Recent advances in surface endothelialization of the magnesium alloy stent materials
Chngjing Pn ,Xuhui Liu ,Qingxing Hong ,Jie Chen ,Yuxin Cheng ,Qiuyng Zhng ,Lingjie Meng,Jun Di,Zhongmei Yng,Lingren Wng
a Faculty of Mechanical and Material Engineering,Jiangsu Provincial Engineering Research Center for Biomaterials and Advanced Medical Devices,Huaiyin Institute of Technology,Huai’an 223003,China
b The Affiliate Huai’an Hospital of Xuzhou Medical University,Huai’an 223003,China
Abstract Magnesium and its alloy have good mechanical properties and biodegradability,and have become the hotspot of the next-generation biodegradable vascular stent materials.However,their rapid degradation in vivo and poor biocompatibility are still the bottlenecks of clinical applications for the cardiovascular stents.In particular,how to induce the repair and regeneration of the vascular endothelial with normal physiological functions on the surface of the magnesium alloy stent materials represents the key to its clinical application in the fiel of cardiovascular stents.It has been believed that it is an ideal way to completely solve the postoperative complications through constructing the multifunctional anti-corrosive bioactive coating on the magnesium alloy surface to induce the formation of vascular endothelium with normal physiological functions.However,how to construct a corrosion-resistant multifunctional bioactive coating with the good endothelial regeneration abilities on the magnesium alloy surface still faces a great challenge.This paper mainly focused on highlighting and summarizing the recent advances in the surface endothelialization of the magnesium alloy materials for the vascular stent,including the bio-inert coating,in-situ immobilization of bioactive molecules on the surface,polymer coating loaded with bioactive factors,novel multifunctional polymer coating,bioactive micropatterns,bioactive layer with glycocalyx-like structure,NO-releasing coating and bioactive sol-gel coating.The advantages and disadvantages of these strategies were discussed and analyzed.Finally,in the senses of future development and clinical application,this paper analyzed and summarized the development direction and prospect of surface endothelialization of the magnesium alloy vascular stents.It is anticipated that this review can give the new cues to the surface endothelialization of the cardiovascular magnesium alloy stents and promote future advancements in this field
Keywords: Magnesium alloy;Stent;Endothelialization;Surface coating;Surface modification
1.Introduction
Cardiovascular diseases (CVD) have become one of the diseases with the highest morbidity and mortality in the world.According to the prediction of the World Health Organization (WHO),more than 23 million people will die of the cardiovascular diseases every year in the world by 2030 [1].Coronary atherosclerotic heart disease is one of the major cardiovascular diseases [2].In the past few decades,bare metal stent (BMS) insertion has become one of the main methods to clinically treat the stenotic coronary heart disease thanks to its advantages of small trauma,rapid recovery and lower economic burden [3].However,the in-stent restenosis (ISR) rate after the BMS implantation is very high [4].Since 2003,the clinical application of the drug eluting stent (DES) has significantly reduced the ISR to about 10%,which is regarded as the milestone of the interventional therapy [5].However,the non-degradable stents (such as 316 L stainless steel,CoCr alloy,NiTi alloy,etc.) currently used in clinic will permanently remain in the body after the implantation.The leakage of metal ions and the poor biocompatibility of the stents will inevitably lead to the complications such as the inflamma tion,excessive intima hyperplasia and late thrombosis [6,7].On the other side,the anti-proliferative drugs released from DES (such as rapamycin,paclitaxel,etc.) not only inhibit the excessive proliferation of smooth muscle cells (SMCs) and prevent ISR,but also prevent the adhesion and growth of the endothelial cells (ECs),resulting in the delayed endothelial healing,late thrombosis,late restenosis and other clinical complications [8–10].In addition,the biocompatibility of the polymer drug carriers used for DES is insufficient and the degradation,aging and coating peeling after the long-term implantation also cause potential risks to the patients [11,12].
In order to overcome the shortcomings of the nonbiodegradable metal vascular stents,researchers mainly employ the biodegradable polymer to construct the drug-eluting coatings to avoid the clinical complications caused by the non-biodegradable polymer [13,14],or the drugs are stored in the micropores on the BMS surface without polymer carrier.The size of the pores determines the drug content and release rate [15].However,there are still obvious shortcomings.After the drug is released,the vascular intima is not fully covered after the polymer degradation,the metal substrate of the vascular stent will be exposed to the blood,and the normal vascular endothelium cannot completely regenerate due to the limited surface hemocompatibility of the stent,which cannot completely avoid the occurrence of thrombus.The harmful metal ions such as nickel,chromium and cobalt on the metal surface are released into the blood for a long time may also lead to the long-term complications [16,17].In addition,due to the poor controllability of the drug release and the significan increase of metal surface area,the release of harmful ions from the carrier-free DES has a greater impact on ISR,which can reach 13.1% in 8 months [18].
The inherent disadvantages of the non-biodegradable metal stents have prompted researchers to develop new biodegradable vascular stents [19,20].At present,the materials applied to the biodegradable stents mainly include the biodegradable polymers and the biodegradable metals [21].The biodegradable polymer stents (such as polylactic acid (PLA),poly(lactic-co-glycolic) (PLGA),etc.) can be gradually degradedin vivo,and the degradation products are nontoxic and can be discharged with body fluid or absorbed by the human body.However,the strength of the polymer stent is low,and the larger stent strut width is required to provide the adequate support force,which can obviously increase the contact area with the blood vessel wall,leading to the potential restenosis risks [22,23].On the other hand,the degradation rate of the biodegradable polymer is uncontrollable,and the degradation products may also have defects such as immune and inflammator reactions [24].Therefore,in recent years,researchers have paid more and more attention to the biodegradable metals with better mechanical properties because of their prominent advantages [25,26]: (1) They can reduce the blood clot formation and antithrombosis treatment time after the implantation compared with the permanent metal stents;(2) The stent is finall completely absorbed by the human body,which is more suitable for the blood vessels where the stent is easy to be squeezed or broken,such as the femoral artery;(3) The degradation and disappearance of vascular stents can restore the vasomotor and systolic pressure,which is conducive to the formation of positive vascular remodeling in late stage.
Currently,the biodegradable metal stent materials mainly include iron,zinc,magnesium and their alloys.Iron-based alloys have good biocompatibility,high elastic modulus and radial support force [27],but the degradation rate is too slow[28].Zinc is one of the essential trace elements of the human body,which has the functions of repairing and improving the integrity of vascular endothelium and anti-atherosclerosis[29,30].Zinc alloys have appropriate bending strength,good elongation and appropriate degradation rate;however,zincbased stent may produce excessive Zn2+during the degradation,which will have potential toxicity to human body [31].Meanwhile,most of the zinc alloys show work-softening,insufficien work-hardening ability and self-aging [32].The aging of the zinc alloy makes the mechanical properties of the zinc stent unstable.Due to the heterogeneous deformation during compression and expansion of vascular stent,the work-softening may make the stent unable to be firml fi ed on the balloon during compression and the stent is easy to move during the implantation,while the work-hardening may lead to uneven expansion and deformation of the stent.
Compared with iron and zinc-based stents,magnesiumbased vascular stent materials have obvious advantages(Fig.1).Magnesium is the fourth abundant element in the human body [33],it is not only a cofactor of many enzymes,but also plays an important role in inhibiting abnormal nerve excitation,synthesizing protein,reducing hypertension,treating acute myocardial infarction and preventing atherosclerosis [34,35].Magnesium alloy has low density,excellent mechanical properties and goodin vivosupport performance.Magnesium alloy is biodegradable and has potential advantages in overcoming the chronic inflammation late thrombosis and long-term use of antiplatelet drugs caused by traditional non-biodegradable metal stents[36–38].Compared with polymer stents,magnesium alloys have higher strength and better ductility,and therefore are more suitable as the vascular stent materials.The degradation products of magnesium alloy will be discharged from the body or absorbed by the human body.Therefore,the magnesium alloy has become a research hotspot of the cardiovascular stent materials [39,40]
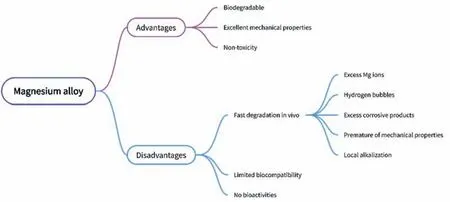
Fig.1.The advantages and disadvantages of the magnesium alloy for the cardiovascular stents.
Although magnesium alloys have shown attractive application prospects in the development of the biodegradable vascular stents,the rapid degradation rate and the poor biocompatibility are still the bottlenecks of their clinical applications [41–43],and these shortcomings are displayed in Fig.1.Magnesium alloy is chemically active,and its standard electrode potential is −2.736 V [44],which is the lowest potential among three biodegradable metals (iron,zinc and magnesium).It is extremely easy to corrode in the physiological environment containing Cl−,resulting in the rapid degradationin vivoand premature loss of the mechanical properties.Moreover,the accumulation of the degradation products may lead to adverse reactions such as the inflammatio [45].Additionally,the magnesium alloy will produce a large amount of hydrogen during the degradation process and the pH value of the surrounding environment will be increased,which is not conducive to the adhesion and growth of cells on the surface,nor conducive to the surface endothelialization after the stent implantation,and thus may cause acute and long-term complications [46–48].On the other hand,the surface of unmodifie magnesium alloy does not have any biomolecules or chemical groups,and its bioactivity is poor,which is difficul to regulate the physiological responses of the surrounding environment to adapt to the physiological microenvironment of human tissues [49,50],especially in blood vessels.All these disadvantages are not helpful for the vascular endothelial regeneration on the magnesium alloy stent surface.
2.Strategies for enhancing corrosion resistance of magnesium and its alloys
For magnesium alloys used in vascular stents,corrosion resistance is always the firs problem to be considered.Serruys [51] pointed out that the ideal biodegradable vascular stent should maintain sufficien support force and mechanical integrity in the process of vascular reconstruction,and gradually degrade after 6–12 months,and completely degrade after 12–24 months.Obviously,the bare magnesium alloy vascular stent has a considerable distance from the considerations.At present,there are two main strategies,including alloying and surface modification have been extensively investigated to improve the corrosion resistance.Generally speaking,the addition of alloy elements can improve the corrosion resistance of magnesium alloy matrix phase or promote the formation and uniform distribution of corrosion resistant phase,improving the corrosion resistance [52].It has been demonstrated that the corrosion resistance of magnesium alloys is closely related to metallographic structure and impurities as well as the surrounding environment [53–55].Therefore,by reducing the content of harmful alloy elements (Co,Ni,Ba,Cd,Al,etc.) and increasing the content of beneficia elements(Ca,Mn,Y,RE,Zn,Sn,Sr,Zr,etc.) to form the corrosionresistant phase and refin the crystalline grains,the corrosion resistance,mechanical properties and biocompatibility of the magnesium alloys can be improved to a certain extent [56].For example,adding calcium to the pure magnesium can form a second phase of Mg2Ca in the alloy.When the concentration of calcium is 0.6%,the grain size can be reduced from 130 μm of the pure magnesium to 30 μm of Mg-Ca alloy,and the distribution of Mg2Ca phase is very uniform,which not only refine the grains,but also plays an important role in solution strengthening [57].Adding 2% Sr to pure magnesium to form the Mg-Sr binary alloy also has a similar effect [58].Since both Ca and Sr are important elements for osteogenesis,magnesium alloys with these two elements are very suitable for the bone implants.In addition,in the past several decades,a large number of binary,ternary or quaternary magnesium alloys,such as Mg-Mn-Zn [59],Mg-Zn-X (X=Ca,Mn,Si) [60],AZ91 [61],Mg-1X (X=Al,Ag,In,Mn,Si,Sn,Zn,Zr) [62],Mg-Li (Al)-(RE) [63],Mg-Mn-Zn-Nd [64],etc.,have been developed to control the degradation rate of magnesium-based biomaterials,maintain their mechanical properties and reduce the clinical side effects.As far as the cardiovascular biomaterials are concerned,the magnesium alloys,including AZ31,WE43,Mg-Zn-Mn,Mg-Nd-Zn-Zr,Mg-Zn-Y-Nd,etc.have been extensively explored.Although these alloys have shown good corrosion resistance and biocompatibility,only WE43 stent of Biotronic company has been used clinically.Moreover,in order to further improve the clinical performances,the stent is still covered by the drug-eluting PLLA coating.On the other side,although there are many alloy elements that can be used to improve the corrosion resistance of magnesium alloys,the corrosion resistance in the physiological environment still needs to be improved.Moreover,most alloy elements cannot effectively improve the biocompatibility of the magnesium alloys,and even the dissolution of some alloy elements under the physiological conditions will cause potential harm to human body.For example,the dissolution of Al,Ag,Zr and Ni may cause toxicities to the surrounding tissues after the implantation [65–67],therefore the content of alloy elements in the biomedical magnesium alloy must be strictly controlled within a certain range.It follows that alloying cannot completely solve the problem of thein vivorapid degradation and the limited biocompatibility of the magnesium alloys.Furthermore,the smelting process of alloying is complicated,which requires special equipment and the properties are difficul to accurately control.Consequently,alloying is usually applied to enhance the mechanical properties of the magnesium alloys.
On the other side,the magnesium alloy surface is usually lack of bioactivities and it is difficul to effectively control the physiological responses of the surrounding environment around the implant to enhance biocompatibility and endothelialization.It is well known that the corrosion degradation behaviors and surface biocompatibility of the magnesium alloy stent materials are closely related to their surface properties,therefore it is of great scientifi significanc to endow magnesium alloys with better bioactivities by surface modificatio to induce the regeneration of natural vascular endothelium.Generally,the surface modificatio mainly includes three strategies: one is to use chemical or electrochemical treatment to form a chemical conversion layer on the surface to improve the corrosion resistance of magnesium alloys [68].The second is to improve the corrosion resistance of the magnesium alloy by changing the surface structure [69].The third is to improve the corrosion resistance by constructing a coating on the surface,such as surface self-assembly,deposition of inorganic coatings [70,71],preparation of layered double hydroxides [72] and polymer coatings [73],etc.The modifie layers formed by these methods can effectively improve the corrosion resistance and also enhance the biocompatibility to a certain extent,but the lack of sufficien biological activity can still lead to coagulation and delayed healing of the endothelium.Many literatures have reviewed the research progress of surface modificatio in improving the corrosion resistance of the magnesium alloys.Here the present paper mainly focuses on the recent advances in surface endothelialization of the magnesium alloys for the cardiovascular stents,and the details of surface modificatio in enhancing the corrosion resistance of magnesium alloys are no longer discussed.
3.The in vivo results of magnesium alloy cardiovascular stents
In 2003,Heublein et al.firstl implanted twenty AE21 magnesium alloy stents in the coronary arteries of 11 pigs[74],and the study showed that the magnesium alloy stents degraded rapidlyin vivoand could significantl induce the early neointima hyperplasia,indicating the feasibility of the magnesium-based alloys for the biodegradable coronary stents.Subsequently,Biotronik Company applied WE43 magnesium alloy to prepare the magnesium alloy vascular stents(adsorbable magnesium stent,AMS,Fig.2a),and 12 magnesium alloy stents and 6 metal stents were implanted into the porcine coronary arteries.Coronary angiography at the 4th and 8th week indicated that the minimum lumen diameter with the AMS was significantl larger than that of the metal stents,suggesting that the performance of the AMS was better than that of traditional non-biodegradable metal stent.The histological analysis at the 8th week also found that the degree of intimal hyperplasia of the pigs implanted with the AMS was significantl lower than that of the metal stent,and there were no serious complications such as in-stent thrombosis (IST),in-stent restenosis (ISR) and stent collapse [75].In 2004,Di Mario et al.implanted the AMS into the coronary arteries of 33 pigs [76].The coronary angiography at the 4th week showed that the minimum lumen diameter (1.49 mm) of the AMS group was significantl larger than that of the control(stainless steel stent,1.34 mm),indicating that the magnesium alloy stent could accelerate the endothelialization while inhibiting the SMCs proliferation.The coronary angiography at the 12th week also found that the minimum lumen diameter of the AMS group increased from 1.49 mm to 1.68 mm,indicating that the endothelialization was beneficia to blood vessel patency and prevention of the neointimal hyperplasia.In order to further improve the corrosion resistance and the ability to inhibit in-stent restenosis,Biotronic company developed a drug-eluting coating using PLLA (paclitaxel as the antiproliferative drug,0.07 μg/mm2,Fig.2b,DREAMS 1 G) on AMS.The results showed that the stent showed good safety and effectiveness.Subsequently,Biotronic company developed the second generation of drug-eluting magnesium alloy vascular stent using poly (L-polylactic acid) as the drug carrier and rapamycin as the antiproliferative drug (1.4 μg/mm2,Fig.2c,DREAMS 2 G).The study demonstrated that the endothelialization and anti-inflammator response of DREAMS 2 G were better than that of DREAMS 1 G after 6 months implantation in the porcine coronary artery [77].The surface coating of the magnesium alloy stent (JDBM,Mg-Nd-Zn-Zr alloy)developed by Shanghai Jiaotong University is polylactic acid coating loaded with rapamycin [78].The animal experiment results suggested that the early biocompatibility of the JDBM stent after implanting into the porcine coronary artery was good.The results of intravascular ultrasound,optical coherence tomography (OCT) andin vitromicro computed tomography (microCT) all showed that the coating significantl improved the corrosion resistance and biocompatibility of the magnesium alloy stents [79].
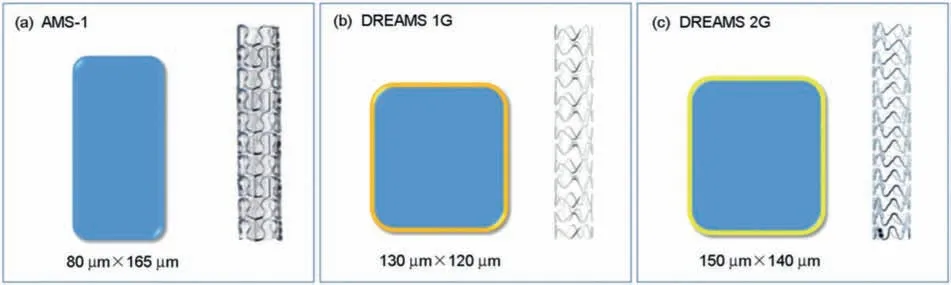
Fig.2.Schematic cross-sectional profile of the magnesium stents struts of non-eluting adsorbable magnesium stent (AMS-1 with 80 mm×165 mm (a);DREAMS 1st generation (DREAMS 1 G) with 130 mm×120 mm struts (b) and DREAMS 2nd generation (DREAMS 2 G) with 150 mm×140 mm struts(c) [89].
After confirmin the safety and effectiveness of the magnesium alloy stents in animals,a large number of clinical studies were carried out to confir their effectiveness and safety in humans [80,81].In 2007 and 2009,Erbel et al.[75] and Waksman et al.[82],respectively reported the results of PROGRESS-AMS,which included 63 patients with simple vascular diseases.The results showed that the magnesium alloy stent was completely degraded after 2 months implantation in human body,resulting in the early loss of radial support force.The late lumen loss (LLL) after 4 months was relatively large (1.88 ± 0.49 mm),indicating that the long-term patency of the blood vessels was limited.It was concluded that the premature degradation of the magnesium alloy stentsin vivoand the early loss of the radial support force can cause the intimal hyperplasia and elastic recoil of the blood vessels.In 2010,Biotronic company carried out a study on the safety and effectiveness of DREAMS 1 G in the human body,and 47 stents were inserted into the coronary arteries of 46 patients with coronary heart disease.The follow-up results showed that DREAMS 1 G was safer and more effective than AMS,and the radial support period of the lumen was 3–6 months.The stents were completely degraded in about 9–12 months.After one year implantation,the target lesion revascularization rate(TLR)was similar to that of DES and far lower than that of AMS.There were no serious cardiovascular adverse events such as IST and cardiac mortality.However,the LLL of the stent was still 0.52 mm,lower than that of AMS while higher than that of DES [83].After 3-year implantation,the LLL was 0.32±0.32 mm,which was better than that of one-year result[84],and no TLR or cardiac death occurred during the follow-up period.In 2013,Biotronik company carried out a prospective,multicenter,non-randomized clinical trial named BIOSOLVE-Ⅱ,in which 123 DREAMS 2 G were implanted in 123 patients with coronary heart disease to evaluate the safety and effectiveness in human body[85].It was found that the lumen radial support period and the complete degradation of DREAMS 2 G was comparable to DREAMS 1G The follow-up results showed that DREAMS 2 G had good safety and effectiveness.The results of coronary angiography at the 6th month [86] and the 12th month[87] were similar.At the 6th month,the LLL and TLR rates were lower than DREAMS 1 G,therefore DREAMS 2 G had obtained the European Conformity (CE) certificatio in June 2016,becoming the firs drug-eluting biodegradable magnesium alloy stent entering the market.The validation trial of DREAMS 2 G (BIOSOLVE-Ⅳ) included 400 patients,and the results showed that the incidence of in-stent thrombosis,target vessel myocardial infarction,and TLR after 12 months implantation were 0.5%,0.5%,and 4.6%,respectively [88],indicating good safety and effectiveness.It also proved the great potential of the magnesium alloy biodegradable vascular stents to replace the traditional DES in clinical application.
4.Surface endothelialization of the non-biodegradable stents
Healthy human vascular endothelial have excellent anticoagulant and can inhibit the excessive proliferation of SMCs[90],it plays a very important role in preventing adverse cardiac events and maintaining blood dynamic balance.Vascular endothelial layer is the key to regulate coagulationanticoagulation balance and intima repair-proliferation balance [91].The stent implantation will inevitably injury the vascular endothelium,leading to a series of side effects such as excessive proliferation of SMCs,inflammator reaction,stent thrombosis,and even in-stent restenosis or implantation failure [92] due to the presence of permanent residual substance for a long time.Fig.3 shows a schematic illustration for ISR and stent thrombosis mechanisms after stent implantation as well as four types of ISR [14].According to the underlining pathophysiological mechanism of the intima healing after the stent implantation,it has been generally believed that the induction of the vascular endothelial with natural physiological function on the stent surface represents an ideal way to completely solve the postoperative complications [93,94].Fig.4 shows the commonly used strategies for the surface endothelialization of the vascular stents.
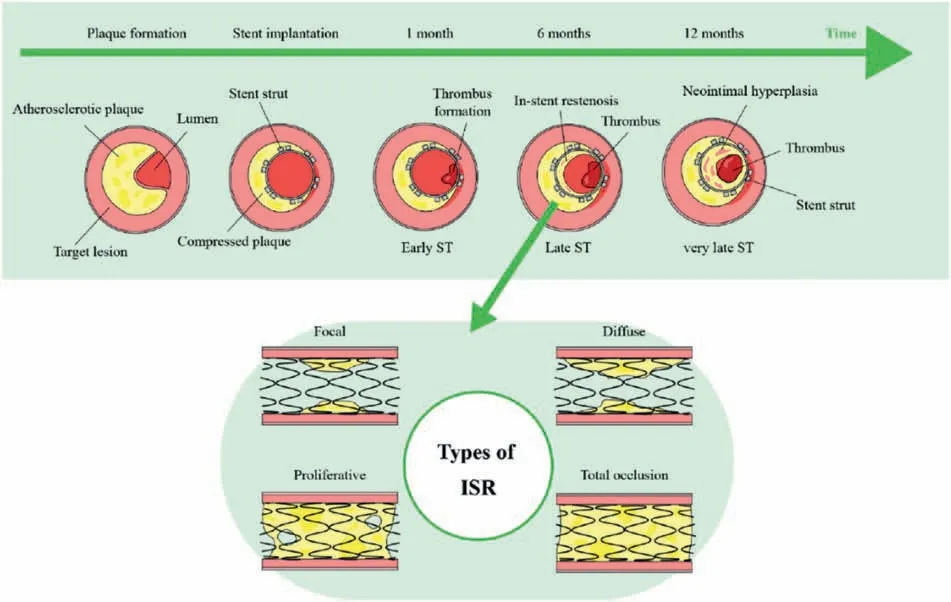
Fig.3.Schematic illustration for mechanisms and types of in-stent restenosis (ISR) and stent thrombosis (ST) [14].
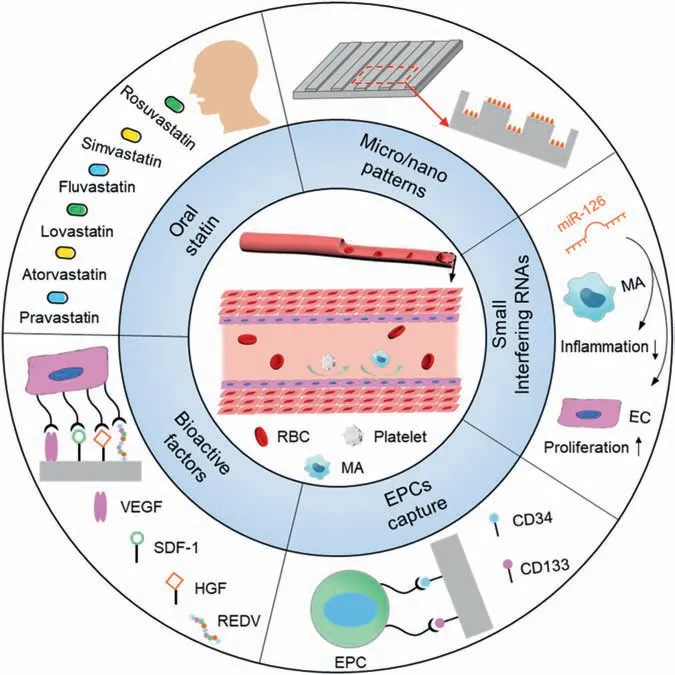
Fig.4.The strategies for surface endothelialization of the cardiovascular stents.
It has been demonstrated that the vascular endothelium is composed of the endothelial cells and glycocalyx layer covering the endothelium surface.Under the shear stress of the blood fl w,the vascular ECs mainly display a regular paving-stone microstructure along the blood fl w direction.Therefore,constructing the regular micropatterns or micronanostructures on the surfaces of the stent materials can guide the ECs to grow along the blood fl w,so as to promote the growth and directional arrangement of vascular ECs to a certain extent and thus facilitate the formation of an endothelial that mimics the arrangement of natural endothelium[95].However,for vascular stents with the complicated threedimensional structures,it is difficul to prepare uniformly oriented microstructures on the stent surface.On the other hand,in order to promote the endothelialization of the stent surface after the implantation,the researchers also tried to promote the early endothelialization by taking oral medicine.At present,statins are mainly used in clinic [96],which can inhibit the SMCs proliferation and migration,and have good antioxidant,anti-inflammator,anti-platelet aggregation and antithrombotic properties.It is beneficia to the ECs growth on the stent surface after the implantation.However,these oral medicines are not targeted.In order to promote the rapid endothelialization,a large amount of medicine is required,which is easy to cause systemic toxicity and other clinical side effects,and thus bring the psychological and economic burdens to patients.
It has been shown that thein-siturepair of the injured endothelium caused by the stent implantation is a complicated dynamic time-ordered process,which is closely related to the spatiotemporal action behaviors of cell chemokines,growth factors,extracellular matrix (ECM) proteins and transmembrane receptor-ligand,etc.Therefore,the introduction of the specifi cellular functional factors that can induce endothelial regeneration on the stent surface,such as vascular endothelial growth factor (VEGF) [97],stromal cell-derived factor-1(SDF-1) [98],hepatocyte growth factor (HGF) [99],Arg-Glu-ASP-VAL (REDV) [100],semaphorin 4D (SEMA4D) [101],etc.can effectively induce and promote the ECs adhesion and proliferation,so as to promote the repair and regeneration of vascular endothelium.However,most of the biomolecules used in this strategy are not highly specific and it is difficul to achieve selective regulation of multiple pathological responses in the complicated physiological environment of the blood vessel.For example,extracellular matrix proteins can simultaneously promote the adhesion and growth of ECs and SMCs.In addition,the activities of these biomolecules are easily lost during the chemical grafting reaction process and the amount of the surface biomolecules is also limited.Consequently,it is difficul to play a long-term regulatory rolein vivo,and there are often a series of problems in practice,such as aggravating inflammator reaction,coagulation reaction and vascular intima hyperplasia.
It is well known that there are a lot of endothelial progenitor cells (EPCs) in human circulating blood that can differentiate into endothelial cells,the specifi antibody,such as CD34 [102] and CD133 [103] that can capture EPCs,can be immobilized on the stent surface to promote the EPCs adhesion,homing and differentiation into endothelial cells,and therefore effectively promote early endothelialization.However,some studies have shown that CD34 and CD133 can also capture monocytes which can differentiate into SMCs [104],leading to the neointima formation and the in-stent restenosis,so it is still difficul to avoid adverse cardiac events [105].At the same time,in order to increase the concentration of EPCs,researchers also tried to promote the regeneration and repair of vascular endothelium through vascular perfusion of thein vitroexpanded EPCs [106];however,whether the perfused EPCs can efficientl adhere to the injury site and differentiate into normal endothelial cells remains controversial,and the perfusion of EPCs may also increase the inflammator response and promote the SMCs proliferation [107],leading to in-stent restenosis and thrombosis.
In recent years,the concept of the precise treatment of the cardiovascular diseases has provided a new idea for the development of the new generation implant/interventional medical devices.Studies have found that the differential expression of manyin vivonon-coding small ribonucleic acid (miRNAs)sequences is closely related to the occurrence and development of the cardiovascular diseases.These miRNAs mainly induce Messager RNA (mRNA) degradation or inhibit gene expression by combining with target sequences on intracellular specifi mRNAs,and then precisely regulate the biological response of one or more types of cells to control disease progression [108].At present,a variety of miRNAs have been found to be closely related to intimal hyperplasia and vascular function after the stent implantation.For example,miR-126,which is highly expressed in the endothelial cells,not only plays an important role in inhibiting inflammatio and promoting the repair of the damaged endothelium,but also plays an important role in promoting the expression of vascular repair related chemokines such as C-X-C motif chemokine ligand 12 (CXCL-12),inhibiting the expression of atherosclerosis risk factor DLK-1(Protein Delta Homolog 1)and restoring the autophagic fl w of injured endothelial cells [109],showing the potential to reverse atherosclerosis.Therefore,thein vivotransport of miRNAs via vascular stent platform has become an important direction of the cardiovascular medical devices and has a good application prospect.For example,Izuhara et al.[110] used poly (lactic acid co glycolic acid)(PLGA) nanoparticles to load miR-126 and then electrostatically immobilized it onto the surface,demonstrating that the nanoparticles can target endothelial cells,accelerate vascular endothelial repair,and inhibit intimal proliferation.However,miRNAs have poor stability in the blood circulation,and there is a risk of inducing immune and inflammator reactions.Although the stability and non-toxicities of miRNAs can be effectively improved by the nanocarriers [111],how to quickly escape from lysosomes after entering cells is still an important problem.Although this direction has potential huge application in the fiel of cardiovascular stents,there are still a lot of fundamental problems to be solved.
5.Surface endothelialization of magnesium alloy stent materials
In theory,the above-mentioned strategies of the surface endothelialization of vascular stents can be directly applied to the magnesium alloy vascular stent materials to achieve the endothelium repair and regeneration.However,magnesium alloys are active in the physiological microenvironment.When the above methods are applied to the surface modificatio of the magnesium alloys to promote endothelial regeneration,the biomolecules immobilized on the surface are easy to lose and inactivate with the corrosion degradation of magnesium alloys.Consequently,it is difficul to achieve the long-termin vivocontrol of biological responses,resulting in the limited ability to promote the rapid repair and regeneration of the endothelium.In order to enhance thein-situregeneration and repair of vascular endothelium,it is firs necessary to improve the corrosion resistance of the magnesium alloys.Since the corrosion degradation behaviors of magnesium alloys are closely related to the biocompatibility,and both are closely related to the surface properties,it is of great scientifi significanc to improve the corrosion resistance of the magnesium alloys and promote the capability of thein-siturepair and regeneration of the injured vascular endothelium through surface modification Generally speaking,improving the corrosion resistance of the magnesium alloy in the physiological environment plays an important role in enhancing the biocompatibility.There are many reviews [112–115] about the methods for improving the corrosion resistance;however,only improving the corrosion resistance is not enough to ensure the excellent ability to promote endothelial repair and regeneration.It is often necessary to endow the magnesium alloy surface with multifunctional bioactivities through other surface biofunctionalization strategies,and thus achieve regulating the multiple physiological responses after implantation and ultimately promoting the rapid repair and regeneration of the endothelium.
5.1.Construction of the bio-inert coatings to enhance surface endothelializaton
It is well known that the plasma protein adsorption on the surface occurs firs after the vascular stent is inserted into the blood vessel.The adsorbed plasma protein has a decisive impact on the subsequent interface biological reactions.The contact of the protein with the vascular stent often leads to the adsorption and functional changes of plasma protein,leading to the clinical side reactions such as the coagulation and SMCs proliferation [116].For example,the fibrinoge adsorption on the surface likely leads to the thrombus formation.Therefore,the endothelialization of the magnesium alloy stent materials can be improved to a certain extent by preparing a bio-inert coating that can prevent the protein and blood cells adhesion.Polyethyle glycol (PEG) is a kind of bioinert polymer with the excellent ability to prevent plasma protein non-specifi adsorption.After micro arc oxidation(MAO)treatment,PEG was immobilized on the magnesium alloy surface through thiol-ene photochemical reaction to improve the blood compatibility and corrosion resistance [117],and the results indicated that,as compared to the pristine magnesium alloy,the corrosion current density of the modifie magnesium alloy was reduced by three orders of magnitude and the hydrogen evolution was reduced by 61.5%.Moreover,there was almost no platelet adhesion on the surface,which was favorable for the ECs growth due to the good blood compatibility and hydrophilicity.However,due to the huge corrosion effect of MAO on magnesium alloy during the treatment,micro arc oxidation may be difficul to be directly used for the surface modificatio of magnesium alloy vascular stent.The zwitterionic molecules are another kind of bio-inert substances with excellent hydrophilicity and blood compatibility.In order to introduce the zwitterionic molecules on the magnesium alloy by self-assembly,Ye et al.[118] firstl grafted phosphorylcholine (PC) or sulfobetaine (SB) onto the end of self-assembled silane molecules,and then immobilized the synthesized organic molecules on the magnesium alloy surface(Fig.5).The results showed that the platelet adhesion and activation were significantl reduced on the modifie magnesium alloy surface.At the same time,owing to the formation of a better corrosion-resistant coating on the surface,the corrosion current density was significantl reduced.Therefore,the author believed that this strategy can be used for the biodegradable magnesium alloy stent to promote endothelial regeneration.Unfortunately,the bio-inert coating often only has short-distance effect.Although it has good hydrophilicity and blood compatibility,it also can prevent the ECs adhesion and growth to a certain extent with the exception of preventing the proteins and platelets adhesion and activation.In particular,it usually lacks bioactivities and cannot actively regulate and promote the ECs growth and induce endothelial regeneration,therefore,the long-term patency after the implantation needs to be improved [119],and further biological modificatio is required.
5.2.In-situ immobilization of bioactive factors by wet chemical method
The introduction of bioactive molecules on the magnesium alloy surface to construct the multifunctional bioactive coatings with the ability to regulate the microenvironment responses around the implant represents one of the main ways to improve the biocompatibility and promote thein-situendothelium repair.This strategy is to introduce different active groups or biomolecules on the magnesium alloy surface to construct the different multifunctional surfaces to achieve the regulation of blood and cell physiological microenvironment,thereby promoting the ECs growth and endothelialization.
5.2.1.In-situimmobilization of bioactive factors via surface self-assembly
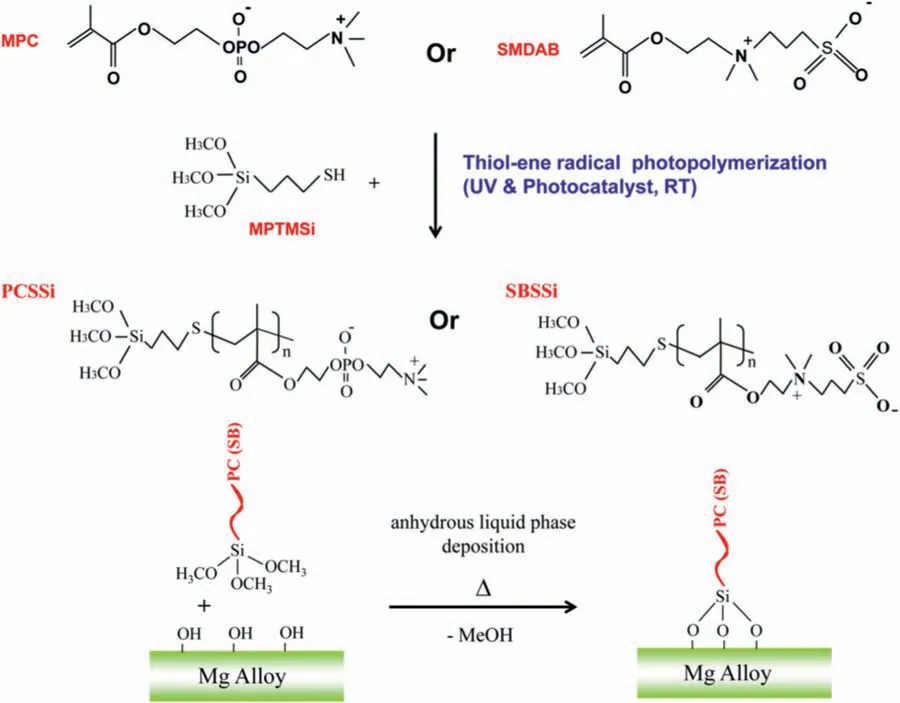
Fig.5.The structures of siloxane functionalized phosphorylcholine (PCSSi) or sulfobetaine (SBSSi) macromolecules and the schematic diagram for surface modificatio [118].
In general,there are no chemical groups on the magnesium alloy surface,so it is impossible to directly introduce bioactive factors on the surface through covalent grafting.Self-assembly is a popular surface modificatio method to form a strong chemical bond between the surface and the organic molecules with the different chemical groups [120].The chemical groups can be further used to carry outin-situchemical coupling and grafting bioactive molecules (such as extracellular matrix proteins,cell adhesion polypeptides,cell growth factors,etc.) to construct the multifunctional surfaces[121,50],leading to the promotion of the endothelial regeneration and repair.In our previous work,we firstl studied the corrosion resistance and biocompatibility of the magnesium alloys modifie by the different self-assembled molecules (3-aminopropyltrimethoxysilane,3-phosphonopropionic acid and dopamine),and found that 3-phosphonopropionic acid modifie magnesium alloy had the best corrosion resistance and can better promote the ECs adhesion and growth due to the covering effect and partial phosphating effect of the selfassembly molecules [122].Therefore,16-phosphoryl hexadecanoic acid with the similar chemical structure was further immobilized on the chemically treated magnesium alloy surface,and then the antifouling molecule (PEG),anticoagulant molecule (heparin) and extracellular matrix protein (fi bronectin) were sequentially grafted on the magnesium alloy surface(Fig.6)to simultaneously improve the corrosion resistance,blood compatibility and endothelial cell growth [123].The results indicated that this strategy can be used for the surface modificatio of the magnesium alloy stent materials to promote the repair and regeneration of the damaged endothelium.Similarly,in order to specificall promote the ECs adhesion and growth,the silane molecule was introduced on the chemically-treated Mg-Zn-Y-Nd surface by self-assembly[124],and then heparin,REDV(Arg-Glu-ASP-VAL)and anti-CD34 were covalently grafted onto the surface.The biocompatibility results showed that the modifie magnesium alloy can not only significantl reduce the platelet adhesion and hemolysis rate,but also obviously promote the ECs adhesion and growth.Moreover,the modifie surface can enhance nitric oxide (NO) release of endothelial cells and reduce the adhesion of macrophages,exhibiting a better anti-inflammator .Therefore,it can be used for the surface endothelialization of the magnesium alloy vascular stent materials.
Although the bioactive coating constructed by introducing bioactive molecules based on self-assembly has obtained goodin vitroandin vivoexperimental results,the surface biomolecules will also be rapidly lost with the corrosion degradation of the magnesium alloys.How to ensure the long-term existence of the bioactive moleculesin vivo,so as to achieve complete endothelialization before the corrosion degradation of magnesium alloys is completed,is still a huge challenge.At the same time,due to the limited number of chemical groups introduced by surface self-assembly,the amount of surface biomolecules is often difficul to meet the requirements of thein vivolong-term regulatory.Moreover,most of biomolecules are grafted by means of surfacein-situchemical reaction,which may lead to the loss of bioactivities,resulting in the poorin vivoperformances.
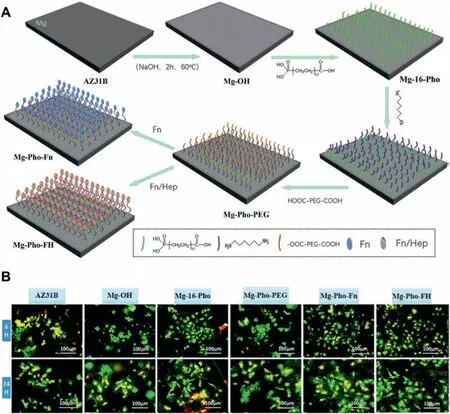
Fig.6.(A) The schematic diagram of the immobilization of PEG,heparin and fibronecti on the magnesium surface based on self-assembly of 16-phosphonohexadecanoic acid.(B) The cell adhesion behaviors on AZ31B,alkali heat treated (Mg-OH),self-assembly of 16-phosphonohexadecanoic acid(Mg-16-Pho),PEG modifie (Mg-Pho-PEG) and heparin/fibronecti modifie magnesium alloys (Mg-Pho-FH) [123].
5.2.2.Immobilization of bioactive factors via polydopamine coating
Dopamine is a high adhesion molecule inspired by mussel feet [125].Under alkaline conditions (pH 8.0–8.5),dopamine can self-polymerize into a closely adhered polydopamine(PDA) coating on the solid surfaces when it is immersed in dopamine solution.At the same time,the PDA coating can also undergo Michael addition reaction or Schiff base reaction with the substances containing amine and thiol groups[126,127],thereby it can be further used as a functional intermediate layer to introduce the bioactive molecules or directly immobilize the bioactive molecules containing amine or thiol groups,leading to the improvement of the corrosion resistance and biocompatibility of magnesium alloy,as well as the promotion ofin-situendothelium regeneration and repair.In general,this strategy does not require special chemical reaction conditions,and the PDA coating on the surface has a strong binding force with the substrate.Therefore,it has a significan effect on improving the corrosion resistance of magnesium alloys and can be used to functionalize the magnesium alloys to promote endothelial repair and regeneration.
Liu et al.[128] treated the Mg-Zn-Y-Nd alloy surface with hydrofluori acid (HF),followed by preparation of the PDA coating on the surface.The corrosion resistance and cell growth behaviors on the modifie surface were investigated in detail.The results showed that the modifie magnesium alloy not only had good corrosion resistance,but also could preferentially promote the ECs growth,indicating that it could be used for the endothelialization of the magnesium alloy stent materials.Furthermore,with the aim of further enhancing the biocompatibility,the sulfonated hyaluronic acid(HA) was grafted onto the PDA-modifie surface.The results indicated that HA can significantl improve the anticoagulant and promote the ECs growth,indicating that the coating can be used as a potential candidate to promote the endothelial regeneration after magnesium alloy stent implantation [129].Furthermore,in order to endow the magnesium alloy surface with multifunctional biological characteristics,Wang et al.[130]prepared a corrosion-resistant chemical conversion layer on the magnesium alloy surface by alkali heat treatment and hydrofluori acid treatment,and then the PDA coating was constructed on the surface.Finally,the mixture of silk fibroi (SF),heparin and GREDVY was one-step grafted on the PDA-modifie magnesium alloy surface by the microdrop deposition method (Fig.7).The as-prepared magnesium alloy can significantl prevent the platelet adhesion and aggregation,reduce the hemolysis rate and prolong the coagulation time,showing excellent blood compatibility.In addition,thanks to the ability of heparin to selectively promote the ECs growth and the specifi action of REDV on promoting the ECs growth,this multifunctional bioactive surface can promote the reendothelialization of the magnesium alloy vascular stent materials.
Due to the rule that dopamine can self-polymerize on almost all solid surfaces to form a reactive coating,the PDA coating is also used as an intermediate layer for immobilizing the bioactive molecules on the polymer coating or inorganic coating prepared on the magnesium alloy to simultaneously improve the corrosion resistance and biocompatibility.For example,after the polyelectrolyte oxidation (PEO) treatment,Wei et al.[131] applied solvent evaporation method to fabricate poly (L-lactic acid) (PLLA) coating on the magnesium alloy surface,then the PDA coating as the intermediate layer was prepared on PLLA surface to further immobilize heparin,which not only endowed the magnesium alloy with excellent blood compatibility and corrosion resistance,but also could promote ECs growth while inhibit the SMCs proliferation,therefore,the authors believed that this method could be used for the rapid endothelialization of the magnesiumbased stents.Similarly,after preparing the inorganic coating on the magnesium alloy surface,heparin can also be immobilized by the chemical reaction of the PDA layer.For example,the Mg-Al layered double hydroxide (LDH) coating was constructed by Li et al.[132] on the magnesium alloy surface through the hydrothermal method,and then PDA coating was prepared on the LDH coating followed by covalently immobilizing heparin.The results showed that the as-prepared composite coating was more conducive to improve the corrosion resistance and promote the ECs adhesion and migration,moreover,it also had obvious inhibitory effect on hemolysis rate and platelet adhesion,suggesting that the composite coating can be used to enhance the corrosion and biocompatibility of the magnesium alloy stent materials to promote the surface endothelialization.
At the same time,dopamine can also form coordination bonds (such as metal ions) or chemical bonds with other substances containing amine groups in the process of the PDA coating formation,so it can also co-polymerize with other substances to form the PDA coating containing bioactive factors on the surface,and the coating still has the ability to react with the biomolecules to further enhance biocompatibility [133,134].Consequently,the multifunctional bioactive surface can be constructed to regulate the physiological microenvironment responses around the implant and promote the repair and regeneration of vascular endothelium.Bai et al.[135] co-polymerized dopamine and hexanediamine on the magnesium alloy after HF treatment to obtain the modifie surface with rich amine groups,and then PEG was immobilized followed by grafting REDV and CGG-ACH11 (a kind of anticoagulant polypeptide) (Fig.8).The results showed that this strategy not only significantl improved the corrosion resistance of the magnesium alloy,but also constructed a hydrophilic bioactive surface and enhanced the blood compatibility.Moreover,the modifie surface can inhibit the SMCs proliferation and promote the ECs growth.In vivoanimal experiments further proved that this functional coating can promote rapid endothelialization and inhibit intimal proliferation,and had great potential in developing the biodegradable metal stents.Tu et al.[136] prepared a PDA coating chelating Cu2+on the surface of stainless-steel stent by one-step method,and further VEGF was grafted on the surface.The Cu2+on the surface can catalyze the NO release from endogenous NO donors,while the introduction of VEGF further promoted the ECs growth.Thein vivoresults suggested that this coating can reduce the occurrence of in-stent restenosis and promote the regeneration and repair of the endothelium.Although the coating in this study was prepared on the surface of the non-biodegradable stainless-steel stents,due to the self-polymerization property of dopamine,it can be easily used for the surface modificatio of magnesium alloy stent materials to promote the endothelium regeneration and repair.
Although polydopamine coating has attracted tremendous attentions and widely applied in the fiel of surface modifi cation of biomaterials due to its self-polymerization characteristics and chemical reaction activity,the main concern is that,for the surface modificatio of the magnesium alloys,dopamine is usually dissolved in Tris.HCl solution containing more Cl−,which has strong corrosiveness to the magnesium alloy and the chemical conversion coating (such as Mg(OH)2layer) on the surface.Therefore,how to ensure that the magnesium alloy has good corrosion resistance,so as to facilitate the subsequent self-polymerization modifica tion and the immobilization of the biomolecules is the key to the surface modificatio of the magnesium alloy.In addition,the PDA coating is sensitive to substances containing amine groups,and it is easy to react with substances containing amine groups.Therefore,the PDA layer can also promote the non-specifi plasma protein adsorption,which may adversely affect the blood compatibility.It is considered that it may be reduced or eliminated through the further surface biofunctionalization,such as the introduction of PEG,zwitterionic molecules or heparin.
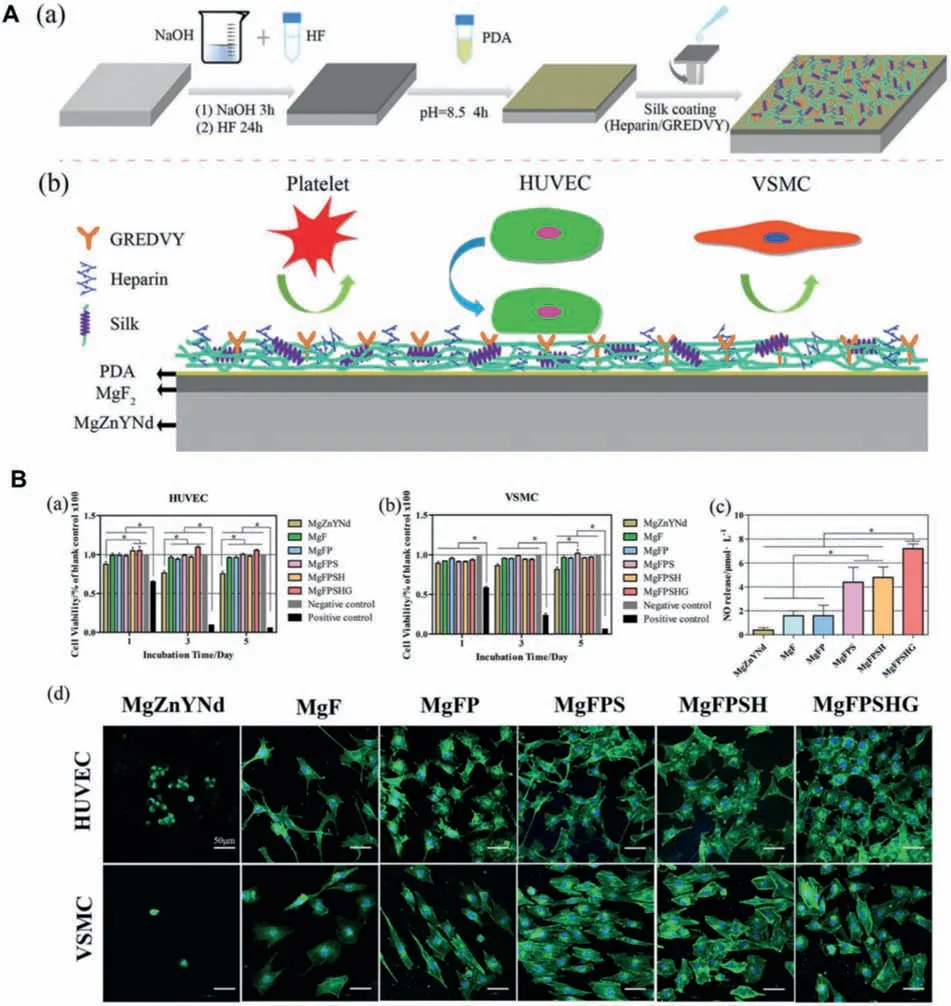
Fig.7.A.The schematic diagram of fabricating the multi-functional coating on Mg-Zn-Y-Nd surface (a) and the mechanism of the influence of the coating on cell behaviors and blood coagulation and platelet adhesion (b).B.Cell viabilities cultured on bare Mg-Zn-Y-Nd,HF-treated (MgF),dopamine coated(MgFP),silk-coated MgFP (MgFPS),silk/heparin-coated MgFP (MgFPSH) and silk/heparin/GREDVY-coated MgFP (MgFPSHG) samples over 5 d incubation:(a) HUVECs and (b) VSMCs;DMEM with serum worked as negative control,DMEM with 10% dimethyl sulfoxide as positive control.(c) NO release of HUVECs in the culture media for different samples;(d) The fluorescen images of HUVECs and VSMCs on different samples surfaces after 24 h culture.[130].
5.2.3.Introduction of bioactive substances by layer-by-layer
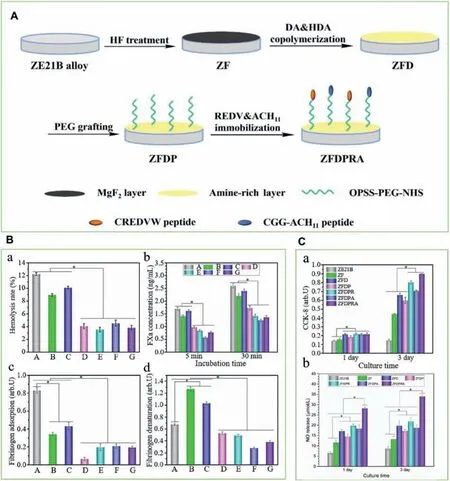
Fig.8.A.Preparation process of the functional coating on ZE21B alloy surface;B.The blood compatibility of the different samples.(a) Hemolysis rate,(b)activated FXa concentration,(c) fibrinoge adsorption,and (d) fibrinoge denaturation;C.Proliferation (a) and NO release levels (b) of HUVECs cultured on the different sample surfaces for 1 and 3 days [135].(A) ZE21B,(B) HF-treated Mg-Zn-Y-Nd (ZF),(C) DA/HAD-treated ZF (ZFD),(D) PEG-grafted ZFD(ZFDP),(E) REDV-coated ZFDP (ZFDPR),(F) ACH11-coated ZFDP (ZFDPA),and (G) REDV/ACH11-coated ZFDP (ZFDPRA).
For the surface modificatio based on the self-assembly or the PDA coating,the subsequent biomolecule immobilization is mostly through covalent grafting.Both of these two strategies not only have a limited amount of surface biomolecules,but also easily lead to the loss of bioactivities during the grafting reaction.Moreover,with the gradual degradation of the magnesium alloy,the bioactive molecules are rapidly lost,so they are greatly restricted in practical applications.Layer by layer (LBL) self-assembly is a surface modificatio technology that relies on the interactions between the different functional groups to form ordered monolayers on the substrate [137].The LBL technology is easy-operation and can form a multilayer structure loaded with the bioactive factors[138].At the same time,the as-prepared multilayer film have special functions,good stability,high structural integrity and complete surface coverage,thereby it has attracted more and more attention for the surface modificatio of the magnesium alloy to promote endothelialization.Kou et al.[139] prepared citric acid/RGD (Arg-Gly-Asp) (CA/RGD) multilayer coating on the polydopamine-modifie magnesium alloy surface by LBL.The results showed that the corrosion current density of PDA/(CA/RGD)2decreased by 95.65%,displaying the good corrosion resistance.Moreover,the coating can signifi cantly inhibit the platelet adhesion,reduce the hemolysis rate and fibrinoge adsorption,exhibiting better blood compatibility.In addition,it can inhibit the SMCs growth while promote the ECs adhesion and proliferation,showing good endothelialization.Similarly,the study of Chen et al.[140] also showed that the citric acid/polydopamine (CA/PDA) multilayer coating on the Mg-Zn-Y-Nd surface can significantl not only inhibit the platelet aggregation/activation,the SMCs growth and the macrophages adhesion/activation,but also obviously promote the ECs growth and migration,indicating the better endothelialization ability.Our group also used LBL technology (LBL) to construct chitosan functionalized graphene oxide (GOCS)/heparin (GOCS/Hep) multilayer coating on the magnesium alloy surface which was treated by alkali heating treatment and the following self-assembly modification The as-prepared coating can not only give the magnesium alloy good corrosion resistance and blood compatibility,but also significantl promote the growth and functional expression of endothelial cells [141],obtaining the multifunctional coating with the better endothelialization.At the same time,biomolecules can also be loaded in the process of LBL.For example,Zhang et al.[142] prepared poly (dimethyldiallyl ammonium chloride)/epigallocatechin gallate/Heparin(PDDA/EGCG/Hep) multilayer coating on AZ31magnesium alloy by LBL (Fig.9).EGCG can chelate magnesium ions to enhance the stability of the coating,PDDA can effectively prevent the corrosion of chloride ions and improve the corrosion resistance,while heparin can improve the blood compatibility and the ability to selectively promote endothelial cells growth.The animal experimental results showed that the as-prepared coating not only exhibited the excellent corrosion resistance,but also could enhance endothelialization and prevent intimal hyperplasia (Fig.8).Therefore,this coating is expected to be applied in the surface coating of the biodegradable magnesium alloy vascular stents.
LBL mainly relies on the weak interaction force,such as electrostatic force,hydrogen bonding and hydrophobic force,to form a multi-layer fil on the surface,which can maintain the activities of the bioactive molecules to the maximum extent during the self-assembly process.Moreover,a variety of bioactive molecules can be loaded into the multilayer to significantl enhance the bioactivities,which represents a promising surface modificatio technology for the magnesium alloys.However,most of the multilayer film constructed by LBL rely on the non-covalent interaction,and the binding force between the layers is weak in the physiological environment.It is easy to fall off in the complicated physiological environment,and the bioactive molecules may be rapidly lost.Therefore,how to ensure the stability of the multilayer fil formed by LBL and the controllable release of the loaded bioactive molecules is an urgent problem to be solved.
5.3.Preparation of bioactive factors-loaded polymer coating to enhance endothelialization
Generally speaking,the polymer coating on the magnesium alloy surface does not change the properties of the substrates,and the coating has good biodegradation,biocompatibility,drug loading and cell regulation ability.It is one of the most effective methods to improve the corrosion resistance and biocompatibility of the magnesium alloy [143,144].Polymer coatings on the magnesium alloy surface mainly include natural polymers (such as chitosan [145],silk fibroi [146])and synthetic polymers (such as PLA [147],PLGA [148],polycaprolactone (PCL) [149],polyurethane (PU) [150],poly(trimethylene carbon) (PTMC) [151],etc.).Generally speaking,natural polymers have better biocompatibility,less toxicity and side effects,but their mechanical properties and corrosion resistance are limited.When they are used to load the bioactive molecules,the drug release behaviors are not easy to control.In contrast,synthetic polymers can achieve better mechanical properties and drug release behaviors,and the degradation rate is also easier to control.However,the biocompatibility of the synthetic polymers needs to be improved,and the residues of small molecule additives in the synthesis process will bring potential toxicity.Although the application of polymer coatings has significantl improved the corrosion resistance of the magnesium alloys,the coatings often need to be further biofunctionalized or loaded bioactive substances(or drugs) to regulate the physiological reactions after the implantation,so as to improve the biocompatibility and realize thein-siturapid repair and regeneration of the vascular endothelial.
By blending bioactive factors with polymer to prepare the bioactive composite coatings on the magnesium alloy surface is one of the important and effective methods to improve the biocompatibility of polymer coatings.Silk fibroi is an important natural polymer with good biodegradability and biocompatibility.Niidome et al.[152] prepared a silk fibroi (SF)coating containing sirolimus (SRL,a drug for preventing intimal hyperplasia) on the HF treated magnesium alloy surface to improve the corrosion resistance and biocompatibility.The results indicated that the sirolimus-loaded SF coating can prevent local and deep corrosion of the magnesium alloy,thus extending the radial support strength of the stent.Moreover,the composite coating also had excellent ECs adhesion and the abilities to inhibit platelet adhesion,so it can be used for the surface modificatio of magnesium alloy vascular stent for the endothelial repair and regeneration.In our previous work,the natural polymer chitosan was used to functionalize graphene oxide (GO),and then the chitosan-functionalized GO (GOCS) was loaded zinc ions and propranol to obtain GOCS@Zn/Pro-complex,which was finall covalently immobilized on the self-assembled modifie magnesium alloy surface to enhance the corrosion resistance and biocompatibility.Thein vitroexperimental results indicated that the modifie magnesium alloy not only had excellent blood compatibility and antibacterial properties,but also had the excellent ability to promote ECs adhesion and proliferation as well as VEGF and NO expression of endothelial cells (Fig.10),suggesting that this coating can be applied to the magnesium alloy vascular stent materials to promote the surface endothelialization and reduce the inflammator reactions [153].
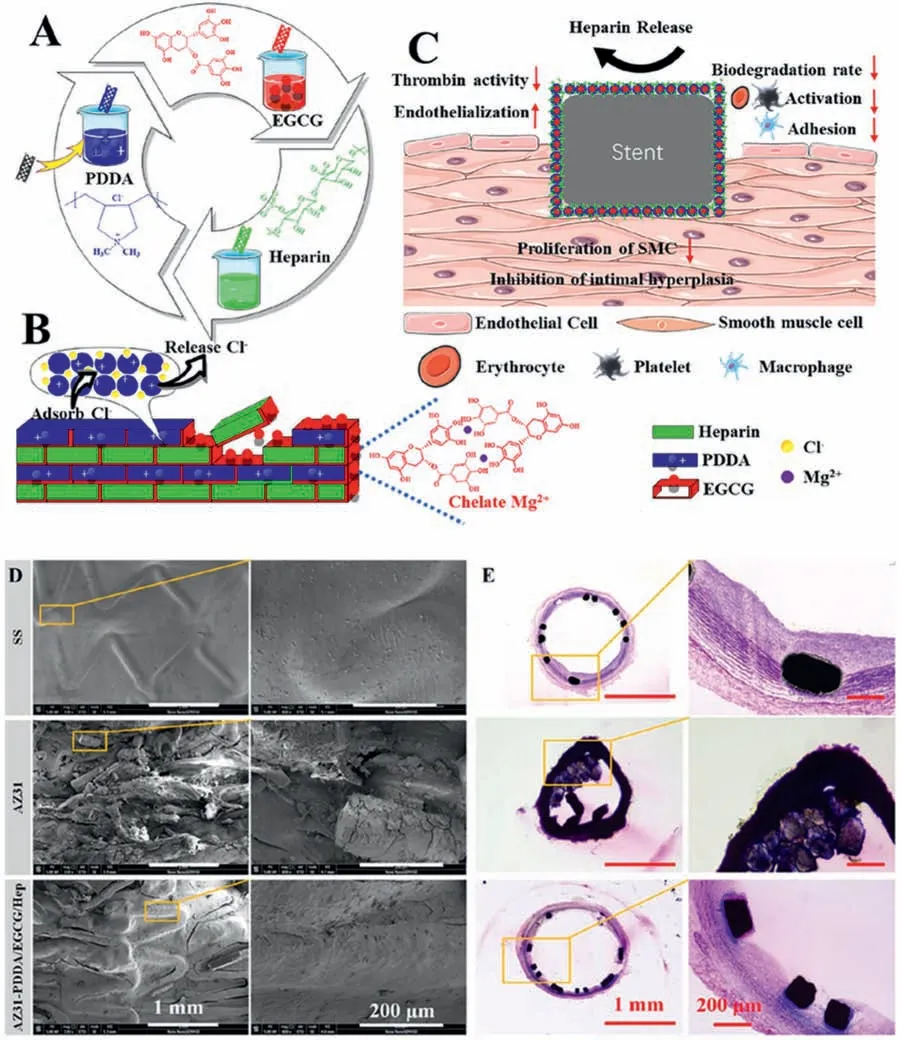
Fig.9.(A) Schematic diagram of the AZ31 plates coated with PDDA/EGCG/Hep using the layer-by-layer (LBL) method for corrosion control and vascular repair.(B) Cross-sectional morphology of the conversion coating.(C) The sandwich-like coating releases heparin,promotes endothelialization,suppresses the proliferation of SMCs,decreases the coagulation activity,and inhibits the adhesion and activation of platelets,erythrocytes and macrophages.(D) SEM images of vessel wall after implantation,scale bar,1 mm and 200 μm.(E) H&E staining result of stents,scale bar,1 mm and 200 μm [142].
As a synthetic biodegradable polymer,PLGA is widely used for drug carrier and is very suitable for the surface coating on the magnesium alloy surface to enhance the corrosion resistance,but its biocompatibility needs to be improved.To this end,Lin et al.[154] constructed a sandwich structure coating of PLGA/gallic acid/PLGA on the ZK60 magnesium alloy surface.The gallic acid slowly released from the coating can capture free radicals to prevent the oxidation reaction,and selectively promote the ECs proliferation while inhibit the SMCs growth.The results indicated that this structure can not only improve the corrosion resistance,but also promote the endothelialization,therefore,it has great potential application in developing magnesium alloy stents to prevent late stent restenosis.Poly (trimethylene carbon) (PTMC) is another kind of synthetic biomedical polymer with the good biocompatibility and biodegradability.Its degradation products are neutral,which can reduce the occurrence of inflammator reaction.Meanwhile,the degradation of PTMC is characterized by surface erosion,which can reduce the blood penetration and thus facilitate the binding between polymer coating and magnesium matrix during the coating degradation.After fluorinatio treatment and self-assembled 3-aminopropyl triethoxysilane (APTES) on the magnesium alloy surface,Tang et al.[155] prepared the paclitaxel loaded PTMC coatings with two molecular weights(50,000 g/mol and 350,000 g/mol,PTMC5 and PTMC35) on the modifie surface.As compared with PLGA (molecular weight 100,000) coating,PTMC coating was uniform and degraded gradually from the surface to the inside.The corrosion current density of PTMC35 coating was only 0.05 μA/cm2,which was significantl less than PLGA (0.11 μA/cm2) and PTMC5 coating (0.13 μA/cm2).In addition,PTMC35 coating had a more stable and sustained drug release ability and can effectively inhibit the SMCs proliferation.Therefore,PTMC35 coating can be used as the drug-eluting coating for the magnesium-based stents to improve the corrosion resistance and inhibit the proliferation of vascular intima,and thus effectively promote the vascular endothelial regeneration.Similarly,in order to simulate theinvivoservice state of the stent,Ye et al.[156] prepared the PTMC coating loaded with atorvastatin calcium (ATVC) on the magnesium alloy wire,and the degradation behavior and the ECs growth behavior were studied using the microfluidi model.As compared to the uncoated AZ31,the PTMC-ATVC coated AZ31 showed good corrosion resistance and endothelial cell migration and proliferation in the microfluidi model with a fl w rate of 10 μL/min.Thein vivoresults of rats also proved that PTMC-ATVC coating can actively regulate the ECs and SMCs growth behaviors,reduce intimal proliferation and inflammator reaction.Consequently,the coating can be used for the magnesium alloy vascular stent materials to inhibit the excessive intima hyperplasia and promote the rapid repair and regeneration of the damaged endothelium.

Fig.10.The SEM images (A) and quantities (B) of the platelets attached on blank Mg,alkali heating treated Mg (Mg-OH),16-phosphoryl hexadecanoic acid modifie Mg (Mg-PHA) and the Zn/Pro-loaded GOCS-modifie Mg (Mg-GOCS@Zn/Pro) surfaces.(C) The cGMP expression of platelets on the different samples.(D) and (E) show the hemolysis rate and APTT of the different samples.(F) Fluorescence images of endothelial cells for 1 d and 3 d (green:cytoplasm;blue: nucleus).CCK-8 values (G),VEGF (H) and NO (I) concentrations of endothelial cells cultured on the different samples for 1 d and 3 d[153].
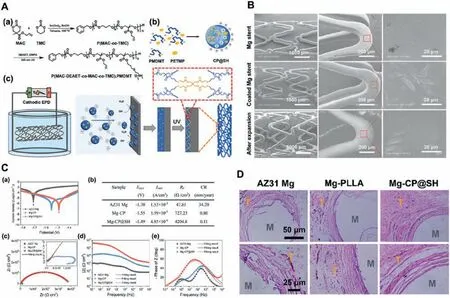
Fig.11.(A) Schematic illustration of stepwise fabrication of thiol-ene photo-crosslinked aliphatic polycarbonate (APC) coating on AZ31 Mg stent by the EPD method: (a) synthetic route of the cationic polycarbonate P(MAC-DEAET-co-MAC-co-TMC) (PMDMT),(b) the preparation of CP@SH colloidal particles,and(c) electrodeposition of CP@SH colloidal particles on a Mg-alloy stent and photo-crosslinking via UV irradiation;(B) SEM images of bare AZ31 Mg stent(top) and CP@SH-coated Mg stents before (middle) and after (bottom) expansion.(C) Electrochemical corrosion test of AZ31 Mg,Mg-CP,and Mg-CP@SH samples in SBF (37±0.5 °C) for 4 h (a) Potentiodynamic polarization,(b) corrosion data analyzed from (a);(c) EIS Nyquist plots,(d) EIS Bode plots of|Z| vs.frequency,(e) Bode plots of phase angle vs.frequency.(D) Representative micrographs of histological H&E-stained sections of the implants: AZ31 Mg,Mg-CP@SH,and Mg-PLLA;M area represents Mg rod;T area represents the tissue [158].
Due to the complicated mesh structure of stent,it is still a great challenge to prepare high-performance polymer coatings without defects on the magnesium alloy stent surface to control the corrosion rate using traditional methods,such as dipping coating and spinning coating.Electrophoretic deposition (EPD) is an effective method to prepare uniform polymer coatings on the surface with the complicated structure(such as stent) [157].In a recent report,Pan et al.[158] reported the method of preparing aliphatic polycarbonate(APC)coating on the magnesium alloy surface by combining electrophoretic deposition(EPD)and photo crosslinking technologies (Fig.11A).The results showed that the APC coating prepared by EDP had the uniform and controllable thickness and could enhance binding force with the magnesium alloy stent surface (Fig.11B),and the subsequent photo crosslinking further improved the mechanical properties and durability of the coating.The magnesium alloy has uniform and slow corrosion behaviorin vitroandin vivo(Fig.11C).At the same time,APC coating can not only effectively promote the ECs adhesion and proliferation,but also improve the blood compatibility and tissue compatibility (Fig.10D).Therefore,this study provided a promising strategy for maintaining the mechanical integrity of magnesium alloy stents and enhancing their surface biocompatibility and endothelialization.
Although polymer coatings have been widely applied in controlling the degradation behavior and improving the biocompatibility of the magnesium alloys,and a large number ofin vitroresults andin vivoanimal experiments have also confirme the excellent performances and application prospects of the polymer coatings,the biocompatibility of both natural and synthetic polymers needs to be improved and their biodegradation behaviors should also be controlled precisely.On the other hand,how to prepare polymer coatings with excellent biodegradability,drug release behaviors and biocompatibility,so that the corrosion rate of magnesium alloy stent materials can be controlled during thein vivolong-term service,is still facing great challenges.In addition,some key factors about polymer coating,such as the binding force with the substrates,the permeability and degradation,also need to be considered,especially when it is applied to the vascular stents.
5.4.Synthesis of novel multifunctional polymer to construct the bioactive coating
Owing to the limited biocompatibility of the abovementioned natural and synthetic polymers,incorporating the bioactive factors(or bioactive chemical groups)into natural or synthetic polymer chains to synthesize novel multifunctional biodegradable polymers to construct the bioactive coatings on the magnesium alloy surfaces represents one of the important ways to improve the biocompatibility and promote the endothelial regeneration and repair.Gu et al.[159] synthesized two biodegradable elastic polyurethanes,poly (carbonate polyurethane) urea (PCUU) and poly (ester polyurethane)urea (PEUU),to construct the drug-eluting polymer coating on the magnesium alloy stent.In the acute blood-contacting tests,PCUU coating on the surface can significantl reduce the platelet adhesion,and the paclitaxel release from the coating successfully inhibited the rat SMCs proliferation.Due to the excellent elasticity,anticoagulant and good drug release behaviors,it has become an attractive polymer for preparing the drug elution coating on the magnesium alloy biodegradable stent.Furthermore,in order to endow the polyurethane with more biological activities,Liu et al.[160] introduced arginine (Arg) and leucine (Leu) into the polyurethane chain to synthesize a two-block polymer for the surface modifica tion of the magnesium alloy vascular stents.As compared with PLGA coating,this polymer had both hydrophilic and hydrophobic chain segments,and the prepared coating had stronger binding force with the substrate.The corrosion resistance,blood and cell compatibility of the polymer-coated magnesium alloy were also significantl improved (Fig.12).Meanwhile,Arg-Leu-PEUU coating can also promote the nitric oxide (NO) release of the human umbilical vein endothelial cells (HUVECs),indicating its better ability to promote endothelial regeneration and repair.Therefore,this polymer can be used to fabricate the polymer coating on magnesium alloy stent to provide better protection and rapid endothelialization.
Plasma polymerization is a relatively simple technology that uses plasma discharge to ionize and dissociate monomers to produce various kinds of active species,followed by forming the polymer film on the surface through addition reaction between these active species or between active species and monomers [161].Polymer coatings with different chemical compositions and biological characteristics can be formed on the magnesium alloy surface by plasma polymerization,and the surface biological function can be further constructed by other methods,such asin-situgrafting [162].Qi et al.[163] prepared polypropylene amine (PAAam) coating with a thickness of about 250 nm on the magnesium alloy vascular stent surface through plasma polymerization.The coating was dense,non-porous and rich in amine groups,and it can significantl enhance the corrosion resistance of the magnesium alloy.Moreover,the coating can obviously promote the adhesion,spreading and proliferation of endothelial cells,indicating its ability to promote endothelialization.Because the surface amine groups can be further functionalized,this method can provide a good strategy to promote the surface endothelialization of magnesium alloy vascular stents.For example,in order to improve the properties of this coating,Huang et al.[164] used acetylene (C2H2) as a cross-linking agent to fabricate a thin,uniform,pinhole-free and hydrophobic C-F coating on the magnesium alloy surface,followed by depositing the hydrophilic PAAam layer on the C-F coating through plasma polymerization.The results showed that the corrosion resistance of the modifie magnesium alloy was significantl improved because of existence of the C-F coating,and it can also effectively promote the adhesion and proliferation of HUVEC,indicating its good ability to promote endothelialization.
5.5.Fabrication of ECM micropattern to enhance endothelialization
It is well known that the vascular endothelial is mainly composed of the endothelial cell arranged along the blood fl w and the glycocalyx hydration layer.This special endothelial cell arrangement plays an important role in maintaining the hemostasis-thrombus balance,preventing thrombosis in the early stage and intimal hyperplasia in the chronic period after the stent implantation.Therefore,the preparation of regular bioactive micropatterns on the magnesium alloy surface can regulate the growth direction and even functional expression of the endothelial cells,thus promoting the endothelialization.
Zhou et al.[165] fabricated the gradient surfaces from nanometer to micrometer on the biodegradable magnesium by chemical etching,and then carried out hydrofluori acid treatment and polydopamine deposition to obtain gradient surfaces with the different roughness,the good corrosion resistance and biocompatibility.Through the high-throughput imaging,the effects of gradient surfaces on the adhesion and proliferation of ECs and SMCs were studied.On the surfaces with the different roughness,endothelial cells and SMCs have different adhesion,spreading and growth behaviors.From nanometer to submicron roughness,the surface can significantl promote the ECs proliferation and functional expression without affecting the corrosion behavior of the magnesium alloys,while the SMCs growth and proliferation on the surface were greatly inhibited.In particular,when the microroughness was between 1.0 and 2.0 μm,the magnesium alloy with the ridge/valley network texture structure can significantl improve the selectivity of ECs to SMC (Fig.13),therefore,this method can provide a potential strategy for realizing the rapid endothelialization of the magnesium alloy stent materials.Because hyaluronic acid (HA) can regulate the growth behavior of SMCs and ECs and promote the surface endothelialization,Liu et al.[166] constructed a hyaluronic acid (HA)bioactive micropattern coating on the magnesium alloy surface (Fig.14).The results showed that the micropatterned ECM coating not only improved the surface endothelialization of magnesium alloy,but also had better blood compatibility,anti-proliferation and anti-inflammator .
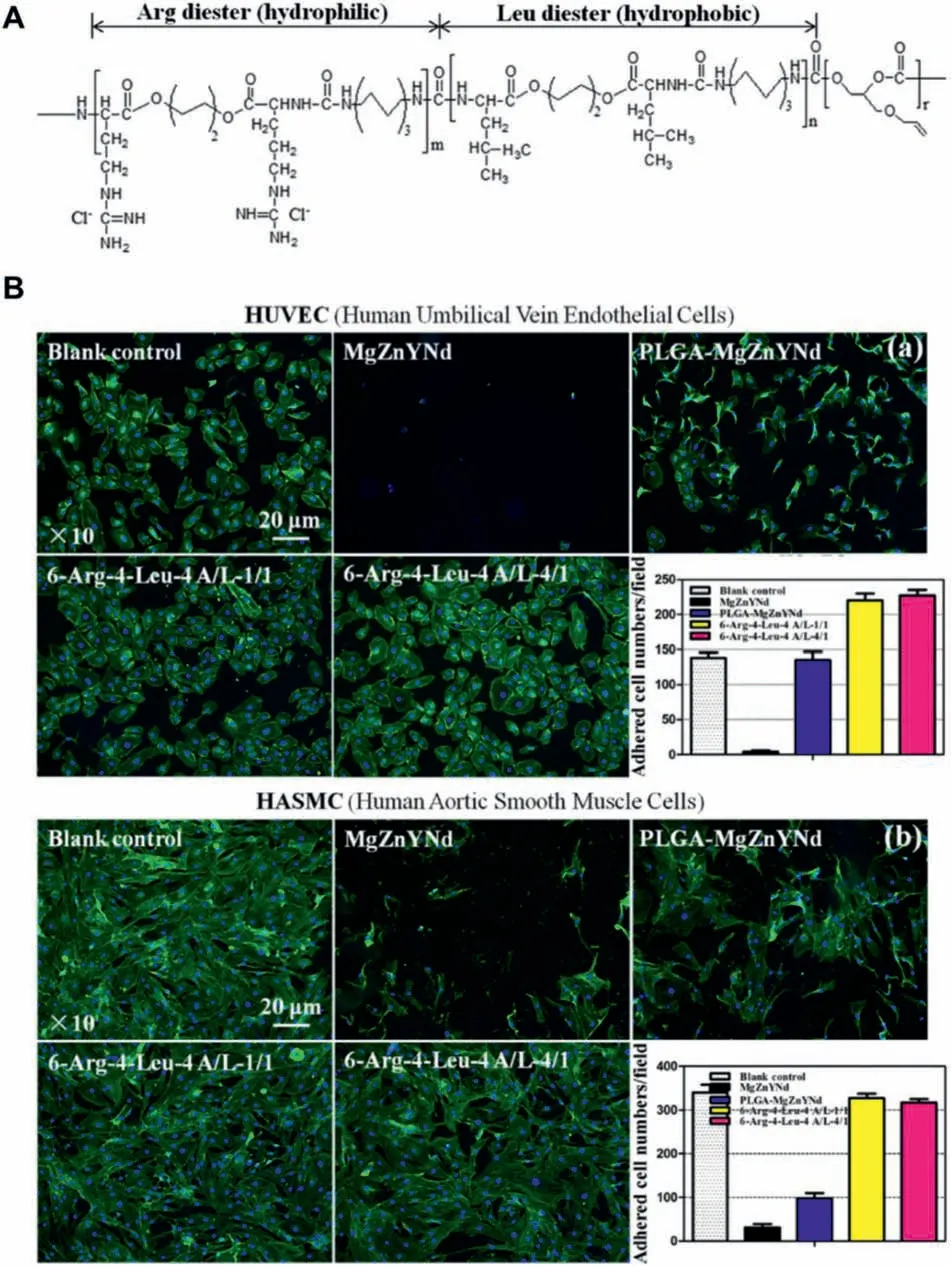
Fig.12.(A) Chemical structure of Arg-Leu-PEUU;(B) Actin (green,dyed with phalloidin-FITC) and nuclei staining (blue,dyed with DAPI) of both (a)human umbilical vein endothelial (HUVEC) and (b) human aortic smooth muscle cell (HASMC) after seeding onto Arg-Leu-PEUU coated MgZnYNd for 24 h (n=5).Bare MgZnYNd and PLGA-coated MgZnYNd were used as the biomaterial controls along with a culture plate as blank control.Inserts at lower right corners are calculated adhered cell numbers [160].
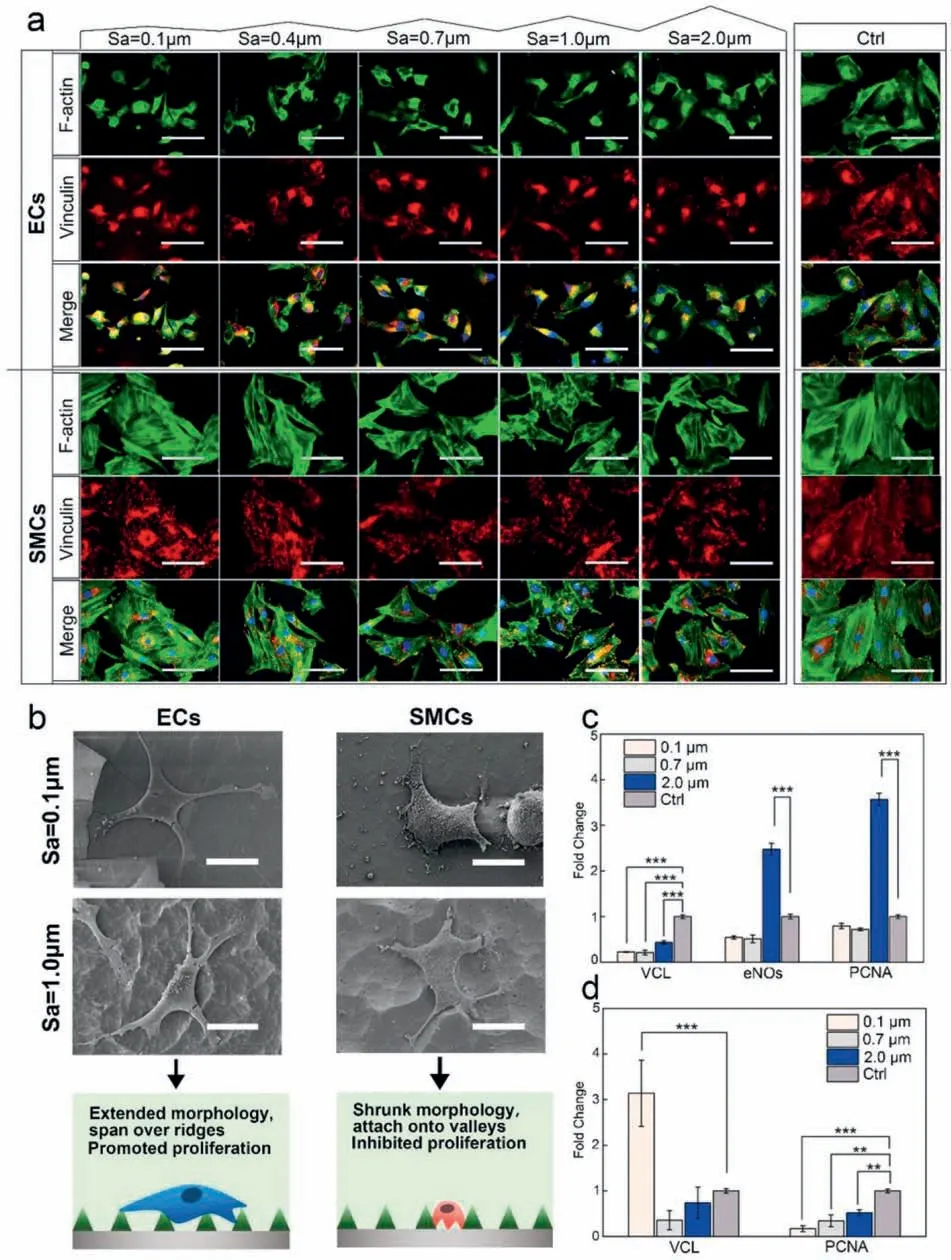
Fig.13.Immunofluorescenc staining (a) and SEM imaging (b) of ECs and SMCs after 1 day culture on the gradients,including F-actin (green),Vinculin(red),and DAPI (blue) staining (scale bar of fluorescenc images represented 50 μm,and scale bar of SEM images represented 20 μm).The gene expressions of vinculin (VCL),eNOs and PCNA in ECs (c) and SMCs (d) measured with RT-qPCR (∗indicates P < 0.05,∗∗P < 0.01,and ∗∗∗P < 0.001) [165].
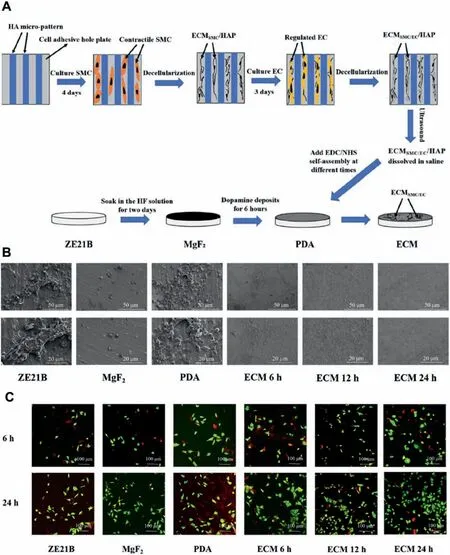
Fig.14.(A) Preparation processes of HA micropattern coating onto ZE21B.(B) SEM images of components of blood adherent on ZE21B,MgF2,PDA,and micropatterned (ECM 6 h,ECM 12 h,and ECM 24 h) samples.(C) The fluorescen images of HUVECs (green) and SMC (red) on the different surfaces[166].
The preparation of the bioactive micropatterns on the magnesium alloy surface can effectively regulate the growth behavior and functional expression of vascular ECs and SMCs.Therefore,it plays an important role in studying the cell behaviors and endothelialization process of the magnesium alloy vascular stent materials.However,the cell growth behavior is not only related to the dimensions of the micropatterns on the surface,but also related to the bioactive substances on the surface.Although the bioactive micropatterns with different dimensions and bioactivities can be constructed by combining advanced surface functionalization technology and surface processing technology,the degradation of magnesium alloy under the physiological conditions may reduce its regulatory ability during its service(for example,the micropatterns gradually disappear.).In addition,due to the complicated threedimensional mesh structure of vascular stents,it is not easy to prepare the regular bioactive micropatterns on the stent surface,therefore,its application in clinical practice is greatly limited.
5.6.Multifunctional bioactive coating with glycocalyx structure
Studies have shown that the vascular endothelial surface of the healthy human is covered by a negatively charged gel-like glycocalyx hydration layer mainly composed of glycosaminoglycans (such as heparan sulfate,hyaluronic acid,etc.) [167].The glycocalyx layer has excellent hydrophilicity and is a natural barrier between the vascular endothelium and blood plasma.It can not only prevent the adhesion of plasma proteins and blood cells on the endothelial surface,maintain the normal structure and function of vascular ECs,but also increase the endothelial NO synthase (eNOS) activity and exhibit the excellent hemocompatibility [168].It follows that the biocompatibility can be significantl improved by constructing the biomimetic layer with the glycocalyx structure on the stent surface.In order to construct a biomimetic coating with the endothelial glycocalyx on the stent surface,Lyu et al.[169]prepared 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-chelated Cu2+complex (DOTACu2+),followed by sequentially immobilizing DOTA-Cu2+and hyaluronic acid (HA) on the surface of plasma polymerized polypropylene amine modifie vascular stents.The introduced Cu2+can catalyze the NO release from NO donorsin vivo,so as to improve the anticoagulant and endothelial repair of the stent.Qiu et al.[170] sequentially grafted heparin and selenocystamine on the stent surface to achieve the similar effects,showing that this kind of the biomimetic coating with the endothelial glycocalyx structure has the great potential in the surface modificatio of magnesium alloy stents.
Hyaluronic acid (HA) is a very important glycosaminoglycan molecule that constitutes the endothelial glycocalyx layer.It has good anticoagulant and anti-inflammator properties [171],and can prevent the bacteria adhesion [172].HA is negatively charged and has good hydrophilicity,which can provide a good growth environment for endothelial cells[173].Meanwhile,some studies have shown that HA can promote the transformation of SMC from the synthetic phenotype to the contractile phenotype [174],and thus prevent in-stent restenosis by inhibiting the SMCs proliferation.Therefore,HA has been widely studied and applied on the surface modificatio of the cardiovascular implants.For example,HA derivative was immobilized on the magnesium alloy surface by Lee et al.to construct the glycocalyxlike layer [175].The electrochemical experimental results showed that the coating had a significan effect on inhibiting the early corrosion of the magnesium alloy,but the coating was peeled off during implantation in rats,resulting in the local and continuous corrosion,indicating that the binding force of this coating was weak.In order to overcome this disadvantage,Li et al.[176] grafted the different molecular weights (MWS: 4×103,1×105,5×105and 1×106) on the polydopamine (PDA) coated magnesium surface,and the results showed that the coating with the molecular weight of 1×105Da had better corrosion resistance and endothelialization (Fig.15).In addition,PDA/HA coating enhanced the blood compatibility,anti-proliferation and anti-inflammator of the magnesium alloy (Mg-Zn-Y-Nd,ZE21).
Although the vascular glycocalyx layer plays a very important role in maintaining the blood dynamic balance and vascular endothelial function,there are few studies about the construction of the hydrated layer with the endothelial glycocalyx structure on the magnesium alloy surface.Obviously,the structure of the endothelial glycocalyx hydration layer cannot be fully simulated by immobilizing glycosaminoglycans (such as heparan sulfate,hyaluronic acid,heparin,etc.)on the magnesium alloy surface,although they are the main components of the endothelial glycocalyx layers.It is considered that the hydrophilicity of these glycosaminoglycans is not enough,and moreover it is difficul to form the gellike villous structure of the endothelial glycocalyx layer.It is expected to achieve this effect in combination with other polymers with good hydrophilicity (such as PEG,chitosan,etc.).
5.7.NO releasing coating
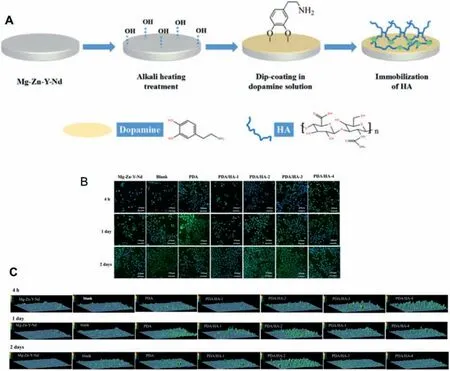
Fig.15.(A) Scheme of preparing PDA/HA coating on Mg-Zn-Y-Nd substrate;(B) Immunofluorescenc staining images of CD31 antibody (green color,endothelial cells marker) and DAPI (blue color,nucleus marker) in the Mg-Zn-Y-Nd,PDA,PDA/HA-1 (4×103 Da),PDA/HA-2 (1×105 Da),PDA/HA-3(5×105 Da),PDA/HA-4 (1×106 Da) and the blank groups after being cultured for 4 h,1 day and 2 days,respectively;(C) The expression quantity of CD31 of HUVEC in different samples by calculating fluorescenc intensity using Ipwin32 [176].
It has been demonstrated that the healthy vascular endothelium can continuously release NO gas signal molecules with the help of eNOS to maintain blood homeostasis[177]and inhibit thrombosis and intimal hyperplasia.However,the eNOS activities of the pathological or damaged endothelial cells is reduced,and the NO release also significantl decreases.Therefore,the sustained releasing NO at the injury site can promote thein-siturepair and functional reconstruction of the injured vascular intima [178].At present,there are two ways to endow the cardiovascular materials with NO release ability.One is to introduce chemical molecules that can release NO (such as S-nitroso-N-acetyl-DL-penicillamine,nitrosothiol,etc.) on the surface to construct a NO-releasing coating[179],but the NO release dose of this coating is difficul to accurately control,and the coating also does not have longterm sustained release ability.Moreover,the released NO is easy to combine with the active oxygen in the body and lose its activity [180].On the other hand,there are a large number of endogenous NO donors in human blood (such as nitrosoglutathione,S-nitrosocysteine,etc.).In thein vivoenvironment,glutathione peroxidase can catalyze the decomposition of endogenous NO donors to release NO with the help of thiol molecules [181].Therefore,another strategy to fabricate the NO releasing coating is to build the NO catalytic release coating,which can catalyze the NO release from thein vivodonors by introducing a catalyst on the surface,so as to achieve the use ofin vivophysiological environment to induce endothelial repair and regeneration.It has been shown that immobilizing organic molecules containing selenium on the surface can catalyze the NO release,leading to promoting the rapid repair of endothelium[182].In addition,a variety of transition metal ions also have the ability to catalyze the NO release [183],of which Cu2+is a representative.The preparation of the bioactive coating containing Cu2+on the surface of cardiovascular materials can catalyze the stable NO release from the donors for a long timein vivo,and thus improve the blood compatibility and promote endothelial regeneration[134].In order to further improve the biocompatibility,on the basis of introducing the compounds containing selenium or metal ions (such as Cu2+),the biocompatibility can be further improved by introducing the bioactive molecules such as heparin,hyaluronic acid,extracellular matrix protein and cell growth factors [184,185].
Although a great progress has been achieved in NO releasing and catalytic coatings,the current reports mainly focus on the non-degradable vascular stents,such as 316SS and Co-Cr.Theoretically,the current strategies for NO releasing or catalytic coatings can be applied to the magnesium alloy stent surface to endow with good endothelialization.However,the corrosion resistance of the magnesium alloy is limited,which may lead to corrosion degradation during the construction of NO releasing coating,resulting in the poor long-term effect of the coating.At the same time,the loss of surface catalyst caused by corrosion degradation may also reduce the effect of catalytic NO release,therefore,how to ensure the long-term catalytic release function of the NO release coatingin vivois still an important issue for the magnesium alloy vascular stents.
5.8.Bioactive sol-gel coatings
Sol-gel method is a chemical synthesis method for preparing the networks through continuous hydrolysis and condensation [186],which is generally divided into inorganic and organic methods.Compared with the traditional methods,solgel method has many unique advantages.One of its most significan advantages is that it can form covalent bonds with the metal surface,which has the good adhesion strength with the substrate.In addition,low cost,simple preparation,low processing temperature,easy control of molecular structure and coating properties are also the advantages of sol-gel coating.López et al.[187] applied organic sol-gel technology and dip coating method to prepare silica coatings on the ZE41 magnesium alloy surface.Three different concentrations of tetraethoxysilane (TEOS) sol solutions were used to prepare sol-gel coatings,and the coatings were then treated at 400 °C and 500 °C,respectively,to enhance the binding force of the coatings with the substrate.The corrosion experimental results in 3.5wt.% NaCl solution showed that,as compared with the pure magnesium,the polarization resistance of the coating treated at 500 °C was three times of the pristine magnesium alloy,and the corrosion current decreased by about 70%.Unfortunately,during the heat treatment,the residual water and solvent in the coating could evaporate rapidly,which can lead to the occurrence of the porous structure and cracks in the solgel coating.As sol-gel technology is wet chemical technology,biomolecules or bioactive corrosion inhibitors can also be loaded in the process of preparing the sol-gel coating,so as to improve the corrosion resistance and biocompatibility of the magnesium alloys.For example,Li et al.[188] constructed a variety of sol-gel coatings loaded with the corrosion inhibitors on the magnesium alloy surface by dip coating (Fig.16).The results indicated that paeonol condensation tyrosine (PCTyr) Schiff base was the most effective inhibitor among the corrosion inhibitors.The coating can improve the corrosion resistance and biocompatibility of the magnesium alloy and promote the ECs adhesion and growth.Therefore,Schiff base inhibitors are expected to replace drugs in drug-eluting stents,so as to achieve controlled degradation,improve blood compatibility and rapid endothelialization,and provide reference for subsequent research and lay a foundation for the clinical application of magnesium alloy biodegradable vascular stents.
6.The application prospects of the coatings for magnesium alloy stent
Medical magnesium alloy is considered as the firs candidate for the manufacture of the next generation biodegradable cardiovascular stents because of its excellent mechanical properties and biodegradability,and it has attracted more and more attention.However,the corrosion resistance and biocompatibility are limited in thein vivophysiological environment,therefore,the in-stent restenosis after the stent implantation remains high.The main reason is that the vascular endothelial layer is inevitably injured by the stent implantation,leading to the SMCs over-proliferation,which further leads to the occurrence of complications such as ISR and thrombosis.It has been generally believed that the rapid reconstruction of the vascular endothelial layer with normal physiological function on the stent surface after the implantation is the fundamental way to solve the clinical complications.In order to promote the surface endothelialization of magnesium alloy vascular stent materials,a large number of surface modificatio technologies have been used to simultaneously improve the corrosion resistance and biocompatibility,thereby promoting the endothelial repair and regeneration on the stent surface.Here we mainly focus on reviewing the latest advances in constructing the bioactive coatings on the magnesium alloy stent materials to promote surface endothelialization and analyzing the advantages and disadvantages of various surface modificatio technologies,as shown in Table 1.

Table 1 The advantages and disadvantages of the different coatings on the magnesim alloy materials for cardiovascular stent.
Generally,all surface modificatio methods can simultaneously improve the corrosion resistance and biocompatibility of the magnesium alloys,and promote the regeneration and repair of vascular endothelium to a certain extent,however,with the degradation of magnesium alloy,the surface biological activities of the coatings will inevitably gradually lose before the complete endothelial layer is formed on the stent surface,ultimately leading to the incomplete regeneration of vascular endothelium or the formed endothelial layer without normal physiological function.Therefore,although the great research advances of the surface modificatio in promoting the endothelialization of magnesium alloy vascular stent materials have achieved,how to effectively design and regulate the corrosion resistance and biocompatibility of the stents to adapt to the physiological changesin vivostill needs a lot of investigation.
At the same time,although the surface modificatio technologies discussed in this review have shown good application prospects in promoting the surface endothelialization of magnesium alloy stent materials,not all surface modifi cation technologies are suitable for the vascular stent with the three-dimensional mesh structure.The stent usually has a tubular three-dimensional network structure,and its strut is usually very thin.Due to the poor corrosion resistance of the magnesium alloy,many surface modificatio technologies and pretreatment methods cannot be effectively applied to the magnesium alloy stents.For example,surface chemical treatment (including alkali heat treatment,hydrofluori acid treatment,phosphating treatment,etc.) and surface electrochemical treatment (such as micro arc oxidation,anodic oxidation,etc.) often produce a large corrosion on the surface before the surface corrosion-resistant passivation layer is generated.Consequently,when these methods are directly applied to the magnesium alloy stent,the structural integrity and mechanical properties of the stent itself may be damaged seriously,making the mechanical design and size design of the stent more difficult For another example,although the surface bioactive micropattern can effectively guide endothelial cells to grow along the blood fl w direction,it is not easy to construct micropatterns with precise dimensions on the stent surface,which greatly limits its application.

Fig.16.The schematic diagram of preparation of PCTyr Schiff base loaded sol-gel coating on magnesium alloy and its mechanism of promoting corrosion resistance and endothelialization [188].
In general,almost all metals will form a surface oxide layer in the natural environment,which is conducive to the surface modificatio reaction based on selfassembly.The self-assembly is usually carried out in the organic solvents containing the organic molecules (such as 3-aminopropyltrimethoxysilane and 3-phosphonopropionic acid.),which has less corrosion on the magnesium alloy.Therefore,the introduction of the bioactive molecules on the stent surface after self-assembly to construct multifunctional bioactive surface to promote endothelialization has a good application prospect for the cardiovascular stent.Moreover,the modifie layer has excellent binding force with stent due to the fact that all molecules are introduced on the surface by covalent bonding.However,although this strategy has achieved good experimental resultsin vitroandin vivo,the number of bioactive molecules on the stent surface is usually inadequate,so its long-termin vivoservice performance needs to be improved,and it has not been applied in the clinic(including nondegradable metal stents) so far.Dopamine can self-polymerize to form a reactive intermediate layer on the stent surface without any pretreatment,and the polydopamine layer has a strong binding force with substrate and can also be used to further graft a variety of bioactive molecules to promote endothelialization,obtaining effects similar to those of based on self-assembled surface modification Although the LBL technology can greatly increase the loading amount of the bioactive molecules,the binding force between the bioactive multilayers is limited,and the bioactive substances are easy to lose during the process of the implantation and service.Since LBL does not require special equipment and is carried out in solution in most cases,a uniform coating can be formed on the stent surface.If the adhesion between the multilayer can be further improved (e.g.,via chemical crosslinking),it is expected to be used for the surface modificatio of magnesium alloy vascular stent to promote endothelialization.
Among all the surface coating technologies,polymer coating has the most promising application for the cardiovascular stent due to its biodegradability,drug loading and good biocompatibility.However,due to the fact that the stent must withstand the large compression and expansion force during the implantation,it is still a challenge to prepare a polymer coating with excellent mechanical properties and good binding force with the surface of the tubular magnesium alloy stent.At present,the preparation methods of polymer coating on the magnesium alloy surface,such as dip coating and spinning coating,are difficul to obtain the uniform coating with excellent mechanical properties and binding force on the stent surface.Ultrasonic atomization spraying [189],electrospinning [190],electrophoretic deposition (EPD) mentioned above and plasma polymerization should be feasible methods.
In addition,both glycocalyx-like bioactive coating and NOreleasing coating exhibit great potential in promoting endothelialization due to their excellent hemocompatibility,the former usually has excellent hydrophilicity and can obviouslyprevent non-specifi protein adsorption and therefore cannot promote endothelialization well,the latter can catalyze endogenous donor to release NO gas signal molecule to signifi cantly enhance the blood compatibility and endothelialization.As a consequence,the combination of these two strategies can construct the endothelial biomimetic surface and provide a great potential strategy to achieve endothelialization of magnesium alloy stents.
7.Concluding remarks and future perspective
Magnesium alloys have great potentials in the biodegradable vascular stents due to their excellent biodegradability and the mechanical properties.Extensive in-depth studies have been carried out in the world,but the limited corrosion resistance andin vivoendothelialization of the magnesium alloys are still the bottlenecks of the clinical applications.Introducing a variety of bioactive components on the magnesium alloy surface to construct the multi-functional bioactive corrosionresistant coating that can inhibit coagulation and accelerate thein vivorepair of the damaged vascular intima is the main research direction of the surface modificatio of the magnesium alloy vascular stent materials,and represents one of the effective ways to improve thein vivoservice performances of the magnesium alloy for the cardiovascular stents.
Although the great progresses have been obtained in the improvement of the corrosion resistance and endothelialization of the magnesium alloys by using various technologies such as preparation of bio-inert coating,in-situimmobilization of bioactive molecules on the surface,polymer coating loaded with bioactive factors,new multifunctional polymer coating,surface bioactive micropattern,multifunctional bioactive layer with glycocalyx structure,NO gas signal molecule release coating and bioactive sol-gel coating,the clinical side effects after the stent implantation are a very complicated sequential cascade reaction,which is regulated and controlled by various factors and multiple signal pathways [191].In combination of the complexity ofin vivocorrosion behaviors of the magnesium alloy,the endothelialization of the magnesium alloy stent materials is far from reaching the clinical requirements.It is worthy that the corrosion resistance should be firstl considered because all the current methods could reduce the corrosion resistance to some degree.Therefore,the surface endothelialization of the magnesium alloy vascular stent materials needs to combine with various surface modificatio methods to regulate the physiological microenvironment response from multiple signal pathways to inhibit thrombosis and promote thein-siturepair and regeneration of the injured intima.In addition,it is worth noting that,although most of the current surface modificatio methods have achieved good resultsin vitroandin vivo,only the magnesium alloy drug-eluting stent of Biotronik Company has been clinically applied.Therefore,how to apply the current surface coating technologies to the magnesium alloy stent to improve its biocompatibility and promote endothelialization is an important subject worthy of further study.
In summary,for magnesium alloy vascular stents,the main direction at present should be how to reduce or avoid the occurrence of restenosis,thrombosis and other complications in the process of stent service,so that its degradation is conducive to the interaction with vascular tissue and the realization of endothelial regeneration and rehabilitation at the site where the stent is placed.It is a key scientifi problem for researchers to deeply understand the interaction rules between the magnesium alloy biodegradable vascular stents and host,and then to study the regulation strategies,such as the rapid endothelialization,inhibition of the proliferative reaction,ensuring the continuous compatibility between the stents and vascular tissues after being covered by intima,and effectively realizing the complete endothelial recovery of the lesion site.At the same time,although the current surface modificatio technologies have achieved a lot of excitingin vitroresults,more extensive and detailedin vivoresearch is also necessary for the clinical application of the magnesium alloy stent materials.
Declaration of Competing InterestThe authors declare that they have no known competing financia interests or personal relationships that could have appeared to influenc the work reported in this paper
Acknowledgments
This work was financiall supported by the National Natural Science Foundation of China (31870952) and Natural Science Foundation of Jiangsu Province of China(BK20181480).
 Journal of Magnesium and Alloys2023年1期
Journal of Magnesium and Alloys2023年1期
- Journal of Magnesium and Alloys的其它文章
- Development of high-strength magnesium alloys with excellent ignition-proof performance based on the oxidation and ignition mechanisms: A review
- Development and application of magnesium alloy parts for automotive OEMs: A review
- Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications
- Exploring the contribution of oxygen reduction reaction to Mg corrosion by modeling assisted local analysis
- Microstructures,mechanical properties,corrosion,and biocompatibility of extruded Mg-Zr-Sr-Ho alloys for biodegradable implant applications
- Fabrication of a model specimen for understanding micro-galvanic corrosion at the boundary of α-Mg and β-Mg17Al12
