Comparative study of extrudability,microstructure,and mechanical properties of AZ80 and BA53 alloys
Sng-Cheol Jin ,Je Won Ch ,Jongin Go ,Jun Ho Be ,Sung Hyuk Prk,∗
aSchool of Materials Science and Engineering,Kyungpook National University,Daegu 41566,Republic of Korea
b Department of Materials Science and Engineering,Kyoto University,Kyoto 606-8501,Japan
c Advanced Metals Division,Korea Institute of Materials Science,Changwon 51508,Republic of Korea
Abstract The extrudability,microstructural characteristics,and tensile properties of the Mg–5Bi–3Al (BA53) alloy are investigated herein by comparing them with those of a commercial Mg–8Al–0.5 Zn (AZ80) alloy.When AZ80 is extruded at 400 °C,severe hot cracking occurs at exit speeds of 4.5 m/min or more.In contrast,BA53 is successfully extruded without any surface cracking at 400 °C and at high exit speeds of 21–40 m/min.When extruded at 3 m/min (AZ80–3) and 40 m/min (BA53–40),both AZ80 and BA53 exhibited completely recrystallized microstructures with a 〈10–10〉 basal texture.However,BA53–40 has a coarser grain structure owing to grain growth promoted by the high temperature in the deformation zone.AZ80–3 contains a continuous network of Mg17Al12 particles along the grain boundaries,which form via static precipitation during natural air-cooling after the material exits the extrusion die.BA53–40 contains coarse Mg3Bi2 particles aligned parallel to the extrusion direction along with numerous uniformly distributed fin Mg3Bi2 particles.AZ80–3 has higher tensile strength than BA53–40 because the relatively fine grains and larger number of solute atoms in AZ80–3 result in stronger grain-boundary and solid-solution hardening effects,respectively.Although BA53 is extruded at a high temperature and extrusion speed of 400 °C and 40 m/min,respectively,the extruded material has a high tensile yield strength of 188 MPa.This can be primarily attributed to the large particle hardening effect resulting from the numerous fin Mg3Bi2 particles.
Keywords: Mg–Bi–Al alloy;Extrusion;Hot cracking;Second phase;Tensile properties.
1.Introduction
As environmental regulations and social demands for the weight reduction of vehicles gradually increase,Mg alloys have attracted considerable research attention.This is because the density of Mg is approximately two thirds that of Al and one quarter that of steel.Therefore,the automobile weight can be effectively reduced by replacing components made of Al alloys or steel with Mg alloy components.Extruded Mg alloys possess a fine microstructure and exhibit higher mechanical properties compared with cast Mg alloys owing to the formation of fin grain structure through dynamic recrystallization (DRX) during hot extrusion [1–3];hence,extruded Mg alloys have been extensively researched.For the wide industrial application of extruded Mg alloy products,simultaneously achieving high strength and price competitiveness is essential.High-alloyed extruded Mg alloys have higher mechanical strength than their low-alloyed counterparts because of various hardening effects induced by alloying elements,e.g.,solid-solution,precipitation,and dispersion hardening [4–6].However,commercial high-alloyed Mg alloys,such as AZ80,AZ91,and ZK60,display significantl lower extrudability than commercial Al alloys [7–13].The decrease in productivity due to low extrusion speed increases the price of the fina extruded products.Consequently,extruded commercial high-alloyed Mg alloy products have low price competitiveness.
To overcome this drawback of commercial high-alloyed alloys,high-alloyed Mg–Bi-based alloys containing no rareearth (RE) elements have been recently developed.The Mg3Bi2phase formed in Mg–Bi alloys has a melting point of 823 °C,which is approximately twice as high as the melting points of Mg17Al12and MgZn2phases formed in the abovementioned commercial alloys (i.e.,AZ80,AZ91,and ZK60) [8,14–17].Moreover,the incipient melting of a Mg–9Bi (wt.%) binary alloy occurs at 550 °C,a higher temperature than that of AZ80 alloy (433 °C).In our previous study[18],we found that adding Al to a Mg–5Bi (wt.%) alloy significantl improves the tensile and compressive strength of the extruded material.However,excessive Al addition can deteriorate extrudability owing to the formation of the Mg17Al12phase or the material ductility.Recently,we have developed an RE-free high-alloyed Mg–5Bi–3Al (BA53,wt.%) alloy while considering the mechanical properties,extrudability,and material price simultaneously [7,19].The total alloying content of BA53 (8.0 wt.%) is similar to that of AZ80(8.5 wt.%).Therefore,herein,characteristics of the recently developed BA53 and commercially used AZ80 are compared in terms of available extrusion speed,microstructural features,and tensile properties.
2.Experimental procedure
Commercial AZ80 (Mg–8Al–0.5Zn–0.2Mn,wt.%) and recently developed BA53 (Mg–5Bi–3Al,wt.%) were investigated in this study.Three cast billets of each alloy were fabricated via the conventional mold casting method in a mixed atmosphere of CO2and SF6gas.A pure Mg ingot and alloying element granules with a high purity of>99.9% (Bi,Al,Zn,and Mn) were melted in a graphite crucible using an induction melting furnace.The molten alloy was held at 720 °C for 20 min for stabilization,and then poured into a steel mold (diameter: 105 mm) preheated to 200 °C.All cast billets were homogenized in an electric furnace at 410 °C for 24 h.The homogenized billets were each machined into a cylindrical sample with a diameter and a length of 68 and 120 mm,respectively,for hot extrusion.The machined samples were preheated at 400 °C for 1 h in an electric furnace and subsequently subjected to direct extrusion using a 300-ton hydraulic horizontal extrusion machine at 400 °C with an extrusion ratio of 50.The extrusion experiments were performed at ram speeds of 1.0,1.5,and 2.0 mm/s for AZ80 and 7,10,and 13.3 mm/s for BA53;the corresponding exit speeds were 3.0,4.5,and 6.0 m/min for AZ80 and 21,30,and 40 m/min for BA53,respectively.The following equation allows to calculate the average strain rate (´ε) applied during the extrusion process [20,21]:
whereDBis the billet diameter,DEis the extrudate diameter,VRis the ram speed,ER is the extrusion ratio.Based on the Eq.(1),the average strain rates under the above-mentioned extrusion conditions were 0.35,0.52,and 0.69 s−1for AZ80 and 2.42,3.46,and 4.60 s−1for BA53,respectively.A flat faced die with a circular hole (diameter of 9.9 mm) was used for extrusion and the extruded bars that exit the extrusion die were naturally air-cooled to room temperature (RT;23 °C).
Microstructural characteristics of the homogenized billets and extruded materials were analyzed through optical microscopy,field-emissio scanning electron microscopy (FESEM),X-ray diffraction (XRD),field-emissio electron probe microanalysis (FE-EPMA),and electron backscatter diffraction (EBSD).All samples for these microstructural analyses were taken from the center region of the homogenized billets and extruded materials,and details regarding sample preparation methods can be found elsewhere [22].Automated EBSD scans were performed in a 600×600 μm2area with a step size of 1 μm using Tex-SEM Laboratories (TSL) data acquisition software.The obtained EBSD data were analyzed using TSL orientation imaging microscopy analysis software;only reliable EBSD data with confidenc indexes of>0.1 were used.For uniaxial tensile testing,dog-bone-shaped specimens with gage dimensions of 6×25 mm (diameter×length)were machined at the center of the extruded materials;the longitudinal direction of the specimens was parallel to the extrusion direction (ED).Tensile tests were performed using a Shimadzu AGS-100kNX universal testing machine at RT under a strain rate of 1×10−3s−1.
3.Results and discussion
3.1.Maximum extrusion speed of AZ80 and BA53
Fig.1 shows the surface quality and hot cracks of AZ80 and BA53 extruded at different speeds.AZ80 extruded at 3.0 m/min has a yellowish surface owing to the slight surface oxidation during extrusion;however,there are no surface cracks.In AZ80 extruded at 4.5 m/min,many hot cracks,with an average spacing of 2.2 mm,formed along the direction perpendicular to the ED.AZ80 extruded at 6.0 m/min has a dark black surface owing to excessive surface oxidation and severe hot cracking occurs,causing certain parts of the surface to peel (Fig.1a).Accordingly,at an extrusion temperature of 400 °C,the maximum extrusion speed that can be achieved without surface cracking of AZ80 is below 4.5 m/min.For BA53,although oxidation occurs on the surface during extrusion,this alloy is successfully extruded at high speeds ranging from 21 to 40 m/min without any surface cracks (Fig.1b).The degree of surface oxidation of extruded BA53 increases as the extrusion speed increases;however,the surface oxidation of BA53 extruded at 40 m/min is less pronounced than that of AZ80 extruded at 6.0 m/min despite a 6.6-fold faster extrusion speed in BA53.These results demonstrate that BA53 has a substantially higher maximum extrusion speed than AZ80 and superior resistance to surface oxidation during extrusion.The microstructural characteristics and tensile properties of AZ80 extruded at 3.0 m/min (denoted as AZ80–3) and BA53 extruded at 40 m/min (denoted as BA53–40),both of which are fabricated at the highest extrusion speed with no hot cracking among the tested conditions,are compared below.

Fig.1.Photographs showing (a) AZ80 extruded at 3.0,4.5,and 6.0 m/min and (b) BA53 extruded at 21,30,and 40 m/min.
3.2.Microstructure of homogenized billets and extruded AZ80–3 and BA53–40
Fig.2 shows optical and SEM micrographs of the homogenized AZ80 and BA53 billets.The homogenized AZ80 billet has an equiaxed grain structure and contains several Al8Mn5particles (4–10 μm) (Fig.2a);these microstructural features are consistent with previous results [23].No Mg17Al12particles are observed in the homogenized AZ80 billet,which indicates that Mg17Al12particles present in the as-cast state are fully dissolved intoα-Mg matrix during homogenization treatment.In contrast,in the homogenized BA53 billet,numerous fin lath-shaped Mg3Bi2particles with a length ranging from 0.5 to 3 μm are distributed in the grains,and relatively coarse rod-like Mg3Bi2particles are present along the grain boundaries (Fig.2b).The presence of fin and coarse Mg3Bi2particles in homogenized BA53 billets is also reported in our previous studies [19,24].Although the homogenization treatment is conducted at the same temperature and time,the amount of undissolved second-phase particles in the BA53 billet is considerably higher than that in the AZ80 billet,which can be attributed to the higher thermal stability of Mg3Bi2phase than Mg17Al12phase.
Fig.3 shows inverse pole figur maps and ED inverse pole figure of AZ80–3 and BA53–40.Both materials exhibit an equiaxed grain structure with no elongated grains owing to the occurrence of complete DRX during extrusion.The high extrusion temperature of 400 °C facilitates the grain boundary bulging phenomenon required for nucleating recrystallized grains,which consequently promotes DRX behavior during extrusion [25–27].Moreover,the adopted extrusion ratio is fairly high at 50;hence,a high strain (3.91) sufficien for complete DRX is imposed during extrusion.The average grain size of BA53–40 (32.1 μm) is higher than that of AZ80–3(17.0 μm).Although the initial extrusion temperature is the same,the actual temperature in the deformation zone close to the die exit increases as the extrusion speed increases.This is due to the fact that as extrusion speed increases,the amount of heat induced by friction and deformation during extrusion increases [11,27,28].This heat-induced temperature increment is strongly related to the process parameters,i.e.,temperature,strain,and strain rate.In this study,because the temperature and strain are fi ed in all extrusion experiments,the degree of heat-induced temperature increment varies depending on the strain rate applied in each extrusion experiment.The average strain rate in BA53–40 (4.60 s−1) is ∼13 times higher than that in AZ80–30 (0.35 s−1).Moreover,the heat-induced temperature increment is proportional to the logarithmic strain rate [21,29].Therefore,the deformation temperature increment generated during extrusion is considerably higher in BA53–40 compared to AZ80–3.The higher temperature in the deformation zone causes more vigorous growth of the DRXed grains owing to the increased mobility of grain boundaries.Increased grain boundary mobility eventually results in coarser grain structure in BA53–40.Notably,when a high-alloyed Mg–7Sn–1Al–1Zn (TAZ711,wt.%) alloy is extruded at 350 °C at an exit speed of 27 m/min,the average grain size of the extruded material is significantl large (84.1 μm) [8].Compared with the grain size of extruded TAZ711,BA53–40 has a considerably smaller grain size (32.1 μm) despite being extruded at a higher temperature(400 °C) and higher speed (40 m/min).As shown in Fig.3,both AZ80–3 and BA53–40 have a〈10–10〉basal texture,with one prismatic pole of the majority of grains almost parallelly aligned to the ED.Furthermore,the maximum texture intensities (Imax) of AZ80–3 and BA53–40 are also similar (6.6 and 5.9,respectively).Consequently,despite considerably different extrusion speeds,the difference between the texture of these two materials is insignifican because complete DRX occurs in both materials.
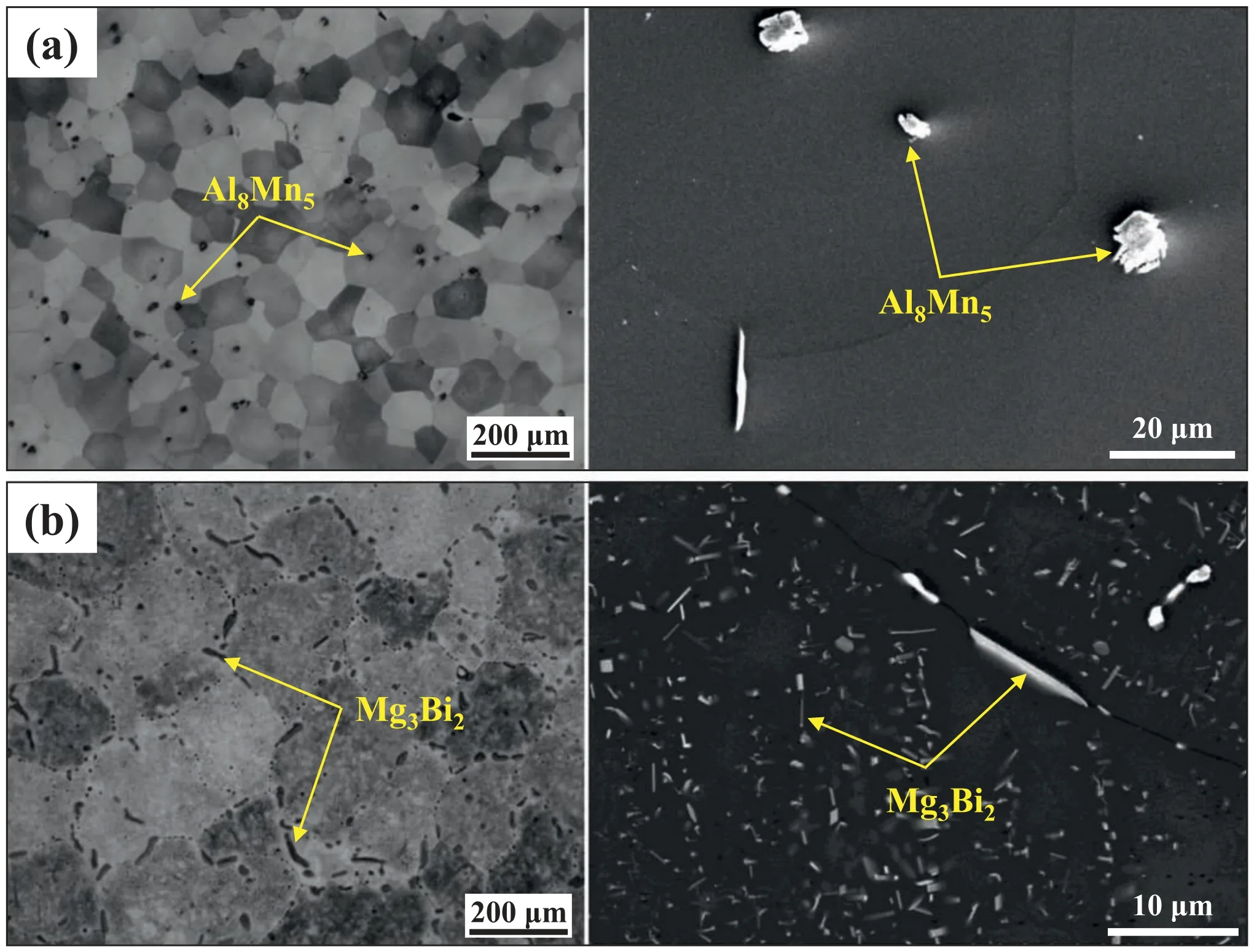
Fig.2.Optical and SEM micrographs of homogenized (a) AZ80 and (b) BA53 billets.
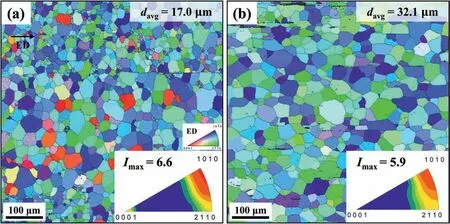
Fig.3.Inverse pole figur maps and ED inverse pole figure of (a) AZ80–3 and (b) BA53–40.ED, davg,and Imax denote extrusion direction,average grain size,and maximum texture intensity,respectively.
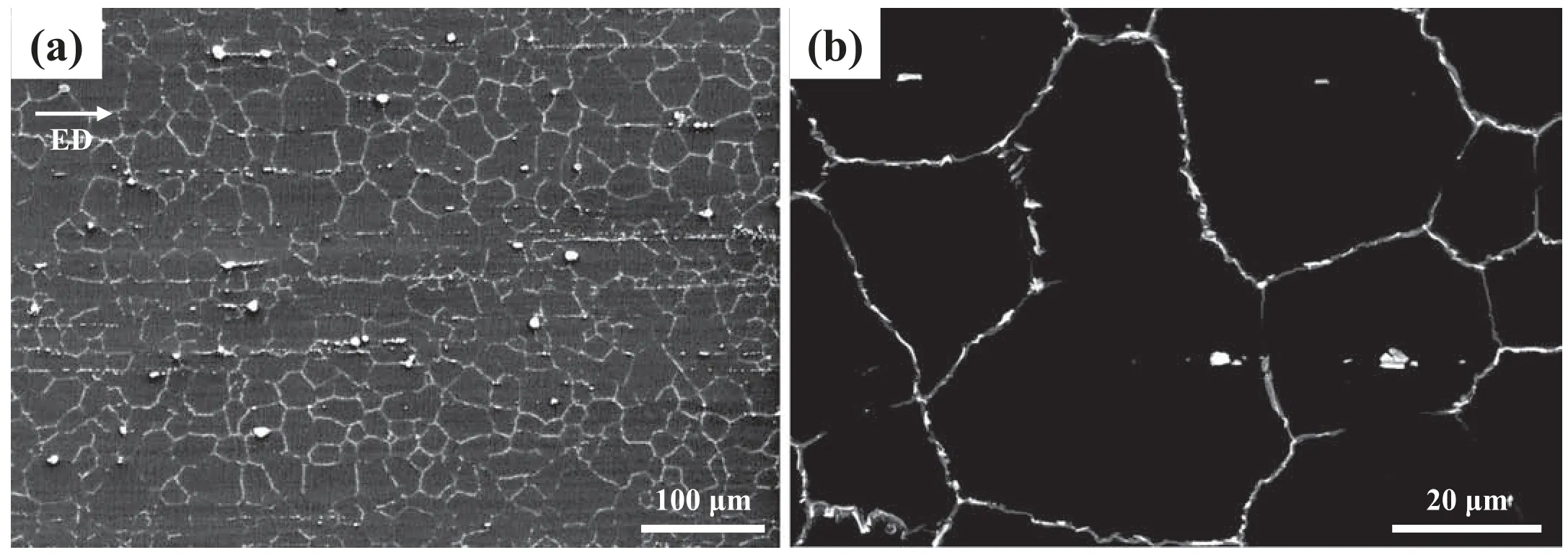
Fig.4.SEM micrographs of AZ80–3 at (a) low and (b) high magnification
3.3.Second-phase characteristics of extruded AZ80–3 and BA53–40
Fig.4 shows SEM micrographs of AZ80–3,which reveal that a continuous network of second-phase particles is formed along the grain boundaries.In accordance with the Mg–0.5Zn–xAl (x=0–30 wt.%) equilibrium phase diagram created using FactSage software (Fig.5a),both the homogenization temperature (410 °C) and extrusion temperature(400 °C) lie in theα-Mg single-phase region.This indicates that (i) Mg17Al12phases formed during the solidifica tion stage of the casting process in as-cast AZ80 are completely dissolved into the matrix via homogenization treatment at 410 °C and (ii) the formation of fin Mg17Al12particles through dynamic precipitation barely occurs during the extrusion of the homogenized AZ80 billet at 400°C.The complete dissolution of Mg17Al12phases during homogenization treatment is confirme form the absence of Mg17Al12particles in the homogenized AZ80 billet (Fig.2a).In addition,when Mg17Al12particles are formed through dynamic precipitation during extrusion,they are not formed along the boundaries of DRXed grains.According to the equilibrium phase diagram,the Mg17Al12phase can precipitate at temperatures below 350 °C in AZ80 (see the green point in Fig.5a).After the AZ80 billet in the container exits the extrusion die,the extruded material is naturally air-cooled to RT.During this cool-down stage,static precipitation of the Mg17Al12phase occurs because of the decrease in the solubility limit of Al in the Mg matrix.The reasons why these static Mg17Al12precipitates are formed at the grain boundaries rather than inside the grains are as follows.Among various lattice defects,grain boundaries provide the most prominent high-diffusivity paths[30,31].In addition,the activation energy for grain-boundary diffusion of Mg (92 kJ/mol) is lower than that of the latticeself diffusion of Mg (135 kJ/mol) [32].Furthermore,DRXed grains,formed via the nucleation of dislocation-free grains and their growth,generally have low dislocation density [33–36];accordingly,dislocation-pipe diffusion,with a fairly high diffusivity,is also limited in the DRXed grains of AZ80–3.Therefore,Al solute atoms supersaturated in the Mg matrix can be easily concentrated at grain boundaries,and therefore,static Mg17Al12precipitates form along the grain boundaries during the natural cooling of AZ80–3.
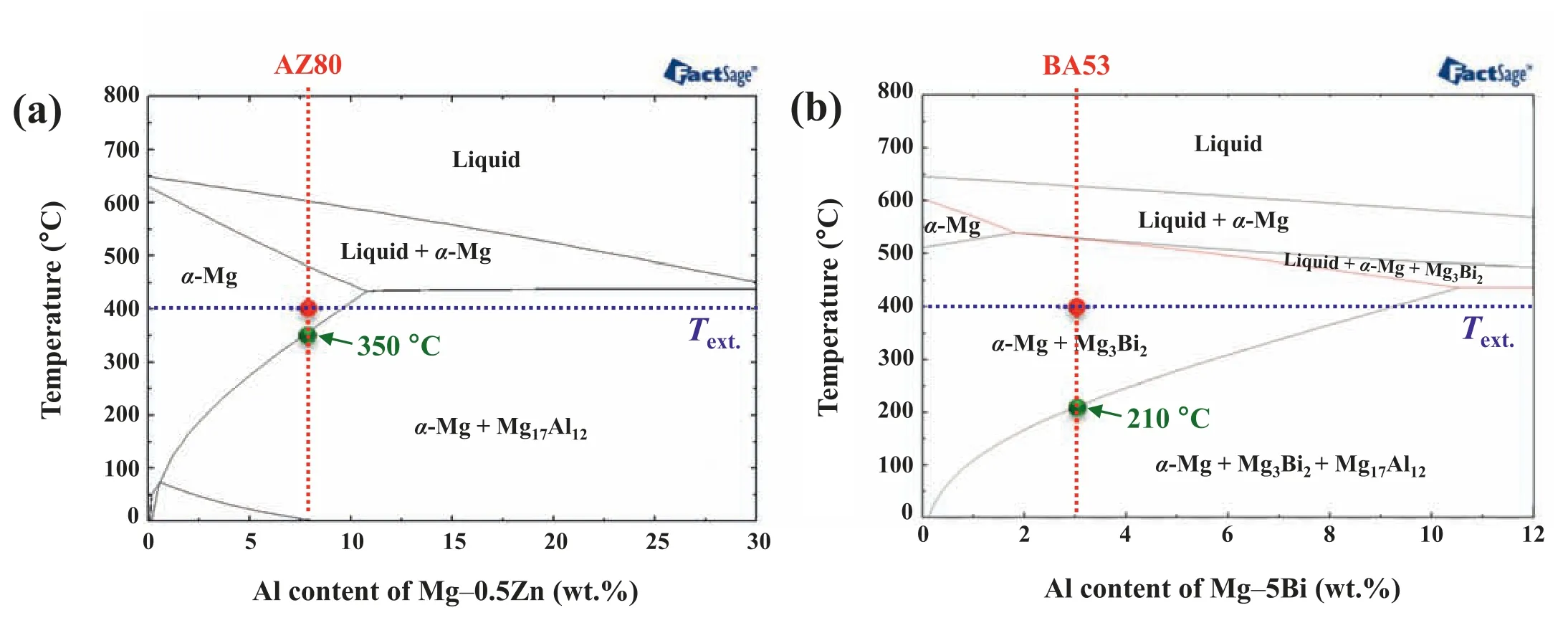
Fig.5.Equilibrium phase diagrams of (a) Mg–0.5Zn–xAl (x=0–30 wt.%) and (b) Mg–5Bi–xAl (x=0–12 wt.%) calculated using FactSage software. Text.denotes extrusion temperature (400 °C).
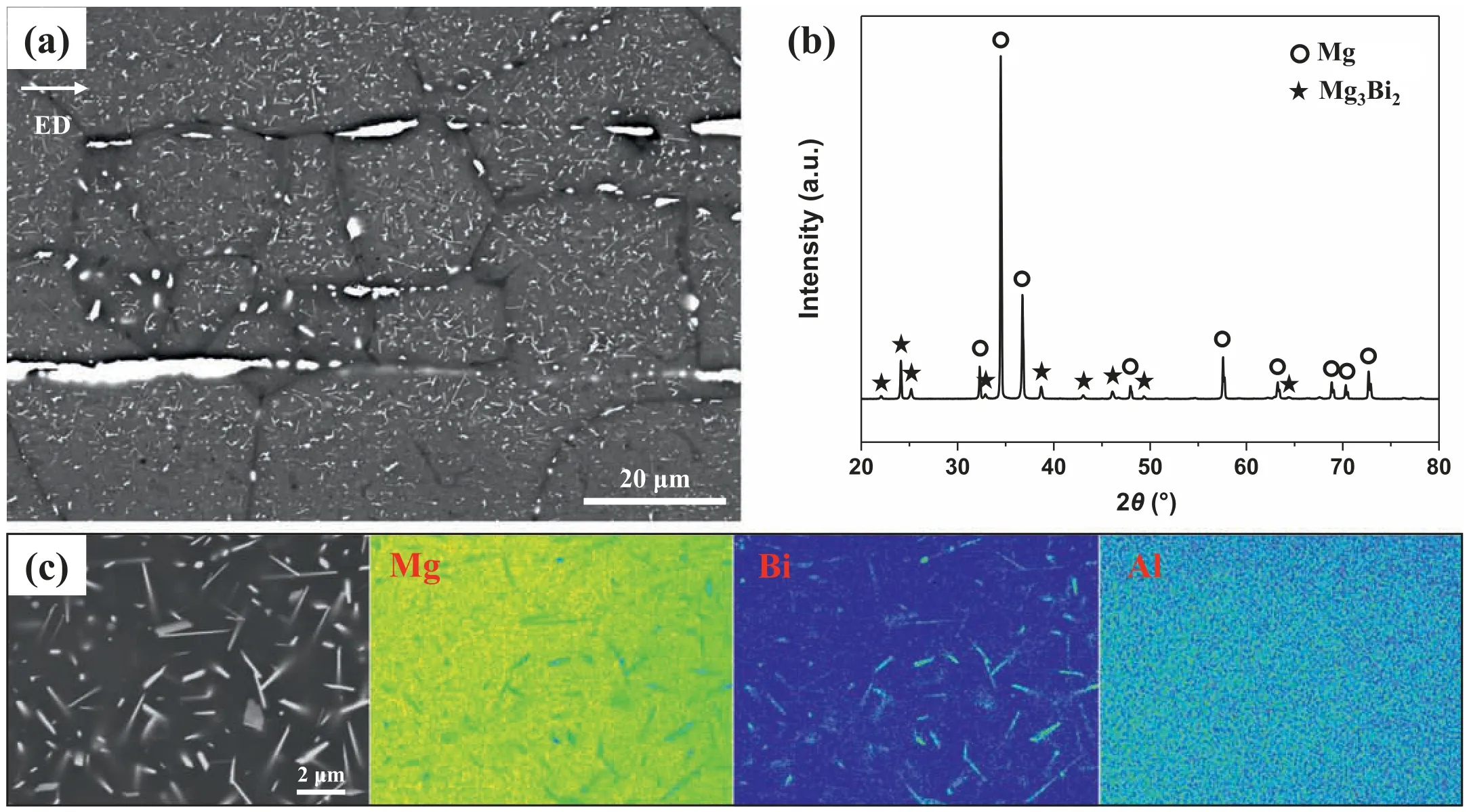
Fig.6.Second-phase particle characteristics of BA53–40: (a) SEM micrograph,(b) XRD pattern,(c) backscattered electron image showing fin particles in grains and corresponding EPMA scanning maps of Mg,Bi,and Al elements.(For interpretation of the references to color in this figur legend,the reader is referred to the web version of this article.)
Fig.5b shows a Mg–5Bi–xAl(x=0–12 wt.%)equilibrium phase diagram created using FactSage software.Unlike AZ80,both the homogenization and extrusion temperatures lie in a two-phase region ofα-Mg and Mg3Bi2in BA53.The twophase region at the homogenization temperature suggests that the homogenized BA53 billet contains undissolved Mg3Bi2particles;it is confirme from the presence of numerous Mg3Bi2particles in the homogenized BA53 billet (Fig.2b).Herein,because the extrusion temperature (400 °C) is lower than the homogenization temperature (410 °C),the undissolved Mg3Bi2particles in the homogenized billet remain after extrusion,without the dissolution of existing particles.Moreover,the time available for precipitating new Mg3Bi2particles during extrusion is insufficien owing to the high extrusion speeds(21–40 m/min).Therefore,additional formation of Mg3Bi2particles through dynamic precipitation does not occur during extrusion.An SEM micrograph of BA53–40 is shown in Fig.6a.The micrograph shows the presence of both coarse particles rearranged along the ED during extrusion and numerous uniformly distributed fin particles.XRD results reveal that both fin and coarse particles in BA53–40 are of the Mg3Bi2phase (Fig.6b),which is consistent with the equilibrium phase diagram of BA53.The size and morphology of the fin particles are nearly the same as those of the undissolved Mg3Bi2particles in the homogenized billet (Fig.2b).The EMPA mapping result confirm that all fin particles are Mg3Bi2phase (Fig.6c).Unlike in AZ80–3,Mg17Al12particles do not form during natural air-cooling in BA53–40.In accordance with the equilibrium phase diagram,the Mg17Al12phase can precipitate at temperatures below 210 °C in BA53(see the green point in Fig.5b);this temperature is signifi cantly lower than 350 °C at which Mg17Al12precipitation can occur in AZ80.A considerable time (at least a few hours)is required for precipitating Mg17Al12phase at temperatures below 210 °C [37,38].However,as the cooling time from 210 °C to RT is very short (<5 min) owing to the small diameter of the extruded material (9.9 mm),static precipitation of Mg17Al12phase does not occur in BA53–40.
3.4.Tensile properties and hardening mechanisms of extruded AZ80–3 and BA53–40
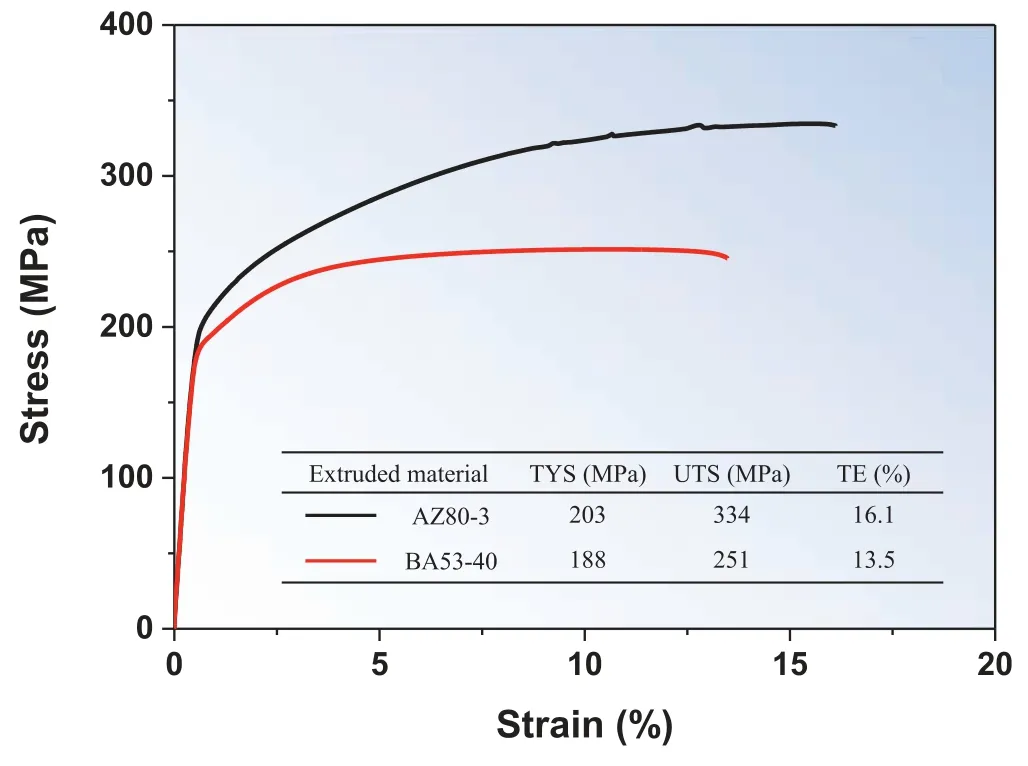
Fig.7.Tensile stress–strain curves and tensile properties of AZ80–3 and BA53–40.TYS,UTS,and TE denote tensile yield strength,ultimate tensile strength,and tensile elongation,respectively.
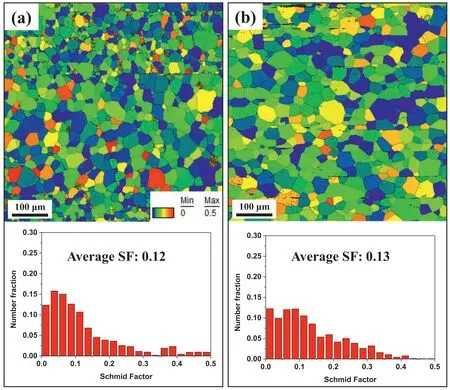
Fig.8.Maps and distributions of Schmid factor (SF) for basal slip under tension along the ED of (a) AZ80–3 and (b) BA53–40.
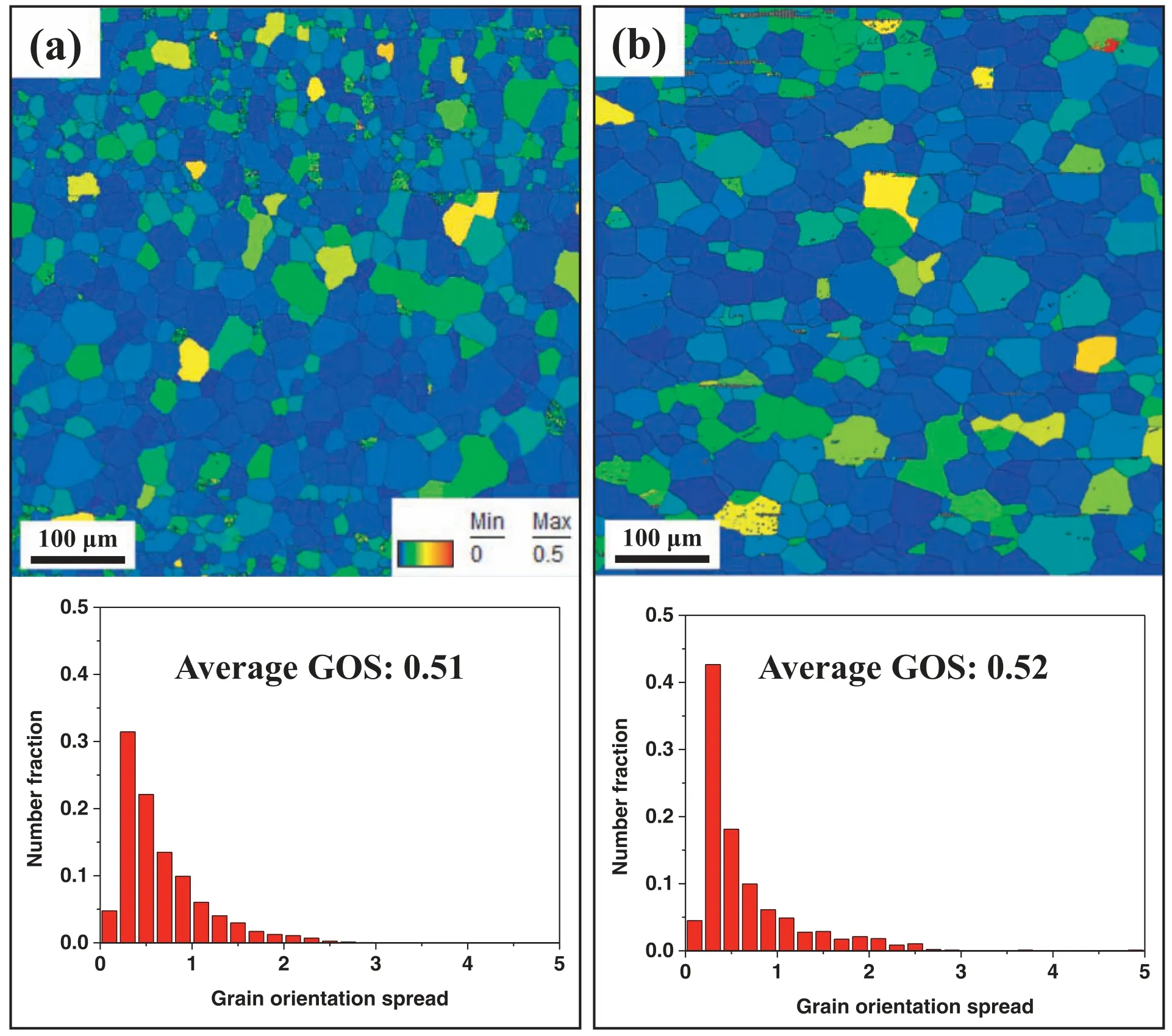
Fig.9.Maps and distributions of the grain orientation spread (GOS) of (a) AZ80–3 and (b) BA53–40.
Fig.7 shows the engineering tensile stress–strain curves and corresponding tensile properties for AZ80–3 and BA53–40.The tensile yield strength (TYS) and ultimate tensile strength (UTS) of AZ80–3 (203 and 334 MPa,respectively)are higher than those of BA53–40 (188 and 251 MPa,respectively).Because the two materials have similar texture distribution and intensity,their texture hardening effect would be similar.Fig.8 shows the Schmid factor (SF) map and the SF distribution for basal slip under tension along the ED of AZ80–3 and BA53–40.Although the SF distribution is slightly different between these two materials,their average SF values are almost identical (0.12 and 0.13 for AZ80–3 and BA53–40,respectively).This indicates that the texture hardening effect of the two materials is nearly identical.
Dislocations accumulated in a material also affect the strength of the material via the strain hardening effect.Fig.9 shows the grain orientation spread (GOS) map and the GOS distribution of AZ80–3 and BA53–40.The GOS value is measured as the average deviation value between the orientation of each detected point in a given grain and the grain’s average orientation [39,40].Accordingly,the GOS value is proportional to the stored strain energy (or dislocation density)of a material [41].Similar to the average SF values,the average GOS values of AZ80–3 and BA53–40 are nearly identical(0.51 and 0.52,respectively).At a given strain,the number of dislocations generated during plastic deformation generally increases as the applied strain rate increases [42,43].When the difference in dislocation slip behavior caused by the different chemical compositions of AZ80 and BA53 is not considered,more dislocations will be generated during extrusion in BA53–40 than in AZ80–3 because of the considerably higher strain rate in BA53–40.However,based on the measured GOS values,the two materials have almost identical dislocation densities.This is because dislocations that accumulated at the early stage of extrusion are annihilated by complete DRX [33,34] and dislocations accumulated in DRXed grains through additional deformation at the late stage of extrusion are annihilated via a vigorous recovery process at the high extrusion temperature of 400 °C.Consequently,the strain hardening effects of AZ80–3 and BA53–40 are almost identical.
The fine grain structure of AZ80–3 than that of BA53–40 plays a crucial role in its higher strength.According to the dislocation pile-up model [44,45],smaller grain size causes dislocation pile-up behind the grain boundary to a smaller extent,and therefore,a higher applied stress,i.e.,a higher material yielding stress,is required for dislocations to pass through the grain boundary.The Hall–Petch or Meyers–Ashworth models explain that the strength of a polycrystalline metallic material proportionally increases to the reciprocal square root of the material’s grain size as long as the grains are sufficientl large to contain pileups [46–49].The average grain size (davg) of AZ80–3 (17.0 μm) is 53% that of the BA53–40 material (32.1 μm);accordingly,the reciprocal square root of the grain size of the former is ∼1.4 times that of the latter.Moreover,Mg alloys have more pronounced grain-boundary hardening behavior compared with Al alloys because the Hall–Petch coefficien of Mg alloys is higher than that of Al alloys[50–53].Therefore,the enhanced grain-boundary hardening effect from fine grains is the primary factor in the higher strength of AZ80–3.
In addition,solid-solution hardening by Al solute atoms can contribute to the increase in the strength of AZ80–3.An increase in the amount of Al in Mg generally increases the strength of the material by the solid-solution hardening effect of Al solute atoms [54].The difference in the atomic radii of Mg (173 pm) and Bi (230 pm) is larger than that between Mg and Al (184 pm).Hence,the solid-solution hardening effect of Bi solute atoms in Mg is expected to be higher than that of Al solute atoms in Mg.However,of the 5 wt.% Bi contained in BA53,a considerable amount of Bi is consumed by forming numerous Mg3Bi2particles.Although all of the 3 wt.% Al contained in BA53 is present in the form of solute atoms in BA53–40,the total amount of Al and Zn solute atoms in AZ80–3 is significantl higher than that of Bi and Al solute atoms in BA53–40.Therefore,it can be deduced that the higher strength of AZ80–3 is a combined result of higher grain-boundary and solid-solution hardening effects.However,notably,the TYS of BA53–40 (188 MPa) is only 7.4% lower than that of AZ80–3 (203 MPa),despite the 13.3-fold higher extrusion speed of the former.
In BA53–40,numerous fin Mg3Bi2particles are uniformly distributed through the material (Fig.6),whereas in AZ80–3,Mg17Al12particles are mostly distributed along the grain boundaries (Fig.4).This larger number of second-phase particles and their more homogeneous distribution in BA53–40 result in a substantially higher particle hardening effect in BA53–40 than in AZ80–3.Consequently,the relatively small difference in TYS between the two materials is attributed to the enhanced second-phase particle hardening effect by numerous fin Mg3Bi2particles in BA53–40.These results demonstrate that BA53 has a maximum extrusion speed of more than ten times that of commercial AZ80.In addition,extruded BA53 has a high TYS of 188 MPa,despite a highspeed extrusion of 40 m/min,and is comparable to AZ80 extruded at the considerably lower speed of 3 m/min.
The comparison of AZ80–3 and BA53–40 also provides the following meaningful insight: if grains of extruded BA53 can be refine by adding alloying elements and/or controlling process conditions,the strength of high-speed-extruded BA53 is expected to be substantially improved compared with that of commercial extruded AZ80 fabricated at low speeds.Therefore,further research on BA53 is required to realize the widespread application of extruded Mg products with simultaneous high productivity and high strength.
Conclusions
This study systematically compared the extrudability,microstructural characteristics,and tensile properties of commercial AZ80 and the recently developed BA53 alloy.When direct extrusion is performed at 400 °C at an exit speed of 4.5 m/min or more,severe hot cracking occurs in AZ80.In contrast,BA53 is successfully extruded at 400 °C under high exit speeds,ranging from 21 to 40 m/min,without any surface cracks.Both AZ80–3 and BA53–40 materials with no surface cracks have a completely DRXed grain structure and typical〈10–10〉 basal texture.In AZ80–3,a continuous network of Mg17Al12particles along grain boundaries is formed through static precipitation of the Mg17Al12phase during natural aircooling after exiting the extrusion die.BA53–40 contains numerous fin lath-shaped particles and relatively coarse particles aligned parallel to the ED.These particles originate from undissolved Mg3Bi2particles in the homogenized BA53 billet.The tensile strength of AZ80–3 is higher than that of BA53–40 because fine grains and more solute atoms in AZ80–3 result in higher grain-boundary and solid-solution hardening effects during tension,respectively.Despite the high extrusion speed of 40 m/min,BA53–40 has a fairly high TYS of 188 MPa,which is primarily attributed to the considerable particle hardening effect from numerous fin Mg3Bi2particles.
Declaration of Competing InterestThe authors declare that they have no conflic of interest.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant (No.2019R1A2C1085272)funded by the Ministry of Science,ICT,and Future Planning(MSIP,South Korea) and by the Materials and Components Technology Development Program(No.20011091) funded by the Ministry of Trade,Industry,and Energy (MOTIE,South Korea).
 Journal of Magnesium and Alloys2023年1期
Journal of Magnesium and Alloys2023年1期
- Journal of Magnesium and Alloys的其它文章
- Development of high-strength magnesium alloys with excellent ignition-proof performance based on the oxidation and ignition mechanisms: A review
- Development and application of magnesium alloy parts for automotive OEMs: A review
- Recent advances in surface endothelialization of the magnesium alloy stent materials
- Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications
- Exploring the contribution of oxygen reduction reaction to Mg corrosion by modeling assisted local analysis
- Microstructures,mechanical properties,corrosion,and biocompatibility of extruded Mg-Zr-Sr-Ho alloys for biodegradable implant applications
