox virus,but the mechanism of infection remains unclear.Smallpox vaccine should be administered to acutely exposed pregnant women and newborns.Several novel recombinant vaccinia immunogloblin (rVIG) and human-specific monoclonal antibodies are being developed for the prevention and treatment of monkeypox virus infection.After the fetus was delivered,the newborn should take a bath as soon as possible to remove the amniotic fluid and dirt from the body.The appropriate isolation protocol for the newborn should be selected according to the infection status of the mother.It is not known whether monkeypox virus is present in breast milk,and pasteurized breast milk can be given to newborns when breastfeeding is considered.Conclusion This review presents an overview of monkeypox in the perinatal period and guides the future research direction.
Keywords Fetal·Maternal·Monkeypox·Newborn
Introduction
Monkeypox virus is a zoonotic orthopoxvirus that causes fatal infections in humans with clinical manifestations similar to those of smallpox.The first case of infection was reported in a nine-month-old infant in 1972 [1].In May 1980,the World Health Organization (WHO) announced the global eradication of smallpox and the simultaneous cancellation of smallpox vaccination [2],which left humans defenseless against the monkeypox virus,and it is now believed that the re-emergence of monkeypox virus in humans is related to vaccine disruption and the overall decline in human immunity [3].Although monkeypox infection varies among populations in different parts of the world(ethnicity,age distribution,smallpox vaccination status)[4],the re-emergence of the monkeypox virus is a major challenge to human health,as the new coronavirus is still rampant worldwide and its shadow has not yet lifted [5].It can be widely transmitted through respiratory droplets and body fluids;therefore,monkeypox is considered the most threatening orthopoxvirus to human health [6].While there are few studies or case reports on maternal and neonatal outcomes,we would like to discuss the prevention and treatment of monkeypox from a maternal—fetal medicine perspective.
Updated epidemiology of monkeypox
In 2022,monkeypox outbreaks were detected in 104 countries (including confirmed or suspected cases of monkeypox),of which 97 had confirmed transmission and seven continued to have only suspected cases.In total,there were 41,743 confirmed and 2605 suspected cases (as of 2022-08-23)[7].Of the 41,743 confirmed cases,only 228 reported a travel history,and most confirmed cases did not account for a clear travel history.Of the confirmed cases,98% were male,and the mean age was 41 years.Human-to-human transmission of the monkeypox virus can occur through close contact with foci,body fluids,and respiratory droplets from infected individuals or animals.Current outbreaks are concentrated in men who have sex with men and may be associated with anal and genital injuries.Although epidemiological investigations suggest this direction,it is not clear how the monkeypox virus is specifically transmitted through sexual contact.Evidence of human-to-dog transmission of the monkeypox virus has been reported.This finding highlights the need to isolate pets from positively infected individuals to avoid secondary monkeypox transmission through pets [8].Limited reports suggest that maternal—fetal infection with monkeypox may lead to fetal or neonatal death [9],which may be related to vertical transmission of the monkeypox virus.The timing of the vertical transmission of the virus and the mechanisms leading to fetal death or serious complications are not known.With a rapid increase in the number of infections worldwide,this group needs to be brought into focus.
Two studies in the Democratic Republic of Congo on the incidence of monkeypox point to a 20-fold increase from 1981—1986 (0.72 cases per 10,000 people) to 2006—2007 (14.42 cases per 10,000 people) [10],a 45%increase between 2008 and 2013 [11].The monkeypox outbreak in the United States in 2003 was the first outbreak outside Africa [12,13];a total of 71 cases of monkeypox were detected in six states.Since May 2022,thus far,14,050 infections have been confirmed in the United States,making it the country with the highest number of infections worldwide [14].A deterministic compartmental model consisting of eight mutually exclusive compartments was proposed by Olumuyiwa et al.[15] which can be applied to evolve the transmission dynamics of monkeypox viruses.The researchers analyzed the parameters of the model and found that the human-to-human contact rate was the most sensitive parameter for monkeypox virus transmission.Shortly before,the team presented a deterministic mathematical model using classical and fractional-order differential equations [16].Using nonlinear least squares,the researchers fitted new cases of monkeypox in Nigeria from January 2019 to December 2019.By inputting different parameters,the model eventually achieved the best dynamic fit for the monkeypox epidemic in Nigeria after a large number of iterations.These parameters will likely provide important reference information for the prevention and control of the monkeypox epidemic in Africa.These two models are important for predicting monkeypox transmission and suggest the importance of strict isolation measures for monkeypox prevention and control.
Sign and symptoms
The incubation period after infection with monkeypox is 5—21 days [17].Prodromal symptoms usually include fever,malaise,and headache,and swollen lymph nodes may be an important clinical feature of monkeypox infection [18].Lymph node enlargement can be generalized or limited to a few areas,such as the neck and axillae [17].Most patients develop a rash 1—3 days after the onset of prodromal symptoms.The rash progresses through four stages,macular,papular,vesicular,and pustular,and lasts for about 2—3 weeks.The disseminated rash is centrifugal (more lesions on the extremities and face).Lesions may also appear on the palms of the hands and soles of the feet [17].These are the clinical manifestations of most infected patients.Because of the similarity of the lesion pattern to other viral infections,the first diagnosis of monkeypox infection cannot be made based on the lesions alone.
Very few studies have been reported on pregnant women and newborns.However,not long ago,researchers reported the results of a prospective observational study.The study population was drawn from patients diagnosed with monkeypox virus infection at Coller Hospital in the Democratic Republic of Congo between March 2007 and August 2011.Of the 244 patients clinically diagnosed with suspected monkeypox infection,216 were confirmed positive by polymerase chain reaction (PCR),including five women who were pregnant at the time of diagnosis of monkeypox infection.Three deaths occurred in children (two aged 5—11 years and one aged < 5 years).Four of the five pregnant women had fetal deaths (80%).Unfortunately,the authors did not describe the clinical symptoms and laboratory characteristics of the four women in detail [19].
Another study described symptoms and pregnancy outcomes in four pregnant women with monkeypox infection[9]: one mild,two moderate,and one severe according to the WHO classification of disease severity.The highest number of lesions was 1335 in pregnant women,and the lowest was 16.Routine blood and biochemical tests were normal in four patients,with only a decrease in albumin levels.Two pregnant women at gestational ages of 6 and 6—7 weeks suffered miscarriages (on days 24 and 16 postinfection,respectively).Autopsy of a stillborn fetus revealed diffuse patchy skin lesions involving the head,trunk,and extremities (including the palms and soles),as well as fetal edema,marked hepatomegaly,and peritoneal effusion.Hemorrhage was present on the surface of the maternal chorionic lobe in a punctate and diffuse manner.The authors examined monkeypox virus in the cord blood,placenta,fetal histopathology,and fetal ascites,suggesting that the fetus died from placental infection with the monkeypox virus [9].A rapid rise in maternal viral load can occur after fetal arrest (from 102to 106copies/mL).This is the largest number of reported cases of monkeypox virus infection in pregnant women.It is hoped that an increasing number of scholars will focus on cases of mother-to-child infection with the monkeypox virus in the future and expand the clinical phenotype pool.
Serious outcomes
Due to the limited number of reports on fetuses and newborns,we first examined infections in children.At the onset of monkeypox virus transmission,the majority of patients with monkeypox infection start out as children younger than 15 years [20],and more than 80% of patients are under 10 years old [21].Recent studies have found that in addition to those infected between the ages of 21 and 40,a proportion of children under the age of 10 have been infected [3,22].In a report from Central Africa,children under 10 years of age accounted for 30.8% of cases,and the overall morbidity and mortality rate was 7.7%.Severe complications (including severe pneumonia,encephalitis,and eye infections)occur primarily in children younger than eight years of age,pregnant women,and immunocompromised individuals.The mortality rate is estimated to be between 1% and 11%[23].During the 2003 US outbreak,a family infected with monkeypox demonstrated the spectrum of the disease: a sixyear-old child was hospitalized with encephalitis;the child’s mother was symptomatic and had multiple skin lesions;and the child’s father,who had been vaccinated against smallpox,had only two skin lesions and mild flu-like symptoms[24].Young age and non-vaccination against smallpox are associated with disease severity [22].The data suggest that pregnant women and newborns are priority groups for prevention and treatment.
There are limited reports of extremely high adverse pregnancy outcomes in pregnant women infected with monkeypox virus [9].Of the four pregnant women,only one delivered a healthy newborn;two had miscarriages early in pregnancy,and one fetus died in utero.A case of congenital monkeypox was reported by Zaire et al.The pregnant woman presented with fever and rash at approximately 24 weeks of pregnancy,and monkeypox virus was subsequently isolated from the blister-like skin lesions of the woman.At about 30 weeks of gestation,the mother delivered a 1500 g female infant with a rash resembling monkeypox scattered throughout her body who died of malnutrition six weeks postnatally [25].Smallpox,also an orthopoxvirus,is associated with increased adverse perinatal outcomes in pregnant women,including fetal death,preterm delivery,and spontaneous abortion [26,27].In light of these reports,we need to recognize the seriousness of mother-to-child transmission of the virus.However,the stage of pregnancy at which the monkeypox virus is most likely to spread,the point at which infection transmission begins,and how to effectively interrupt mother-to-child transmission of the monkeypox virus remains unclear.
Vaccination for pregnant women or newborns
The smallpox vaccine provides 85% cross-protection against monkeypox virus infection in humans [28—30].Pregnancy is a contraindication for smallpox vaccination in cases of non-emergency exposure [31].This is mainly because a pregnant woman’s own immune system is altered during pregnancy [32,33],and some adaptive immune responses are down-regulated during pregnancy,such as decreased numbers of T and B cells [34].However,no association has been noted between smallpox vaccination and an increased risk of congenital malformations,spontaneous abortions,preterm births,or stillbirths [35—37].Smallpox vaccination in the first trimester was associated with a small increase in congenital birth defects;however,the association was small,and this finding was based on limited data [36].A 2006 study collected data on infections in pregnant women from three historical smallpox outbreaks and compared the mortality rates of smallpox-infected pregnant women with and without smallpox vaccinations.The results showed that the mortality rate of pregnant women who were vaccinated against smallpox was significantly lower than that of unvaccinated pregnant women [38].Although the lethality of the monkeypox virus is not as high as that of the smallpox virus,the results of this study have implications for the choice of vaccination strategy for pregnant women with monkeypox infections.Future studies with larger sample sizes are required to verify that smallpox vaccination during pregnancy is not associated with the occurrence of congenital malformations,spontaneous abortions,preterm births,or stillbirths.In addition,we investigated whether smallpox vaccination affects special conditions,such as multiple pregnancies and in vitro fertilization.Are pregnant women with gestational hypertension,diabetes mellitus,or autoimmune diseases suitable candidates for smallpox vaccination? These questions deserve further exploration during follow-up.
The US Priority Population Smallpox Vaccination Program,implemented in 2003 [39],established a National Smallpox in Pregnancy Vaccine Registry to track and evaluate outcomes following accidental smallpox vaccination in pregnant women [40].Before a woman is vaccinated,she should be asked if she is pregnant or plans to become pregnant in the next four weeks,and women who answer very positively should not be vaccinated immediately because of the risk of fetal vaccinia [41].For women who are suspected of being pregnant or who want to confirm that they are not pregnant,a urine pregnancy test should be performed on the day of vaccination.If a pregnant woman is accidentally vaccinated or becomes pregnant within four weeks of smallpox vaccination,her doctor should inform her of the possible risks to the fetus.However,vaccination during pregnancy is not a reason for the termination of pregnancy [41].According to the report,of the 52,185 women who received the smallpox vaccine through the US Priority Population Smallpox Vaccination Program,62 received the vaccine within 42 days before becoming pregnant,and 23 became pregnant within four weeks of vaccination.Follow-up results reported early miscarriages in two women.However,the report did not indicate that miscarriages were related to smallpox vaccination [42].Nevertheless,vaccination within six weeks prior to conception or within four weeks after smallpox vaccination should be viewed with caution or avoided.
When emergency exposure occurs,there are no absolute contraindications for smallpox vaccination.Monkeypox infection has a higher probability of causing fetal and neonatal death,and women exposed to the monkeypox virus are advised to receive smallpox vaccination,regardless of whether they are pregnant or breastfeeding [31].The risk of fetal vaccinia due to smallpox vaccination during pregnancy is very low [43,44],although it may lead to preterm delivery and fetal and neonatal deaths [45,46].In contrast to the smallpox vaccine ACAM 2000,JYNNEOS™ is a non-replicating,weakened live vaccinia virus that cannot cause smallpox,monkeypox,or any other infectious disease.Vaccinianeutralizing antibody responses in humans were evaluated to establish the effectiveness of JYNNEOS in the prevention of smallpox and monkeypox.The Advisory Committee on Immunization Practices (ACIP) system evaluated three randomized controlled and 15 observational studies,including a total of 5775 subjects.After comparing geometric mean titers and seroconversion data,the JYNNEOS™ vaccination group was slightly higher than the ACAM2000 group on key variables,while having fewer serious adverse events(including myocarditis and pericarditis) after the initial vaccination.Based on the results of the Grading Recommendations Assessment,Development and Evaluation(GRADE)evaluation and evidence to recommendations(EtR) framework,the ACIP voted unanimously in favor of recommending JYNNEOS™ as an alternative to ACAM2000 for primary vaccination.The ACIP systematically evaluated one additional randomized controlled trial and 17 observational studies,including a total of 6417 subjects,using the same methodology and determined that the JYNNEOS™booster should be used as an alternative to the ACAM2000 booster [47].The Center for Disease Control and Prevention (CDC) recommends JYNNEOS™ smallpox vaccination[48] and prohibits ACAM 2000 [47],especially for infants aged < 1 year,pregnant women,and nursing women [49].
The JYNNEOS™ vaccine was administered by two subcutaneous injections four weeks apart [48].People who have been vaccinated against smallpox in the past may need only one dose.Because there is limited global knowledge of the effectiveness of the JYNNEOS™ vaccine,pregnant women or newborns who have completed vaccination need to continue to take steps to protect themselves from infection by avoiding close skin-to-skin contact,including those infected with the monkeypox virus or exposed to the ACAM 2000 live vaccine [50].From an evidence-based perspective,the data on the use of the JYNNEOS™ smallpox vaccine in pregnant women are not sufficient to clarify the risks associated with vaccination during pregnancy and pregnancy outcomes.The JYNNEOS™ vaccine was tested in combined animal studies of developmental toxicity in rats and rabbits.In one study in female rabbits,three human doses of JYNNEOS™ (a single dose of 0.5 mL) were administered via the subcutaneous route: before conception,on gestation day 0,and on gestation day 14.In three female rat studies,researchers similarly administered two or three human doses of JYNNEOS™ (a single dose of 0.5 mL) via the subcutaneous route to female rats: before mating,on day 0 of gestation,and on day 14 of gestation;before mating,on day 0 of gestation;or on days 0 and 6 of gestation.None of the studies reported vaccine-related fetal malformations,stunting prior to lactation cessation,or effects on maternal fertility [47].
There are limited retrospective investigations on complications associated with smallpox vaccination in newborns.A review reported that among smallpox-vaccinated infants(one year of age) in the United States,there were six cases of encephalitis,one case of progressive vaccinia,14 cases of eczema vaccinatum,507 cases of accidental inoculation,394 cases of generalized rash per million,including hypersensitivity reactions,and five deaths [51].To date,this is the largest cross-sectional study related to live smallpox vaccination in infants,and the dataset also suggested that the risk of adverse vaccine reactions was very high in the group of infants under one year of age after vaccination.The US CDC provides guidelines for smallpox vaccination [52],and there is still no global consensus on which month of life is the most appropriate time for smallpox vaccination in newborns [53—55].Therefore,newborns are vaccinated prophylactically only in the event of monkeypox virus exposure.According to the ACIP recommendations,newborns should also be vaccinated with the JYNNEOS™ vaccine,which has few side effects,to reduce adverse neonatal vaccine reactions.Of course,we expect that evidence from prospective research will support these proposals.
If a pregnant woman or newborn is accidentally exposed to the monkeypox virus,the sooner the exposed person is vaccinated,the better.The US CDC recommends vaccination within four days of the date of exposure to prevent an attack of the disease.If administered within 4—14 days of the date of exposure,vaccination may reduce symptoms of the disease but may not prevent it [30].Successful vaccination provides adequate protection from the disease for more than 95% of individuals for 5—10 years [56].
Antiviral drugs
Several existing mainstream antiviral drugs are unsuitable for pregnant and neonatal populations.The US CDC currently recommends tecovirimat for people with severe monkeypox infections,such as hemorrhagic disease,fusion skin lesions,sepsis,encephalitis,or other conditions requiring hospitalization.One patient with monkeypox infection on Tecovirimat showed a reduction in the duration of illness and viral shedding [57],but the evidence-based grade of the case reports is low,and large clinical trials are needed to confirm the effectiveness of Tecovirimat.Pregnant or breastfeeding women and children younger than eight years of age are defined as being at high risk of severe monkeypox infection [58].However,tecovirimat is not indicated for infants weighing less than 13 kg;therefore,it also includes the neonatal population.This is because the toxicity of tecovirimat to the fetus and its effects on neonatal development are unknown.Although one positive case at 28 months of age was reported in which the child showed no adverse effects following administration of the drug,researchers are concerned that tecovirimat may have toxic effects on immature developing kidneys [58].The drug has not been authorized for use in non-severely infected pregnant women who are breastfeeding,as trace amounts of the drug can be detected in breast milk [59].In studies on fertility and early embryonic development in mice,researchers did not observe any effects of Tecovirimat on female fertility when mice were exposed to dose levels 24 times higher than human exposure.In the reproductive toxicity study of tecovirimat,rabbits were exposed to 100 mg/kg (0.4 times the human exposure at the recommended human dose) and mice to 1000 mg/kg (approximately 23 times higher than human exposure at the recommended human dose),and embryo-fetal toxicity was not observed.However,at a dose of 100 mg/kg in rabbits,researchers detected maternal toxicity in females,which showed weight loss (due to reduced feeding) and death (9/22) [59].As the mechanism of maternal toxicity due to Tecovirimat is not known,this drug should not be used in infected pregnant women or newborns.The causes of mortality or weight loss in maternal rabbits need to be investigated further.
Brincidofovir,taken orally,is an analog of the intravenous drug cidofovir.Compared to cidofovir,brin cidofovir has a somewhat improved safety profile,such as less nephrotoxicity [60].Brincidofovir has been shown to be effective against the monkeypox virus in in vitro and animal studies,but there is a lack of data on its efficacy in treating human cases of monkeypox.Based on the results of animal reproduction studies [61],when pregnant rats and rabbits were administered lower than recommended human doses of brin cidofovir,significant embryotoxicity,reduced embryo-fetal survival,and fetal structural malformations were observed in the experimental group.This suggests that brincidofovir can cause serious adverse effects in the fetus when administered to pregnant women.Moreover,researchers administered a lower dose of brincidofovir than recommended for humans,and some of the rats in the experimental group developed breast cancer or squamous cell carcinoma.In an embryofetal development study of rats and rabbits,pregnant animals were given oral doses of brincidofovir of up to 4.5 mg/kg/day from day 7 to day 20 of gestation,and maternal toxicity was observed in rats at either the 1.5 mg/kg dose or the 4.5 mg/kg dose,mainly in the form of reduced food consumption and slow weight gain.When rabbits were exposed to a dose of 4.5 mg/kg of brincidofovir,in addition to the symptoms observed in rats,morphological changes in the maternal toxicity profile of the rabbits were observed,including changes in appearance and internal organs and skeletal deformities[61].There are no data or reports on the use of brincidofovir in pregnant women or newborns to assess major birth defects,miscarriages,and other adverse maternal and infant outcomes.When brincidofovir was administered orally to lactating rats (4 mg/kg per day or 15 mg/kg twice a week),researchers detected brincidofovir in the milk of mothers but not in the plasma of littermates.There are no reports on the presence of brincidofovir in human breast milk,and there are no data on the effect of brincidofovir on breastfed newborns or maternal fertility.
Vaccinia immunoglobulin (VIG)and orthopoxvirus-specific human monoclonal antibodies
The CDC does not recommend VIG for the prevention or treatment of monkeypox because there are no data from VIG studies in Africa or the United States [62].VIG is currently used primarily in patients with progressive vaccinia [63,64]as first-line treatment for severe complications [43].However,it is a potential treatment for patients with severe vaccinia following smallpox vaccination.There is still a lack of studies on the effectiveness of VIG against monkeypox,and VIG has not been tested in humans against monkeypox.It is also unclear whether administration of VIG to pregnant women affects female fertility or whether it can cause fetal harm [63].Human C immunoglobulin has been widely used in the pregnant population for many years and generally does not have a negative impact on the reproductive system.The risk/benefit of immunoglobulin administration should be evaluated on a case-by-case basis,and we believe that pregnant women or newborns,regarding immunoglobulin use,can refer to the prophylactic process and treatment experience after varicella virus and herpes zoster virus exposure[65—67].
A study developed a recombinant vaccinia immunoglobulin (rVIG) containing 26 specific human immunogloblin G(IgG)1 antibodies against different smallpox virus proteins[68].A mouse model of orthopoxvirus attack was selected as the vehicle for the trial.For the first 24 hours after virus infection,the experimental group was administered 200,100,30,10,3,1,and 0.3 μg rVIG,while the control group was administered 400 μg VIG.Viral titers in the lungs of the mice were measured on day five after infection.The results showed that VIG failed to protect the mice from attack;however,the 200 μg and 100 μg doses of rVIG protected 100%of the mice from death.Doses lower than 100 μg had no protective effect in mice.This suggests that rVIG captures the diversity and specificity of the natural immune response to orthopoxviruses,with higher specificity and activity than VIG.rVIG has a very wide therapeutic window in murine pox,achieving protection in experimental animals from 14 days before to 6 days after a viral attack with a single injection of rVIG.No surviving mice showed morbidity or mortality after receiving another lethal dose of the virus 63 days after the initial attack,indicating that rVIG had allowed the organism to develop immune memory [69].In conclusion,these results established that rVIG might be a potential prophylactic or therapeutic agent for orthopoxvirus infection.
Human-specific monoclonal antibodies are a determining factor in the protective effect against orthopoxvirus attacks in a mouse model.Researchers obtained a set of orthopoxvirus human-specific monoclonal antibodies from poxvirus-immunized subjects and used them to investigate the molecular basis of broadly neutralizing antibodies against different orthopoxviruses [70].The study analyzed in detail the major specific antibodies that cross-neutralized with three viruses,smallpox,monkeypox,and cowpox,and found that most of them (79%,38/48) cross-neutralized at least two orthopoxviruses,with 12 (25%) antibodies neutralizing three orthopoxviruses,including smallpox,monkeypox,and cowpox.The researchers then designed a mixture of two specific human monoclonal antibodies (Mix6 and its derivative Mix4) and found that the use of a mixture of human-specific monoclonal antibodies was more effective than the use of potent monoclonal antibodies alone in the orthopoxvirus neutralization crossover assay.Mix6 and Mix4 showed significant superiority in the prevention of orthopoxvirus infection compared with conventional VIGIV,suggesting that novel mixtures of potent human monoclonal antibodies could be used to treat orthopoxvirus infection.Future research should include larger animal models,such as nonhuman primates.There is still a long way to go from the discovery of novel specific antibody complexes to their application in pregnant women and newborns at a high risk of monkeypox infection.
Delivery and care of newborns
There is no evidence to suggest the mode of delivery best suited for pregnant (infectious) women with monkeypox infection.Since the monkeypox virus may lead to vertical transmission [9],meaning that the newborn is infected in utero (which can be screened for congenital skin lesions by noninvasive ultrasound),cesarean delivery in this case does not provide additional benefit to the mother and baby.When a monkeypox-infected pregnant woman has genital or vaginal lesions or when the pregnant woman is unable to identify genital lesions,these conditions may expose the newborn to monkeypox infection via the birth canal,and cesarean delivery should be recommended.
After delivery,medical personnel should use PCR methods to detect monkeypox virus nucleic acid in specimens of maternal amniotic fluid,umbilical cord blood,rashes,herpes,scabs,and nasopharyngeal swabs while carefully observing the newborn for signs of skin lesions such as rashes and blisters.Some studies have shown that some patients have changes in white blood cells and platelets and may also have elevated transaminase levels,reduced blood urea nitrogen levels,and hypoproteinemia [19].Neonatologists should monitor the serological and biochemical parameters of newborns.Institutions should focus on placental pathology and inflammatory cytokine levels and assess the correlation between viral load and inflammatory cytokine levels to further clarify the mechanisms and evidence of vertical transmission [9].
There are limited evidences on evidence-based care of newborns with suspected or infected monkeypox,and caregivers should wear personal protective equipment (PPE).In addition,some guidelines for maternal management of varicella in pregnancy are worth considering [71].No matter whether the mother is a suspected or confirmed patient,caregivers should adequately wipe,remove amniotic fluid and other soil from the newborn’s body,and bathe the newborn as soon as possible.After delivery,mother-to-child transmission should be eliminated as soon as possible,and caregivers should transfer the newborn to an isolation ward for medical observation or treatment under the following situations.
1.The mother’s nucleic acid result was negative for the monkeypox virus: end isolation for the newborn.
2.The mother’s nucleic acid result was positive,and the newborn’s nucleic acid result was negative;the mother and baby were kept in the same room and isolated for 3 weeks.Care can be provided as usual for the newborn.
3.Positive nucleic acid results for both mother and newborn:
(1) Space: mother and newborn can live in the same room.
(2) Environment: set the room at the right temperature and humidity,minimize movement of people,open the windows regularly,disinfect the newborn's belongings,and use 75% ethanol (medical alcohol) and chlorinated disinfectant water to wipe the floor and furniture.
(3) Daily necessities: heat-resistant bottles and teats should be disinfected at high temperature;contaminated clothing,bed sheets,swaddling clothes,and diapers of newborns should be changed in a timely manner.
(4) Contact: there should be no direct skin-to-skin contact between the mother and newborn.If contact is needed,the mother should always wear gloves and clean clothing,cover all visible skin below the neck,cover newborn skin lesions,keep clean and dry to prevent secondary infection,and change clothing after contact.
(5) Personnel: obstetric and neonatal nursing staff should follow the local healthcare facility’s monkeypox infection prevention and control protocol,master the use and handling of PPE,and be properly disinfected and protected.
4.Mother’s nucleic acid test result is positive,and the neonatal nucleic acid test result remains negative,but the neonate has clinical symptoms such as fever or rash: avoid keeping the mother and baby in the same room and exclude the possibility of other viral infections that may cause fever or rash via tests as soon as possible,such as varicella zoster virus.Care should be taken the same as in the the third situation mentioned above.
Breastfeeding
The benefits of mother-infant rooms,skin-to-skin contact,and breastfeeding for the growth and development of newborns are well known.However,direct contact between a monkeypoxinfected mother and her newborn is not recommended,given the risk of transmission and the potential for severe infection in newborns in close contact with the monkeypox virus [72].Separating a monkeypox-infected mother from her newborn(isolation in a separate room) is the best way to prevent transmission of the virus to the newborn.The WHO recommends that mothers with monkeypox do not breastfeed (do not breastfeed until all crusts on the rash have fallen off),the starting point being to minimize the risk of transmission to the newborn after birth [73].However,it has also been suggested that in low-and middle-income countries,the benefits of breastfeeding may outweigh the increased risk of monkeypox infection in newborns [73].It is unknown whether monkeypox virus is present in breast milk.However,when a newborn needs to be considered for breastfeeding,it is currently believed that the newborn can be given pasteurized maternal breast milk[74].For infections occurring during pregnancy,if a pregnant woman’s lesions or blisters have crusted over and fallen off,a mother who is nucleic acid-negative may breastfeed her newborn directly.If a breastfeeding woman develops a monkeypox infection,newborns who need to be breastfed can be fed sterilized and nucleic acid-negative breast milk after the mother and baby are isolated.
Maternal breastfeeding should be avoided after live vaccination with a replicating vaccine (ACAM 2000) for at least 3 or 4 weeks,evidence for which comes from a case of home transmission after smallpox vaccination reported by JAMA in 2004 [75].On May 4,2003,a US Army soldier followed all precautions to avoid home transmission after receiving a primary smallpox vaccination.Approximately one week later,blisters appeared on his wife’s areola.About 2 weeks later,the breastfed daughter developed papules.A PCR test for the virus yielded positive results.This is the first case worldwide of post vaccination transmission from mother to infant through direct skin-to-skin and skin-to-mucous membrane contact that occurred during breastfeeding.Breastfed infants in close contact with a live smallpox vaccine recipient with replicating smallpox are at potential risk of vaccine infection,and precautions should be taken.
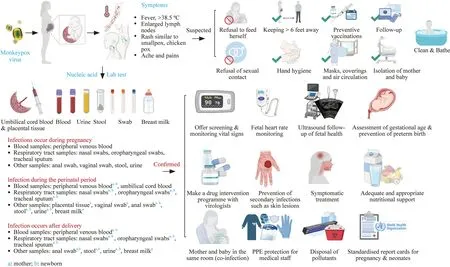
Fig.1 Management of pregnant women and newborns exposed to or infected with the monkeypox virus (Created with BioRender.com and reprint copyright obtained)
There is no evidence that mothers who receive live nonreplicating vaccine (JYNNEOS) transmit the vaccine to their newborns following skin-to-skin and skin-to-mucous membrane contact during breastfeeding.Similarly,it is not known whether JYNNEOS is excreted in human milk or affects infants.However,because the JYNNEOS vaccine does not replicate,it should not present a risk of transmission to infants through breast milk [47].However,caution should be taken for breastfeeding mothers receiving antiviral treatment,as tecovirimat and brin cidofovir have been detected in the milk of animals [59,61].
Guidelines or expert consensus for the maternal and infant management of monkeypox
Currently,there are no specific global guidelines for the pregnant and neonatal populations.We believe that there is a need for a recognized guideline for pregnant women and children,especially infants and children,which can be quickly put into use in healthcare settings worldwide during the unfortunate global outbreak of monkeypox and can be updated when new evidence becomes available.The lack of clinical management guidelines for monkeypox in some regions where monkeypox virus infection is currently disseminated or undetected may lead to a lack of effective management by healthcare providers of specific monkeypox patient populations,such as pregnant women,fetuses,and newborns.If prevention and control fail,the human monkeypox virus can become a serious public health problem.Given the recent effective global advocacy of the human monkeypox virus,this is an excellent opportunity to enhance the prevention of monkeypox infection and interrupt the global pandemic of monkeypox.In this study,we outlined the existing global clinical management guidelines for monkeypox,extract prevention and control methods related to pregnant women and children,and provide the best prevention and control strategies for this specific group of pregnant women and children.Figure 1 shows the management of pregnant women and newborns exposed to or infected with the monkeypox virus.
Information collection for pregnant women and newborn cases
We recommend the use of the Global Monkeypox Clinical Information Collection Form from the WHO [76].Pregnancy and neonatal modules have been specifically added to the WHO Form to systematically collect demographic,symptomatology,and laboratory information on pregnant women and newborns to standardize data collection on the clinical features of monkeypox in outpatient and inpatient cases.This will facilitate the formation of a global cohort to retrospectively summarize the effects of monkeypox on pregnancy outcomes in pregnant women and on neonatal growth and development.This study could provide important information for the development of monkeypox prevention and control strategies for specific populations.
What else should we learn in the future?
Pregnant women are at an increased risk of monkeypox exposure because their immune systems are altered during pregnancy.Available evidence has confirmed that monkeypox can be transmitted vertically from mother to child,but many questions remain unanswered: (1) when is the monkeypox virus transmitted from the mother to the fetus;(2) what happens to the fetus if the mother is infected early in pregnancy;(3) what maternal factors put the fetus at a high risk of vertical infection with the monkeypox virus;(4) what happens to the fetus during labor;(5) is the fetus at a higher risk of monkeypox virus infection during labor than during other stages of pregnancy;and (6) what is the role of immunity against monkeypox during pregnancy in women previously exposed to monkeypox.These clinical problems related to maternal—fetal medicine have yet to be investigated further.Certainly,future research cannot be separated from the ongoing development of new vaccines and potent drugs against the monkeypox virus.Although we collated some elements for the prevention and control of monkeypox in the mother-infant population,it is not sufficient.Governments need to develop detailed prevention and control measures for pregnant women,fetuses,and newborns to prevent the spread of the monkeypox virus among mother and child populations;after all,newborns are the future of the planet.
AcknowledgementsWe sincerely thank all the NICU doctors and nurses who participated in the writing of this review by taking the time to review all the guidelines on monkeypox and completing the extensive reading and review of the literature.
Author contributionsYK,TLK and XFF contributed equally to this paper.YK,TLK and XFF: data curation,writing—original draft.ZP,LCM,HLY,WLS and CGQ : data curation.ZWH: conceptualization,writing—review and editing.
FundingNone.
Data availabilityNot applicable.
Declarations
Conflict of interestThe authors have no conflict of interest to disclose.No financial benefits have been received.
Ethical approvalNot applicable.
 World Journal of Pediatrics2023年3期
World Journal of Pediatrics2023年3期
- World Journal of Pediatrics的其它文章
- Monkeypox virus: past and present
- Stressful life events,psychosocial health and general health in preschool children before age 4
- Number and distribution of eosinophils and lymphocytes in the Japanese pediatric gastrointestinal tract: in search of a def inition for “abnormally increased eosinophils”
- Associations of preterm and early-term birth with suspected developmental coordination disorder: a national retrospective cohort study in children aged 3–10 years
- T-cell and B-cell repertoire diversity are selectively skewed in children with idiopathic nephrotic syndrome revealed by high-throughput sequencing
- Pathologic etiology and predictors of malignancy in children with cervical lymphadenopathy
