基于炎症营养参数的评分系统预测胃上部癌新辅助化疗效果的研究
朱红苹 刘睿清 张宪祥 张茂申 王东升 卢云

[摘要] 目的 探討局部进展期胃上部癌患者新辅助化疗(NACT)前后炎症营养指标与NACT疗效的关系,并建立临床预测模型。方法 选取2013年4月—2022年1月于我院胃肠外科接受NACT的胃上部癌患者117例,根据化疗结果分为有效组与无效组,收集患者的年龄、性别、吸烟史、饮酒史、首发症状、肿瘤部位、肿瘤分化程度、肿瘤临床分期、肿瘤病理类型、NACT前1周内及NACT后1周内的血常规结果等临床资料,经单因素及多因素分析后筛选出影响NACT疗效的因素,进一步构建列线图模型并验证该模型的性能。结果 多因素分析结果显示,患者NACT前后的血浆中性粒细胞与淋巴细胞比值(NLR)差值(△NLR)(OR=2.043,95%CI=1.334~3.127,P<0.05)、血浆血小板与淋巴细胞比值(PLR)差值(△PLR)(OR=1.007,95%CI=1.000~1.014,P<0.05)、血清白蛋白(Alb)差值(△Alb)(OR=0.936,95%CI=0.878~0.997,P<0.05)以及T分期(OR=4.044,95%CI=1.128~14.501,P<0.05)均为影响NACT疗效的独立危险因素。基于多因素分析结果构建胃上部癌NACT疗效列线图预测模型,该模型受试者特征曲线下面积为0.877,绘制的校准曲线及临床决策曲线显示校准度较好且与实际结果较一致。结论 胃上部癌患者△NLR、△PLR、△Alb及T分期为影响NACT疗效的独立危险因素,胃上部癌NACT疗效预测模型具有良好的预测性能和临床应用价值。
[关键词] 胃肿瘤;慢性病指标;营养评价;化学疗法,肿瘤,局部灌注;危险因素;回归分析
[中图分类号] R735.2
[文献标志码] A
VALUE OF A SCORING SYSTEM BASED ON INFLAMMATORY AND NUTRITIONAL INDICATORS IN PREDICTING THE EFFICACY OF NEOADJUVANT CHEMOTHERAPY FOR UPPER GASTRIC CANCER \ ZHU Hongping, LIU Ruiqing, ZHANG Xianxiang, ZHANG Maoshen, WANG Dongsheng, LU Yun (Gastrointestinal Surgery, The Affiliated Hospital of Qingdao University, Qingdao 266555, China)
[ABSTRACT] Objective To investigate the association of inflammatory and nutritional indicators with the efficacy of neoadjuvant chemotherapy (NACT) in patients with upper gastric cancer before and after NACT, and to establish a clinical predictive model. Methods A total of 117 patients with upper gastric cancer who underwent NACT in Department of Gastroenterology in our hospital from April 2013 to January 2022 were enrolled, and according to the efficacy of chemotherapy, they were divided into effective group and ineffective group. Related clinical data were collected, such as age, sex, smoking history, drinking history, initial symptoms, tumor location, degree of tumor differentiation, tumor stage, tumor pathological type, and routine blood test results within one week before NACT and within one week after NACT. Univariate and multivariate analyses were performed to identify the influencing factors for the efficacy of NACT, and then a nomogram model was established and validated. ResultsThe multivariate analysis showed that the difference in plasma neutrophil-to-lymphocyte ratio before and after NACT (△NLR) (OR=2.043,95%CI=1.334-3.127,P<0.05), the difference in plasma platelet-to-lymphocyte ratio before and after NACT (△PLR) (OR=1.007,95%CI=1.000-1.014,P<0.05), the difference in serum albumin before and after NACT (△Alb) (OR=0.936,95%CI=0.878-0.997,P<0.05), and T stage (OR=4.044,95%CI=1.128-14.501,P<0.05) were independent risk factors for the efficacy of NACT. A nomogram predictive model for the efficacy of NACT in upper gastric cancer was constructed based on the results of the multivariate analysis, with an area under the ROC curve of 0.877, and the calibration curve and the clinical decision curve showed that the model had good calibration and consistency with the actual results. Conclusion △NLR, △PLR, △Alb, and T stage are independent independent risk factors for the efficacy of NACT in patients with upper gastric can-cer, and the predictive model for the efficacy of NACT for upper gastric cancer has good predictive performance and clinical application value.
[KEY WORDS] Stomach neoplasms; Chronic disease indicators; Nutrition assessment; Chemotherapy, cancer, regional perfusion; Risk factors; Regression analysis
胃癌属于全球人群中第5大常见和第3高病死率的肿瘤[1],近年来胃上部癌发病率呈上升趋势[2]。既往研究表明新辅助化疗(NACT)可提高肿瘤根治性切除率并改善患者预后[3-4]。目前欧洲肿瘤内科学会(ESMO)指南[5]、美国国立综合癌症网络(NCCN)指南[6]及我国2018版《胃癌诊疗规范》[7]均推荐局部进展期胃癌患者采用NACT治疗。
既往研究表明,中性粒细胞与淋巴细胞比值(NLR)、血小板与淋巴细胞比值(PLR)、淋巴细胞与单核细胞比值(LMR)、系统免疫炎症指数(SII)及血清白蛋白(Alb)等炎症营养指标均可作为评估胃癌患者预后的标志物[8-10]。炎症反应可通过多种机制导致肿瘤生长、侵袭和转移[11],患者营养不良可导致肿瘤进展。既往研究显示,炎症营养指标与肿瘤患者营养不良相关[12]。有研究表明第三腰椎(L3)水平横断面机体组成成分面积能够反映全身营养状况[13]。本研究通过探讨炎症营养指标及其NACT前后差值与NACT疗效的关系,构建相关指标预测胃上部癌NACT效果的临床模型,旨在为医务工作者采取相关治疗措施时提供一定参考。现将结果报告如下。
1 资料和方法
1.1 一般资料
选取2013年4月—2022年1月于我院胃肠外科接受NACT的胃癌患者。患者纳入标准:①年龄18~75岁者;②经胃镜活检病理检查证实为胃上部癌者;③接受NACT治疗者;④NACT前肿瘤临床分期≥cT2或有淋巴结转移者;⑤影像学检查未见肿瘤远处转移者;⑥机体其他器官功能正常者;⑦用胃癌一线化疗药物(主要包括氟尿嘧啶类、铂类与紫杉醇类)治疗者。排除标准:①NACT疗程<2周期者;②行NACT后未手术者;③具有血液系统疾病及其他有血常规异常的疾病者。
收集患者的年龄、性别、吸烟史、饮酒史、首发症状、肿瘤部位、肿瘤分化程度、肿瘤临床分期、肿瘤病理类型以及NACT前1周内、NACT后1周内血常规指标,计算患者NACT前后NLR、PLR、LMR、SII、Alb。获取患者NACT前1个月内以及手术前最后一次CT平扫的L3横断面单幅图像,通过sliceOmatic图像分析软件计算该层面机体组成成分面积(皮下脂肪、内脏脂肪及肌肉组织面积)[14],并计算NACT前后患者L3水平横断面机体组成成分面积的差值。由两位放射科专家通过CT平扫对比患者NACT前后胃上部胃壁厚度的变化,按照实体瘤的疗效评价标准[15]对胃上部癌NACT疗效进行准确评估,评估结果分为完全缓解(CR)、部分缓解(PR)、疾病稳定(SD)和疾病进展(PD),根据NACT治疗结果将患者分为有效组(CR、PR者)和无效组(SD、PD者)。
1.2 统计学分析
采用SPSS 26.0统计学软件进行统计分析,服从正态分布的计量资料以x?±s表示,组间比较采用t检验;计数资料以例(率)表示,组间比较采用χ2检验。采用多因素logistic回归模型分析NACT疗效的影响因素。使用R语言建立预测NACT疗效的列线图预测模型,并对模型效能进行评价。以P<0.05为有统计学差异。
2 结果
2.1 两组患者一般资料比较
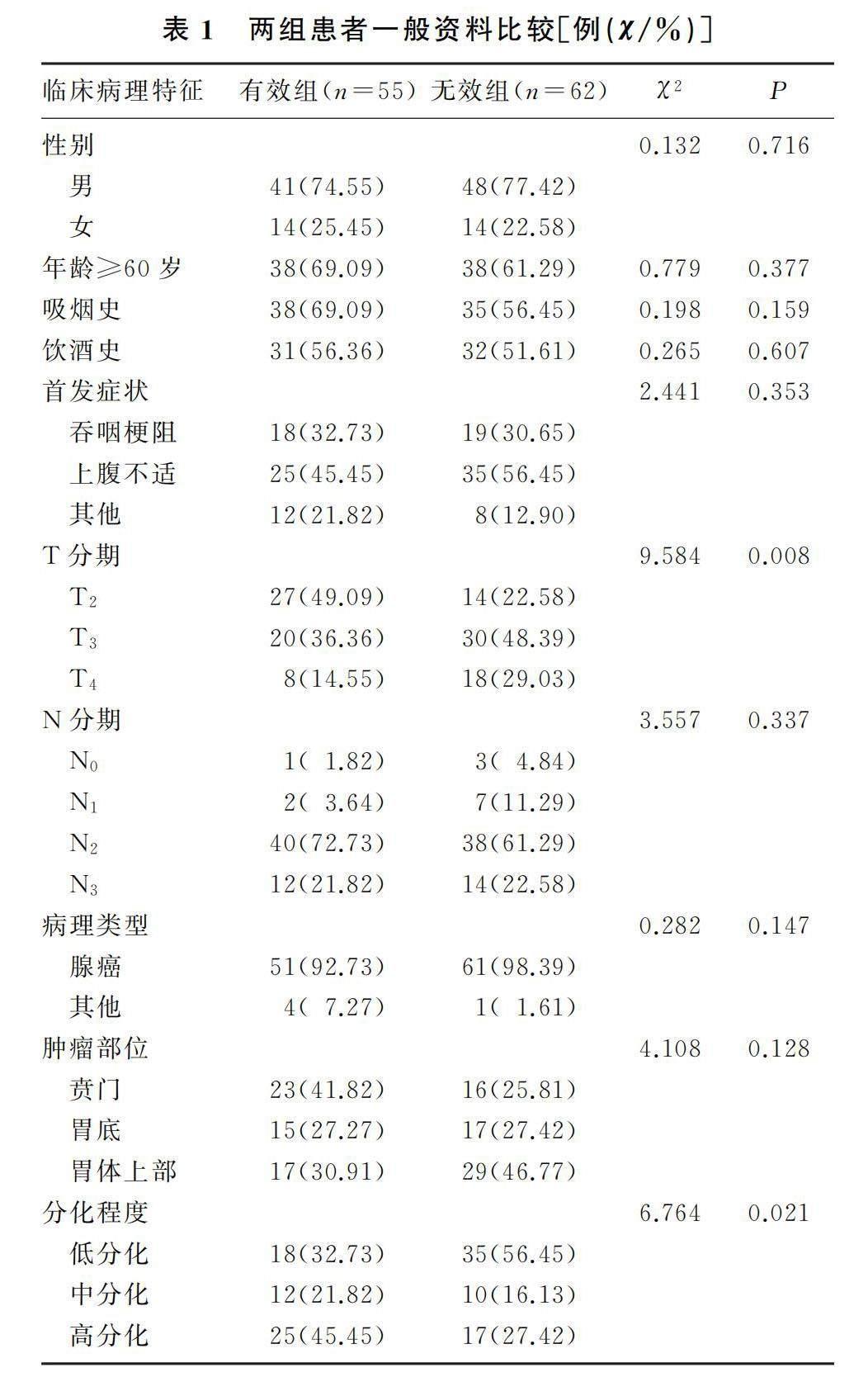
本研究共纳入患者117例,其中男性患者89例,女性患者28例,60岁及以上患者76例。有效组、无效组患者肿瘤T分期及分化程度差异有显著性(χ2=9.584、6.764,P<0.05)。见表1。
2.2 两组患者的炎症及营养指标比较
两组患者的NACT前后NLR差值(△NLR)、PLR差值(△PLR)以及Alb差值(△Alb)相比较,差异均具有统计学意义(t=-3.781~2.496,P<0.05)。见表2。
2.3 影响NACT疗效的多因素logistic回归分析
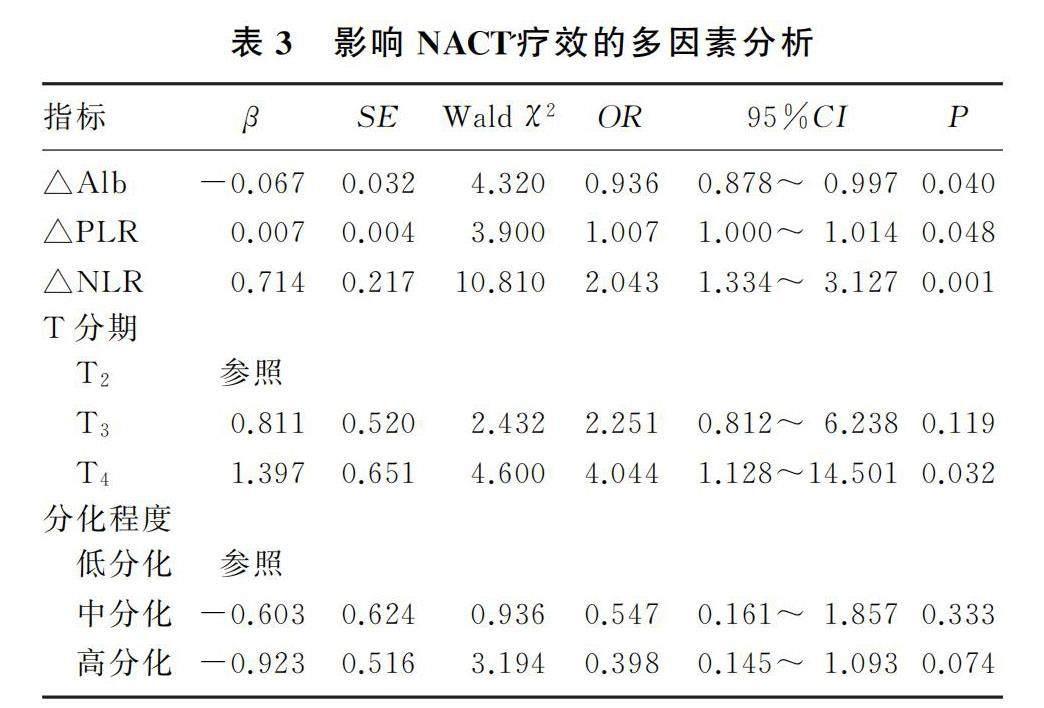
将t检验及χ2检验中P<0.05的影响因素纳入多因素logistic回归模型,分析结果显示T分期、△NLR、△PLR和△Alb为影响NACT疗效的独立危险因素(P<0.05)。见表3。
2.4 列线图预测模型的构建
基于多因素logistic回归分析结果构建预测NACT疗效的列线图模型(图1A);绘制列线图预测模型的受試者特征(ROC)曲线(图B),计算曲线下面积(AUC)为0.877,95%CI=0.812~0.941。采用Bootstrap自抽样法对上述模型进行内部验证,重复抽样1 000次,计算C-index值为0.856,表示该模型具有良好的预测价值。绘制校准曲线及临床决策曲线结果显示,该模型校准度较好,模型和实际结果具有较好的一致性,并且具有明显正向净收益,在预测胃上部癌NACT疗效方面具有较好临床实用性(图1C、D)。
3 讨论
近年来,全球胃上部癌发病率逐渐增高,针对胃上部癌的治疗方案也逐渐个体化以及精准化。目前NACT方案已经成为局部进展期胃癌的首选治疗方案[5-6],且据以往研究,针对胃上部癌的NACT治疗具有良好效果[16]。随着NACT的推广,对其疗效的预测已经成为研究热点。一项研究表明NLR、PLR可以作为NACT疗效的独立预测指标[17],但也有部分学者认为NLR和PLR对肿瘤预后预测并无意义[18]。因此,虽然关于NACT疗效判断的研究较多,但评判标准仍存在争议。本研究综合多数研究中纳入的指标对NACT疗效预测进行探讨,以期得出更准确结果。
炎症反应可导致肿瘤的发生发展,机体炎症反应可引起外周血中性粒细胞、淋巴细胞等血细胞数量异常,并通过相关途径导致肿瘤进展[6]。外周血NLR和PLR作为系统炎性反应指标,已被证实与多种肿瘤的发生发展有关系[19]。因此本研究通过NACT前后NLR、LMR、SII和PLR等炎症指标变化预测NACT疗效具有可行性。机体营养不良易致肿瘤进展,较差的营养状态可加剧肿瘤细胞增殖和肿瘤微环境形成,最终致肿瘤发展及转移。血清Alb及CT平扫L3水平横断面机体组成成分可反映患者机体营养状态[14],故本研究亦将上述两者作为反映患者NACT前后营养状况的研究指标。
本研究NACT有效组的患者NACT后NLR、PLR、LMR和SII明显降低,反映出淋巴细胞数量的增加可能是由于NACT诱导致炎症下调的原因。与炎症标志物相反,NACT后Alb升高,反映患者营养状态得以改善。经研究发现,NACT前低血小板與中性粒细胞比值以及高淋巴细胞与白细胞比值都属于NACT疗效的独立危险因素[20]。有研究显示,NACT后NLR与淋巴细胞计数减少为化疗效果的独立危险因素[21]。此外肿瘤细胞可诱导血小板活化并聚集,使PLR升高,导致肿瘤进展。
ISHIBASHI等[21]研究显示NACT前NLR升高为食管癌预后的独立危险因素,KIM等[22]研究结果表明NACT前NLR升高为胃癌患者预后的独立危险因素,MUNGAN等[23]的研究结果则显示NACT前较高PLR和NLR与胃癌患者不良预后有关。本研究两组患者的T分期、△NLR、△PLR以及△Alb表现出显著性差异,且T分期、△NLR、△PLR和△Alb为胃上部癌NACT疗效的独立危险因素。另外,本研究关注NACT前后相关炎症营养指标的差值因素,并发现相关炎症营养指标差值对胃上部癌NACT疗效具有预测价值,故该模型具有一定创新性。
本研究构建的预测NACT疗效的列线图模型以国人临床数据为基础,因此更适用于我国的胃癌NACT患者,此外既往研究中多以单一因素为基点讨论胃癌NACT疗效的影响因素,本研究系统讨论了多个临床指标,依据每个参数的权重和重要性构建了列线图预测模型,且血常规结果、Alb水平、肿瘤分化程度、肿瘤临床分期等皆为临床工作中易于获取的实用性指标,因此本研究构建的列线图预测模型具有一定的实用性。本研究也存在不足之处,如样本量偏少,且为回顾性研究,未进行外部验证。上述不足需在后期研究中进行完善。
综上所述,T分期、△NLR、△PLR和△Alb为胃上部癌NACT疗效的独立危险因素,基于以上因素构建的临床预测模型则可以准确地判断胃上部癌NACT临床效果。
作者声明:所有作者均参与了研究设计、论文的写作和修改。所有作者均阅读并同意发表该论文,且均声明不存在利益冲突。
[参考文献]
[1]BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA A Cancer J Clin, 2018,68(6):394-424.
[2]LI P, JING J, LI R, et al. Upper gastrointestinal cancer in China: Spatial epidemiologic evidence from screening areas[J]. Cancer Prev Res (Phila), 2020,13(11):935-946.
[3]KUMAR CG P, MUDULY D, IMADUDDIN M, et al. P-282 Factors predicting response to neoadjuvant chemotherapy in gastric cancer[J]. Ann Oncol, 2021,32:S193.
[4]ZHU Z, GONG Y B, XU H M. Neoadjuvant therapy strategies for advanced gastric cancer:Current innovations and future challenges[J]. Chronic Dis Transl Med, 2020(3):147-157.
[5]SMYTH E C, VERHEIJ M, ALLUM W, et al. Gastric can-cer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2016,27(suppl 5):v38-v49.
[6]AJANI J A, D'AMICO T A, ALMHANNA K, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in onco-logy[J]. J Natl Compr Canc Netw, 2016,14(10):1286-1312.
[7]陈俊良,王风华. 2021版CSCO胃癌诊疗指南转移性胃癌更新解读[J]. 中国肿瘤临床, 2022,49(7):325-330.
[8]ZHANG L X, WEI Z J, XU A M, et al. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study[J]. Int J Surg, 2018,56:320-327.
[9]LI K J, XIA X F, SU M, et al. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy[J]. BMC Cancer, 2019,19(1):1004.
[10]CHEN G J, WUXIAO Z J, LIANG Y, et al. Inflammation-based Glasgow prognostic score as an independent prognostic factor in patients with angioimmunoblastic T-cell lymphoma[J]. Chin Med J (Engl), 2021,134(5):579-581.
[11]SINGH N, BABY D, RAJGURU J P, et al. Inflammation and cancer[J]. Ann Afr Med, 2019,18(3):121-126.
[12]AKBOGA S A, GOKCE A, HATIPOGLU M, et al. The relationship between mortality and inflammatory markers and the systemic immune inflammatory index in patients in the intensive care unit with a pneumothorax as a complication of COVID-19 disease[J]. Ir J Med Sci, 2022,191(4):1931-1936.
[13]UOJIMA H, CHUMA M, TANAKA Y, et al. Skeletal muscle mass influences tolerability and prognosis in hepatocellular carcinoma patients treated with lenvatinib[J]. Liver Cancer, 2020,9(2):193-206.
[14]LINDQVIST C, BRISMAR T B, MAJEED A, et al. Assessment of muscle mass depletion in chronic liver disease: Dua-l-energy X-ray absorptiometry compared with computed tomography[J]. Nutrition, 2019,61:93-98.
[15]杨学宁,吴一龙. 实体瘤治疗疗效评价标准:RECIST[J]. 循证医学, 2004,4(2):85-90,111.
[16]RAHMAN S, THOMAS B, MAYNARD N, et al. Impact of postoperative chemotherapy on survival for oesophagogastric adenocarcinoma after preoperative chemotherapy and surgery[J]. Br J Surg, 2022,109(2):227-236.
[17]TANG C, CHENG X, YU S, et al. Platelet-to-lymphocyte ratio and lymphocyte-to-white blood cell ratio predict the efficacy of neoadjuvant chemotherapy and the prognosis of locally advanced gastric cancer patients treated with the oxaliplatin and capecitabine regimen[J]. Onco Targets Ther, 2018,11:7061-7075.
[18]LOCOCO F, CHIAPPETTA M, EVANGELISTA J, et al. Role of peripheral blood markers for detecting response and predicting prognosis in patients with non-small-cell lung cancer undergoing neoadjuvant therapy and surgery[J]. Lung, 2022,200(3):393-400.
[19]HU Y P, WANG S M, DING N H, et al. Platelet/lymphocyte ratio is superior to neutrophil/lymphocyte ratio as a predictor of chemotherapy response and disease-free survival in luminal B-like (HER2-) breast cancer[J]. Clin Breast Cancer, 2020,20(4):e403-e409.
[20]TEZOL O, BOZLU G, SAGCAN F, et al. Value of neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio and red blood cell distribution width in distinguishing between reactive lymphadenopathy and lymphoma in children[J]. Bratisl Lek Listy, 2020,121(4):287-292.
[21]ISHIBASHI Y, TSUJIMOTO H, HIRAKI S, et al. Predictive value of immuno-inflammatory and nutritional measures modulated by neoadjuvant chemotherapy on the response of neoadjuvant chemotherapy and long-term outcomes in patients with esophageal cancer[J]. Oncol Lett, 2020,19(1):487-497.
[22]KIM M R, KIM A S, CHOI H I, et al. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis[J]. PLoS One, 2020,15(7):e0236445.
[23]MUNGAN I, DICLE ? B, BEKTA? ?, et al. Correction to: Does the preoperative platelet-tolymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer?[J]. Mil Med Res, 2020,7(1):12.
(本文編辑 范睿心 厉建强)

