肝细胞癌合并肝硬化门静脉高压症腹腔镜同期联合手术术后肺部感染的危险因素分析
文静 贾哲 赫嵘 张艳华 张宏伟 张珂

摘要:
目的 觀察腹腔镜同期联合手术治疗肝细胞癌(HCC)合并肝硬化门静脉高压症(PHT)术后肺部感染发生率并分析危险因素。方法 回顾性分析2017年1月—2022年2月首都医科大学附属北京地坛医院普外科105例HCC合并肝硬化PHT腹腔镜同期联合手术患者临床资料。记录可能引起肺部感染的30项因素,包括基本情况、疾病因素、手术因素和术后因素。观察手术恢复情况,记录肺部感染发生情况。计数资料两组间比较采用χ2或 Fisher精确检验。Logistic多因素回归分析筛选肺部感染的独立危险因素。结果 105例患者中66例行腹腔镜断流联合肝切除术,39例行腹腔镜断流联合射频消融(RFA),均顺利完成手术,无中转开腹和术后非计划再次手术病例,无术后30 d和住院期间死亡病例,中位住院时间20(14~25)d。肺部感染发生率为25.71%(27/105)。吸烟(OR=3.362,95%CI: 1.282~8.817, P=0.014)、MELD评分(OR=3.801,95%CI: 1.007~14.351,P=0.049)、肿瘤位置(OR=1.937,95%CI: 1.169~3.211,P=0.010)、手术方式(OR=0.006,95%CI: 0.001~0.064,P<0.001)、术中输液量(OR=4.871,95%CI: 1.211~19.597,P=0.026)和术后合并胸水(OR=9.790,95%CI: 1.826~52.480,P=0.008)为肺部感染的独立危险因素。结论 HCC合并肝硬化PHT腹腔镜同期联合手术患者具有较高肺部感染风险。术后合并胸水是引发肺部感染的高危因素,断流联合RFA可显著降低肺部感染风险。应加强术前预康复、围手术期肝功能维护、术中损伤控制和目标导向性液体治疗、减轻术后第三间隙积液,以降低肺部感染发生。
关键词:
癌, 肝细胞; 肝硬化; 高血压, 门静脉; 外科手术; 感染
基金项目:国家自然科学基金(31970566)
Risk factors for pulmonary infection after laparoscopic surgery in treatment of hepatocellular carcinoma with liver cirrhosis and portal hypertension
WEN Jing, JIA Zhe, HE Rong, ZHANG Yanhua, ZHANG Hongwei, ZHANG Ke. (Department of General Surgery, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China)
Corresponding author:
ZHANG Ke, zhangke302@sina.com (ORCID:0000-0002-5006-8674)
Abstract:
Objective To investigate the incidence rate of pulmonary infection after laparoscopic surgery and related risk factors in patients with hepatocellular carcinoma (HCC) comorbid with liver cirrhosis and portal hypertension (PHT). MethodsA retrospective analysis was performed for the clinical data of 105 HCC patients with liver cirrhosis and PHT who underwent laparoscopic surgery in Beijing Ditan Hospital, Capital Medical University, from January 2017 to February 2022. A total of 30 factors that might cause pulmonary infection were recorded, including general information, disease factors, surgical factors, and postoperative factors. Postoperative recovery was observed and the occurrence of pulmonary infection was recorded. The chi-square test or the Fishers exact test was used for comparison of categorical data between two groups, and the multivariate logistic regression analysis was used to investigate the independent risk factors for pulmonary infection. Results Among the 105 patients, 66 underwent laparoscopic devascularization combined with hepatectomy and 39 underwent laparoscopic devascularization combined with radiofrequency ablation (RFA). The surgery was successful for all patients, with no case of conversion to laparotomy or unscheduled reoperation. No death was observed within 30 days after surgery and during hospitalization, with a median length of hospital stay of 20 days (range 14-25 days). The incidence rate of pulmonary infection was 25.71% (27/105). Smoking (odds ratio [OR]=3.362, 95% confidence interval [CI]:1.282-8.817, P=0.014), MELD score (OR=3.801, 95%CI: 1.007-14.351, P=0.049), tumor location (OR=1.937, 95%CI: 1.169-3.211, P=0.010), surgical procedure (OR=0.006, 95%CI: 0.001-0.064, P=0.000), intraoperative infusion volume (OR=4.871, 95%CI: 1.211-19.597, P=0.026), and postoperative pleural effusion (OR=9.790, 95%CI: 1.826-52.480, P=0.008) were independent risk factors for pulmonary infection. Conclusion There is a relatively high risk of pulmonary infection in HCC patients with liver cirrhosis and PHT undergoing laparoscopic surgery. Postoperative pleural effusion is the high risk factor for pulmonary infection, and devascularization combined with RFA can significantly reduce the risk of pulmonary infection. It is recommended to strengthen preoperative rehabilitation, perioperative liver function maintenance, intraoperative damage control, and goal-oriented fluid therapy and reduce postoperative fluid accumulation in the third space, so as to reduce the incidence rate of pulmonary infection.
Key words:
Carcinoma, Hepatocellular; Liver Cirrhosis; Hypertension, Portal; Surgical Procedures, Operative; Infection
Research funding:
National Natural Science Foundation of China (31970566)
肝细胞癌(HCC)合并肝硬化门静脉高压症(portal hypertension, PHT)、食管胃底静脉曲张破裂出血(esophagogastric varices bleeding, EGVB)是外科治疗难点。近10年来同期联合手术治疗HCC合并EGVB研究报道不断增多,证实同期行断流联合肝切除或射频消融(radiofrequeney ablation,RFA)均是安全有效的治疗方式,腹腔镜同期联合手术,降低了手术创伤,加快了术后康复[1]。肝硬化患者接受腹部外科手术,术后肺部感染发生率高于腹腔感染[2]。PHT可造成肺内毛细血管扩张,血管新生和血管内单核巨噬细胞聚集[3],麻醉和手术创伤打击可加剧PHT全身高动力循环[4],合并肺部感染,加重PHT肺部病理改变,引发通气血流比失调,弥散功能障碍以及动静脉分流,严重时诱发肝肺综合征和呼吸衰竭[5]。目前少有关于HCC合并肝硬化PHT同期联合手术肺部感染的研究报道,本研究旨在分析腹腔镜同期联合手术肺部感染危险因素,以期为临床制订针对性围手术期处理措施提供参考。
1 资料与方法
1.1 研究对象 回顾性分析2017年1月—2022年2月首都医科大学附属北京地坛医院普外科连续完成的105例腹腔镜断流联合肝切除或RFA患者临床资料。全部病例均为乙型肝炎后肝硬化,HCC临床诊断参照欧洲肝病学会(EASL)指南[6],术后病理均证实为HCC。手术适应证:(1)年龄18~65岁,肝功能Child-Pugh A/B级,无严重心肺肾及代谢性疾病,ASA分级≤3级;(2)中国肝癌临床分期(2017版)≤Ⅱa期[7];(3)既往有EGVB史,术前内镜评估食管胃底静脉曲张程度为F2~F3级[8],伴红色征阳性,认为经一个疗程内镜注射或套扎治疗,仍然会发生EGVB。超声与腹部增强CT或MRI均无门静脉系统血栓。脾脏内侧缘不超过腹中线,下缘不超过左锁骨中线肋缘下5 cm,CT扫描脾脏不超过7个肋单元,无腹壁曲张静脉团,后腹膜无广泛粗大侧支分流。
1.2 手术方法 肿瘤位于肝表面,易于手术切除,肝切除量不超过两个肝段行断流联合肝切除。肿瘤位置深在,估计肝切除量大于两个肝段行断流联合RFA。静吸复合全身麻醉。脐下缘建立观察孔,腹中线剑突与脐中点建立主操作孔,剑突下建立副操作孔,左侧腋前线肋缘下避开脾脏下缘建立助手辅助孔。根据术前规划和术中探查情况,可在右侧肋缘下建立1~2个操作孔,便于肝切除操作及RFA时腹腔镜超声引导。术中先结扎脾动脉,继而行肝切除或RFA,最后完成脾切除及贲门周围血管离断术。肝切除时采用控制性低中心静脉压技术,不阻断第一肝门。行RFA前腹腔镜超声引导肿瘤穿刺病理活检。扩大脐下缘观察孔取出脾脏及肝标本。于胰尾旁放置腹腔引流管,联合肝切除者,肝断面旁另行放置腹腔引流管,术后观察引流情况。
1.3 预防手术部位感染 手术开始前予以二代头孢菌素预防感染,手术时间超過2 h,术中追加一次预防性抗生素治疗,术后延续该治疗方案。无感染病例术后5天停用抗生素,术后感染病例,根据血液、体液、分泌物等标本细菌培养结果,选择敏感抗生素治疗。
1.4 观察指标 记录基本情况(性别、年龄、吸烟史、合并基础疾病、营养状态、肝储备功能、血常规和肝功能),疾病因素(肿瘤位置、大小、个数、分期,曲张静脉分级,门静脉主干直径,腹水),手术因素(手术方式、手术时间、术中出血量、术中输血和输液量)和术后因素(腹水引流量、合并胸水)。观察手术恢复情况,记录肺部感染发生情况。肺部感染诊断标准采用美国传染病学会和美国胸科学会2016版指南[9]。胸水为术后影像学检查提示胸腔积液伴或不伴有呼吸症状。
1.5 统计学方法 应用SPSS 21.0软件进行数据分析,非正态分布的计量资料以M(P25~P75)描述,计数资料两组间比较采用χ2或 Fisher精确检验。单因素分析有统计学差异的因素,二元逐步向前法Logistic回归行多因素分析。P<0.05为差异有统计学意义。
2 结果
2.1 一般情况 105例患者中66例(62.9%)行腹腔镜断流联合肝切除,39例(37.1%)行腹腔镜断流联合RFA,均顺利完成手术,无中转开腹病例,中位手术时长为270(240~345) min,中位出血量为500(300~600) mL。无术后非计划再次手术病例,无术后30 d和住院期间死亡病例,中位住院时间20(14~25) d。肺部感染发生率为25.71%(27/105)。
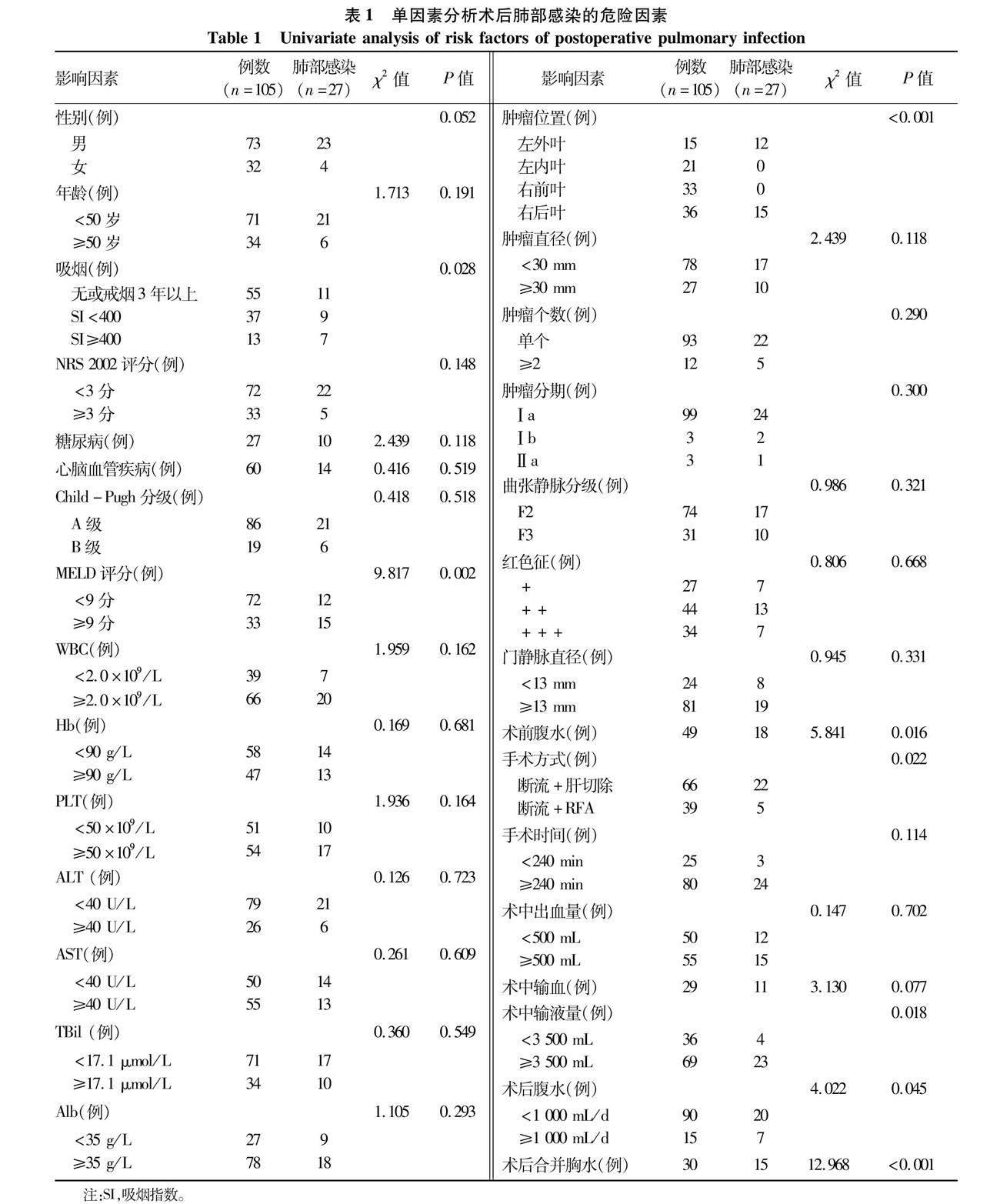
2.2 肺部感染危险因素分析 单因素分析提示,肺部感染与吸烟、MELD评分、肿瘤位置、术前合并腹水、手术方式、术中输液量、术后腹水量和术后合并胸水相关(P值均<0.05)(表1)。Logistic回归多因素分析提示,吸烟、MELD评分、肿瘤位置、手术方式、术中输液量和术后合并胸水为肺部感染独立危险因素(P值均<0.05)(表2)。
3 讨论
外科术后肺部感染发生率为1.3%~17.15%[10],上腹部手术易发生肺部感染,腹腔镜手术可降低肺部感
染发生率[11]。本研究术后肺部感染发生率为
25.71%,提示HCC合并肝硬化PHT同期联合手术患者面临较高肺部感染风险。吸烟、MELD评分、肿瘤位
置、手术方式、术中输液量和术后合并胸水为本研究肺部感染的独立危险因素。手术方式是唯一的保护因素,接受断流联合RFA患者肺部感染风险显著低于断流联合肝切除患者(OR=0.006)。笔者前期一项HCC合并肝硬化PHT开腹同期联合手术安全性与近远期疗效临床研究[12]结果表明:断流联合RFA手术创伤低,对肝功能影响小,术后肺部感染等严重并发症发生率低,且远期无复发生存率与断流联合肝切除无显著差异,是此类患者更为适宜的联合手术方式。
吸烟可诱发炎症介质的释放,抑制气道纤毛运动,减少肺表面活性物质,增加气道黏液分泌,降低支气管黏膜清除能力[13]。本研究中SI每升高一级,肺部感染风险增加3.4倍。按《中国加速康复外科临床实践指南(2021)》[14],吸烟患者术前应严格戒烟4周,并进行准确的肺功能评估,了解肺通气和弥散功能改变,重度吸烟者应在戒烟同时,予以气道雾化吸入和服用沐舒坦等药物,以稀释痰液促进排出,必要时予以预防性抗生素治疗。
Chlid-Pugh分级和MELD评分是肝硬化患者肝储备功能评估与手术风险预测的常用模型[15-17]。Child-Pugh分级对肝硬化手术患者术后死亡具有较高预测价值[18]。肝切除或PHT相关手术,MELD评分≥9分是发生术后严重并发症和手术死亡的敏感预测指标[19]。本研究Chlid-Pugh分级不是肺部感染的危险因素,而MELD评分≥9分肺部感染风险增加3.8倍,提示肝硬化PHT患者术前应用Chlid-Pugh分级进行肝储备功能评估的同时,要结合MELD评分预测其手术风险,并加强围手术期保肝治疗。 肝硬化PHT高动力循环造成全身血容量分布不均,有效循环血量不足。术中低容量可造成脏器灌注不全,而高容量增加肺水肿、肺部感染风险[20]。本研究中术中输液量≥3 500 mL可使肺部感染风险增加4.9倍。欧阳春磊等[21]在断流术中以每搏量变异度评估血流动力学变化,经目标导向性液体治疗进行术中个体化补液,认为每搏量变异度控制在4.5%~8.5%,既可避免容量过低脏器灌注不全,也可避免容量过高,以减轻肺水肿和肠屏障功能损伤,降低术后肺部感染风险。
本研究27例肺部感染者中15例HCC位于肝右后叶,12例位于肝左外叶,其中22例联合肝切除。肝后叶HCC患者发生肺部感染风险是肝左外叶HCC患者的5.8倍。肝右后叶肿瘤切除,需切断镰状韧带、右三角韧带、右冠状韧带并分离肝裸区,膈肌分离范围大。肝膈韧带和脾膈韧带的分离、切断,破坏其内的淋巴管道,造成腹腔淋巴液经胸导管回流增多,导致胸水发生[22]。术后肝断面渗液和腹腔引流管刺激,RFA热传导和消融毁损灶无菌坏死过程,均会影响膈肌淋巴循环,增加胸水风险[23]。
本研究显示,胸水患者发生肺部感染风险是无胸水患者的9.79倍。胸水黏蛋白使胸膜间摩擦力增大,影响肺通气。胸水炎症因子弥散进入肺泡组织间隙影响其顺应性,增加肺通气阻力。胸水使肺不张肺实变,造成通气血流比失调,影响肺换气。胸水限制肺膨胀,削弱咳嗽排痰能力,增加肺部感染风险[24]。肝硬化低蛋白血症血胶体渗透压下降,抗利尿激素活性增强加重水钠潴留是胸水产生的始动因素,膈肌分离损伤和围手术期液体负荷过重是胸水产生的诱发因素。术前准确评估肝功能,加强保肝治疗,改善全身营养;术中准确把握组织分离层次,减小手术创面,降低创伤应激反应;术后及时纠正低蛋白血症,通过水钠摄入控制和小剂量利尿剂对抗醛固酮抗利尿作用,减轻第三间隙积液。由此以降低胸水发生风险,进而降低肺部感染发生。
总之,HCC合并肝硬化PHT腹腔镜同期联合手术患者具有较高肺部感染风险。术后合并胸水是引发肺部感染优势比最高的致病因素,断流联合RFA可降低肺部感染风险。可通過术前预康复、围手术期保肝、术中损伤控制和目标导向性液体治疗、术后减轻第三间隙积液等措施,降低肺部感染发生。本研究为单中心小样本回顾性临床研究,观察指标难免存在偏倚,今后需扩大病例数以校正偏倚。
伦理学声明:本研究方案于2022年6月22日获首都医科大学附属北京地坛医院伦理委员会审批通过,批号:2022-032-01,符合临床研究伦理规范。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:文静负责课题设计,资料分析,撰写论文;贾哲、赫嵘、张艳华、张宏伟参与临床数据库设计和数据收集,修改论文;张珂负责拟定写作思路,指导撰写文章并最后定稿。
参考文献:
[1]LI XC, WU YS, CHEN DK, et al. Laparoscopic hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: A systematic review and meta-analysis[J]. Cancer Manag Res, 2019, 11: 5711-5724. DOI: 10.2147/CMAR.S189777.
[2]MARTIN MATEOS R, GARCIA DE LA FILIA MOLINA I, ALBILLOS A. Pre-surgical risk assessment in patients with cirrhosis[J]. Acta Gastroenterol Belg, 2020, 83(3): 449-453.
[3]WEI L, WAN H. Roles of hemodynamic alterations in portal hypertension and cirrhosis[J]. J Clin Hepatol, 2013, 29(4): 308-310.
魏丽, 万红. 门静脉高压症血流动力学改变的发病机理[J]. 临床肝胆病杂志, 2013, 29(4): 308-310.
[4]GRACIA-SANCHO J, MARRONE G, FERNNDEZ-IGLESIAS A. Hepatic microcirculation and mechanisms of portal hypertension[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(4): 221-234. DOI: 10.1038/s41575-018-0097-3.
[5]CHEN WW, WU SD, JIANG W. Research progress on hepato-pulmonary syndrome[J]. Chin J Clin Med, 2018, 25(5): 810-814. DOI: 10.12025/j.issn.1008-6358.2018.20170692.
陈巍文, 吴盛迪, 蒋炜. 肝肺综合征研究进展[J]. 中国临床医学, 2018, 25(5): 810-814. DOI: 10.12025/j.issn.1008-6358.2018.20170692.
[6]European Association for the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2012, 56(4): 908-943. DOI: 10.1016/j.jhep.2011.12.001.
[7]National Health and Family Planning Commission of the Peoples Republic of China. Diagnosis, management, and treatment of hepatocellular carcinoma (V2017)[J]. J Clin Hepatol, 2017, 33(8): 1419-1431. DOI: 10.3969/j.issn.1001-5256.2017.08.003.
中華人民共和国国家卫生和计划生育委员会. 原发性肝癌诊疗规范(2017年版)[J]. 临床肝胆病杂志, 2017, 33(8): 1419-1431. DOI: 10.3969/j.issn.1001-5256.2017.08.003.
[8]TAJIRI T, YOSHIDA H, OBARA K, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition)[J]. Dig Endosc, 2010, 22(1): 1-9. DOI: 10.1111/j.1443-1661.2009.00929.x.
[9]KALIL AC, METERSKY ML, KLOMPAS M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society[J]. Clin Infect Dis, 2016, 63(5): e61-e111. DOI: 10.1093/cid/ciw353.
[10]CHEN XM, CHEN XP, ZHENG XJ. Application of predictive nursing intervention in prevention of pulmonary infection in elderly patients with gastric cancer after laparoscopic surgery[J]. J Qilu Nurs, 2019, 25(20): 61-63. DOI: 10.3969/j.issn.1006-7256.2019.20.020.
陈吓妹, 陈雪萍, 郑休嘉. 预见性护理干预在老年胃癌患者腹腔镜手术后肺部感染预防中的应用[J]. 齐鲁护理杂志, 2019, 25(20): 61-63. DOI: 10.3969/j.issn.1006-7256.2019.20.020.
[11]KARA S, KPELI E, Y1LMAZ HEB, et al. Predicting pulmonary complications following upper and lower abdominal surgery: ASA vs. ARISCAT risk index[J]. Turk J Anaesthesiol Reanim, 2020, 48(2): 96-101. DOI: 10.5152/TJAR.2019.28158.
[12]ZHANG K, JIANG L, JIA Z, et al. Radiofrequency ablation plus devascularization is the preferred treatment of hepatocellular carcinoma with esophageal varices[J]. Dig Dis Sci, 2015, 60(5): 1490-1501. DOI: 10.1007/s10620-014-3455-1.
[13]CHEN F, LIU BY, CAO XQ, et al. Construction of early warning score for pulmonary infection after radical gastrectomy for gastric cancer[J]. Chin Nurs Res, 2022, 36(8): 1405-1409. DOI: 10.12102/j.issn.1009-6493.2022.08.016.
陈芳, 刘丙云, 曹晓倩, 等. 胃癌根治术后肺部感染早期预警评分表的构建[J]. 护理研究, 2022, 36(8): 1405-1409. DOI: 10.12102/j.issn.1009-6493.2022.08.016.
[14]Chinese Society of Surgery, Chinese Society of Anesthesiology. Clinical practice guidelines for ERAS in China (2021)(Ⅰ)[J]. Med J Peking Union Med Coll Hosp, 2021, 12(5): 624-631.
中华医学会外科学分会, 中华医学会麻醉学分会. 中国加速康复外科临床实践指南(2021)(一)[J]. 协和医学杂志, 2021, 12(5): 624-631.
[15]PENG Y, QI XS, GUO XZ. Child-pugh versus MELD score for the assessment of prognosis in liver cirrhosis: A systematic review and meta-analysis of observational studies[J]. Medicine, 2016, 95(8): e2877. DOI: 10.1097/MD.0000000000002877.
[16]LUO YX, ZHOU T. Value of systemic immune inflammatory index on predicting the prognosis of patients with decompensated liver cirrhosis[J/CD]. Chin J Liver Dis (Electronic Version), 2021, 13(1): 52-58.
罗永祥, 周涛. 全身免疫炎症指数对失代偿期肝硬化患者预后的评估价值[J/CD]. 中国肝脏病杂志(電子版), 2021, 13(1): 52-58.
[17]YANG L, KAN QX, GAO J. Relationship between PALBI, MELD, INR and the prognosis of patients with liver cirrhosis combined with upper gastrointestinal hemorrhage and a multivariate study[J]. J Clin Exp Med, 2022, 21(11): 1137-1141.
杨磊, 阚全香, 高杰. PALBI、MELD及INR值与肝硬化患者合并上消化道出血患者预后的关系及多因素研究[J]. 临床和实验医学杂志, 2022, 21(11): 1137-1141.
[18]JADAUN SS, SAIGAL S. Surgical risk assessment in patients with chronic liver diseases[J]. J Clin Exp Hepatol, 2022, 12(4): 1175-1183. DOI: 10.1016/j.jceh.2022.03.004.
[19]HACKL C, SCHLITT HJ, RENNER P, et al. Liver surgery in cirrhosis and portal hypertension[J]. World J Gastroenterol, 2016, 22(9): 2725-2735. DOI: 10.3748/wjg.v22.i9.2725.
[20]GU ZJ, HU SS, SHI XW, et al. Comparative study of perioperative fluid management in elderly patients with hepatocellular carcinoma complicated with cirrhosis in laparoscopic hepatectomy[J/CD]. Chin Arch Gen Surg(Electronic Edition), 2022, 16(3): 199-204.
顾竹劼, 胡双双, 师小伟, 等. 老年肝癌合并肝硬化患者围手术期不同液体管理在腹腔镜肝切除术中的对比研究[J/CD]. 中华普通外科学文献(电子版), 2022, 16(3): 199-204.
[21]OUYANG CL, REN B, XU C. Effects of different levels of stroke volume variation following goal-directed fluid therapy on short terms of prognosis in cirrhotic patients with portal hypertension under general anesthesia[C]//Proceedings of the 2016 Chinese Society of Integrated Traditional and Western Medicine Anesthesia (CSIA) Annual Meeting, the Third National Symposium on Integrated Traditional and Western Medicine Anesthesia, and the Founding Conference of the Anesthesia Professional Committee of Henan Institute of Integrated Traditional and Western Medicine, Zhengzhou, 2016: 243-246.
欧阳春磊, 任波, 徐晨. 目标导向液体治疗对肝硬化门脉高压症手术短期预后的影响[C]//2016中国中西医结合麻醉学会年会暨第三届全国中西医结合麻醉学术研讨会、河南省中西医结合学会麻醉专业委员会成立大会论文汇编. 郑州, 2016: 261-264.
[22]LIU HP. Study on the right side pleural effusion after primary liver cancer resection of part of the causes and countermeasures[J]. World Latest Med Inf, 2015, 15(48): 36, 39.
刘含平. 探讨原发性肝癌切除术后右侧胸水的部分成因及对策[J]. 世界最新医学信息文摘, 2015, 15(48): 36, 39.
[23]LAI C, JIN RN, LIANG X, et al. Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma[J]. J Zhejiang Univ Sci B, 2016, 17(3): 236-246. DOI: 10.1631/jzus.B1500322.
[24]JANY B, WELTE T. Pleural effusion in adults-etiology, diagnosis, and treatment[J]. Dtsch Arztebl Int, 2019, 116(21): 377-386. DOI: 10.3238/arztebl.2019.0377.
收稿日期:
2022-10-22;錄用日期:2022-12-01
本文编辑:王莹
引证本文:
WEN J, JIA Z, HE R, et al.
Risk factors for pulmonary infection after laparoscopic surgery in treatment of hepatocellular carcinoma with liver cirrhosis and portal hypertension[J]. J Clin Hepatol, 2023, 39(7): 1586-1591.
文静, 贾哲, 赫嵘, 等. 肝细胞癌合并肝硬化门静脉高压症腹腔镜同期联合手术术后肺部感染的危险因素分析[J]. 临床肝胆病杂志, 2023, 39(7): 1586-1591.

