Conservation aquaculture of Ompok bimaculatus (Butter catfish), a near threatened catfish in India
Pradyut Biswas, Alok Kumar Jena, Soibam Khogen Singh
aDepartment of Aquaculture, College of Fisheries, Central Agricultural University (Imphal), Lembucherra, Tripura (W), 792210, India
bAquaculture Division, ICAR-Central Institute of Fisheries Education, Mumbai, 400061, India
Keywords:
Ompok bimaculatus
Ontogeny
Polyculture
Reproductive biology
Threatened
A B S T R A C T
The butter catfish, Ompok bimaculatus, belonging to the silurid family, is widely regarded as an important food fish for aquaculture diversification in India.Furthermore, the species poses a threat due to habitat degradation and other anthropogenic factors, and has been categorised as “near threatened”, therefore, its culture promotion is warranted.To establish a successful breeding programme, a precise understanding of the life history, and biology (reproductive/feeding) of the fish will assist hatchery managers and researchers.Studies in these areas have been undertaken by several groups over the years, but without much coherence, the data are still fragmented.Few attempts on the culture attributes of this catfish have been performed under both mono- and polyculture systems to bring about parallel promotion through aquaculture in a larger part of the subcontinent.Highlighting this, we have tried to analyse and present a consolidated account of the morphological characteristics, feeding habits, reproductive biology, early developmental ontogeny, and culture potentials of O.bimaculatus in India.This review was also prompted by the paucity of information on the cultural aspects of the species.Additionally, based on the reported studies, future directions and perspectives of the culture promotion of the species are proffered to ensure future research initiatives.
1.Introduction
The Indian butter catfish,Ompok bimaculatus(Bloch), commonly known as “pabda”, is a credible species with a wide distribution in some Asian countries, including Pakistan, India, Bangladesh and Myanmar(Talwar & Jhingran, 1991).Currently, the species is described under the“near threatened” category (Ng et al., 2010), and is facing a risk of extinction in the wild due to reasons such as habitat deterioration and population overexploitation.Subsequently, the Conservation Assessment and Management Plan (Molur & Walker, 1998) recommended the initiation of a captive breeding program forO.bimaculatusto restore its population.The unavailability of the catch data ofO.bimaculatusfrom the wild over the two decades is mostly due to a lack of studies and therefore, needs attention.In the past, the species has not received much attention in aquaculture, mainly due to the unavailability of information regarding its breeding and culture techniques.However, success has been achieved quite recently, wherein breeding and seed rearing in India is achieved by the Regional Aquaculture Research Centre of the Central Institute of Freshwater Aquaculture, Kalyani, West Bengal, India(Chakrabarti et al., 2009).Notwithstanding the fact that standardised seed production techniques are already in place, there are only a few Indian states practicing commercial-scale seed production.Under aquaculture practice, the species has a localised pattern, wherein,popularization of culture has been reported only from states such as West Bengal and Tripura.High market value (US$ 8–12 per kg) (Pradhan et al., 2014), good flesh quality, soft bony structure, rich lipoprotein content (Arambam et al., 2020; Biswas et al., 2018; Dhar et al., 2019;Rawat et al., 2018) and fast adaptability to culture conditions are few positive credentials for its prospects as a candidate species.Additionally,it has a good growth rate and, under optimal stocking densities, can reach an average body weight of 80.30 g in eight months (Debnath et al.,2016).Successful expansion of the existing culture practices through diversification of the species demands a set of scientific studies on different biological attributes, such as early developmental ontogeny,food and feeding habits, reproductive biology, and culture requirements.Furthermore, these attributes must be understood to explain the variations in the level of populations as well as to make efforts to enhance stock in the wild.Additionally, information about the reproductive season can be used to plan seasonal closures during the reproductive season.In the context of the need for conservation ofO.bimaculatus, aquaculture can act as a strong tool.In addition, the promotion of aquaculture can bring about a long-term conservation strategy.A very good example of such a strategy relates to the recovery ofOsteobrama belangeri(pengba) in Manipur (India) through a ranching programme assisted by captive breeding in hatcheries and culture production (Das et al., 2016).
In line with the species restoration programme, the inclusion of prioritised endemic fishes specific to the region of abundance can be a wise means for diversification programmes.In line with this, Indian aquaculture over the years has made a paradigm shift from backyard small-scale farming activity to a broader commercial system through intensification of the system and diversification of species of interest.Additionally, to tackle climate change adaptation, such endemic species are being introduced in current practices (Biswas et al., 2018).At present, polyculture of the three/six species combinations using Indian Major Carps (IMC), Catla (Labeo catla), Rohu (Labeo rohita) and Mrigal(Cirrhinus mrigala) and Chinese carps remains a major and focal activity,contributing to more than 87% of the country’s production (Singh et al.,2019), whereas many endemic species preferred in larger pockets of the country are often neglected or stagnant (NFDB, 2016).Meanwhile, from a farmer’s income perspective, carp farming generates less farm cash outflow, and therefore, often acts as a deterrent for its exclusive culture in ponds.Thus, species with high market value can benefit farmers when some portion of the stocked biomass is replaced by high value species.
In India, next to carps, the preference for catfishes such asClarias magur(Magur),Heteropneustes fossilis(Singhi) andO.bimaculatus(Butter catfish) is high.The present level of production is very marginal and practiced in selected regions of the country.The production crisis,coupled with the positive flesh nutritional profile, accounted for a rapid rise in its market value, which is positive from a farming perspective.Acting upon the need for production enhancement, recent research has focused on regional candidate species, especially endemic species, with an overall target to improve fish yield.In line with this, scientists across the Indian subcontinent have made serious efforts to examine the above aspects.However, research efforts are fragmented, and outcomes are not collaborative.Thus, a well-classified document that can link the earlier findings and connect to the present needs is essential.Considering this,an effort to review the different aspects of the research findings and field trials carried out exclusively onO.bimaculatusis attempting to close the existing knowledge gap.
In this review, we synthesized the major aspects of morphological characteristics, feeding habits, reproductive biology, early developmental ontogeny, breeding programs and existing culture practices of the species.This review will prove to be a useful tool for future researchers and aqua-culturists to improve seed production and culture practices, and implement adequate guidelines for sustainable fishery management and conservation of this endangered species.More importantly, based on the available literature and scope for expansion,the task/recommendations on the promotion of this valued fish are mentioned for future research actions.
1.1.General morphology
O.bimaculatus(Bloch, 1794) is a non air breathing freshwater catfish belonging to the family Siluridae of the order Siluriformes.This species has the highest growth among the other three species under the genus,namely,O.pabo,O.pabdaandO.bimaculatus(Pradhan & Barman,2014), making it best suited for aquaculture promotion.Taxonomically,O.bimaculatushas a round black blotch above and behind the base of the pectoral fin, which differentiates it from its counterparts.The species is also reported to have a special electrogenerated system, but of lower magnitude (Morshnev & Ol’Shanskii, 1997).The morphological characteristics ofO.bimaculatushave been documented by various researchers (Acharya & Iftekhar, 2000; Bhuiyan, 1964; Darshan et al.,2019; Day, 1878; Jayaram, 1981; Rahman 1989, 2005; Talwar &Jhingran, 1991).
Diagnosis: The body is elongated, strongly compressed with a depressed head and a rounded snout.It attains a maximum length of 45 cm SL (standard length).Mouth is superior, with a lower jaw longer than the upper jaw.Two pairs of barbels are present, where maxillary barbels extend posterior to (or slightly beyond) the anal fin base.Nostrils widely separated from each other.Teeth found on the jaws and vomer.Pelvic fin does not reach anal-fin origin and bears 7–8 rays.The anal fin has 57 to 75 branched rays, and the caudal fin has 17–18 rays.The Anal fin is with 2–3 spines whereas pelvic fin is with one spine.The caudal fin is deeply forked and the lateral line is complete.Fin formula: D (Dorsal fin).4, P1(Pectoral fin).12–15 (1/11–14), P2(Pelvic fin).7–8, A (Anal fin).66–73.
Coloration: The fish has a silvery body with purple dorsally faintly greenish dark with a tinged of golden yellow and whitish on the abdomen, dorsal side of the body with a transverse dark blotch, behind the operculum on the lateral line.Fins are pale golden; occasionally, the caudal fin is edged gray, and its tips are black.
1.2.Distributions and habits
The fish has an extensive geographical distribution covering several Southeast Asian countries, including Bangladesh, Indonesia (Java,Sumatra), Malaysia, Myanmar, Pakistan, Sri Lanka, Thailand, Vietnam and India, especially northeastern states such as Odisha, Bihar and West Bengal (Jayaram 1999, 2009; Day, 1981; Chakrabarty et al., 2007; Banik& Malla, 2011; Biswas et al., 2018; Dhar et al., 2019).O.bimaculatusis predominantly found as a resident species in Southeast Asian countries,mainly India, Pakistan, Bangladesh, Sri Lanka, Nepal and Myanmar(Fig.1) (IUCN, 2010; Ng et al., 2010).It inhabits lotic freshwater habitats at elevations of 100–2500 m, especially in streams/rivers, lakes,ponds, canals and inundated fields with sluggish to moderate water flow(Banik et al., 2011; Sridhar et al., 1998).Shallow rivers (0.5–1.5 m),often muddy and musky, are the preferred natural habitats of butter catfish (Debnath et al., 2013).It normally breeds in streams, rivers and floodplains during monsoon months.Likewise, Chakrabarti et al.(2012)reported that open Beels and floodplain wetlands such as Mauns with plenty of submerged and floating aquatic weeds are the most suitable habitats, as they provide shelter and hideouts.To some extent,O.bimaculatushas also been recorded in brackish-water environments of South Indian states (Debnath et al., 2013).

Fig.1.Global distribution of butter catfish (O.bimaculatus).The distribution was adapted from the International Union for Conservation of Nature (IUCN, 2010).
1.3.Growth pattern
Studies on the length-weight relationship in wild populations have been reported by several researchers and reveal an isometric or positive allometric growth pattern (Malakar et al., 2013; Malla & Banik, 2015;Mishra et al., 2013; Muhammad et al., 2017; Sivakami, 1987).The general growth pattern in wild populations shows seasonal variation, i)isometric growth in the premonsoon period, and ii) allometric growth in the monsoon and postmonsoon periods (Malla & Banik, 2015; Mishra et al., 2013).Overall, the condition factor (K) in both wild populations ranges between 0.279 and 0.673, and this variation is largely related to the spawning or maturation cycle, rather than feeding activity (Malla &Banik, 2015; Mishra et al., 2013; Muhammad et al., 2017; Parameswaran et al., 1970; Qayyum & Qasim, 1964).
1.4.Sex ratio in wild

Fig.2.A haul of O.bimaculatus from the College of Fisheries, CAU, Tripura, India.
In the wild, equal proportions of both male and female populations(1:1.03) have been reported (Mishra et al., 2013; Rao & Karamchandani,1986).However, in other studies, the dominance of females over males has been observed in wild populations, with a sex ratio of 1:1.5, 1:1.65 or as high as 1:2.93 (Arthi et al., 2013; Malla & Banik, 2015; Qayyum &Qasim, 1964; Sarkar et al., 2017).This indicates the variation in sexO.bimaculatusfeed on crustaceans, fish and molluscs.Preference for insect feed during the early stage of the fish is possibly the reason why cannibalism is more frequent inO.bimaculatus(2nd to 5th day after hatching) in the absence of a sufficient amount of food.This significantly affects larval survival during the early stage, which remains the most critical period during the seed rearing process (Chakrabarti et al., 2012).Furthermore, Debnath et al.(2013) reported the cannibalistic and predatory nature ofO.bimaculatusduring adult stages.They reported better growth when fed live mola carplet, flying barbs, crustaceans and molluscs.ratio/dominance in the wild population and between geographical boundaries (Table 1).
1.5.Food and feeding habits
To establish a successful rearing and culture ofO.bimaculatus,adequate knowledge of their preferred food and feeding habits is essential.Such studies have reported a wide variation in their food and feeding habits (Table 1).Arthi et al.(2011) have reported omnivorous feeding behaviours ofO.bimaculatusduring juvenile and adult stages,mostly feeding on vegetable matter, larvae and adult crustaceans,crustacean nymphs, insects, molluscs and smaller portions of miscellaneous organisms in their study.In contrast, few studies have reported an omnivorous feeding nature ofO.bimaculatus(Arthi et al., 2013; Malla &Banik, 2015; Mishra et al., 2013; Sivakami, 1982).Additionally,Qayyum and Qasim (1964) reported a piscivorous feeding habit, with small fish comprising 60% of the diet, with a smaller proportion of insects, and crustaceans (prawn).Fishes comprisingPethia ticto,Pethia conchonius,Pethia stigma,Esomus danricus,Trichogastersp.,Mystussp.,Osteobrama cotio,Amblypharyngodonsp.and insects (Orthoptera, Hymenoptera, Coleoptera, Odonata, Hemiptera, Ephemeroptera, Plecoptera, etc.) have been observed in the gut ofO.bimaculatus.Meanwhile, an insectivorous nature has also been reported (Hanjavanit& Sangpradub, 2009; Parameswaran et al., 1970; Sangpradub et al.,2014; Sivakami, 1982).Similarly, Rainboth (1996) reported that
1.6.Size and age at maturity
Precise knowledge of sexual maturity and appropriate size for breeding are required for successful captive breeding.Sexual maturity is defined as the minimum size at which mature testes and ovaries are found in the male and female, respectively.The average size of first sexual maturity in both sexes ofO.bimaculatusis considerably different.O.bimaculatusattains maturity at the end of the first year (Debnath et al., 2013; Biswas et al., 2018; Singh, 2019).Many studies have reported the minimum size at first sexual maturity in the wild population(Qayyum & Qasim, 1964; Mishra et al., 2013; Malla & Banik, 2015;Agnihotri et al., 2017; Sarkar et al., 2017), as presented in Table 1.However, the minimum size of 20–23 cm (weight 20–40 g) is documented as the length at first maturity of the fish in captivity (Biswas et al., 2018; Chakrabarti et al., 2012; Debnath et al., 2013).All the studies have documented that males mature earlier than females in both wild and captive stocks.
1.7.Spawning season
Sivakami (1982) and Arthi et al.(2013) reported thatO.bimaculatusbreeds throughout the year with peak spawning activity during the months of October and August and September at the Bhavani Sagar Reservoir and Amaravathy River, Tamil Nadu, respectively.Rao and Karamchandani (1986) and Qayyum and Qasim (1964) reported July to August as the spawning season ofO.bimaculatusat Kulgarhi Reservoir,Madhya Pradesh and Aligarh, Uttar Pradesh, respectively, during their study.Renunuan and Silapachai (2005) documented July to September as the spawning season forO.bimaculatusin their study at the Nong Koh Reservoir, Chonburi Province, Thailand.Mishra et al.(2013) reported June to July as the spawning season ofO.bimaculatusat the Ghaghara River, India.Malla and Banik (2015) documented that the fish breeds during the month of May–August during his study in different lotic water bodies of Tripura, India.Apart from the natural spawning seasons,several other researchers have reported June–August as the spawning season ofO.bimaculatusin captivity (Biswas et al., 2018; Chakrabarti et al., 2012; Debnath et al., 2013).Agnihotri et al.(2017) reported a prolonged spawning season ofO.bimaculatusfrom April to August with peak spawning activity in June in his study in six tropical rivers of the Ganga basin, India.In a recent study, Mishra et al.(2018) documented late June to August as the spawning season of butter catfish obtained from different wild populations of major rivers in India (Brahmaputra,Cauveri, Ganga, Godavari, Krishna, Mahanadi, Narmada, Subernrekha and Tapti) and their tributaries (Amravati, Betwa, Chambal, Ghagra,Gomati, Hooghly, Ramganga, Sharda and Sone).Apart from the natural spawning seasons, several other researchers have reported June to August as the peak spawning season of butter catfish in captivity (Biswas et al., 2018; Chakrabarti et al., 2012; Debnath et al., 2013), while Banik et al.(2011) and Singh (2019) documented June to late July and May to August as the breeding season of butter catfish under captivity in India,respectively.Qayyum and Qasim (1964) documented the single spawning nature of butter catfish in his study at Aligarh, Uttar Pradesh,India, while Rao and Karamchandani (1986) reported butter catfish as a multiple spawner at Kulgarhi Reservoir, Madhya Pradesh.Most researchers have reported the spawning season ofO.bimaculatus,which coincides with the monsoon season (Table 1).

Table 1Life history parameters of O.bimaculatus.
1.8.Gonado-somatic index (GSI) and fecundity
The GSI value gives a precise idea of the maturity stages, which tend to increase as the fish reach maturity and decline after spawning, with a minimum GSI value observed during the resting phase (Mishra et al.,2013, 2016; Malla & Banik, 2015).The maximum average GSI of fully matured males and females of the fish during the peak spawning period is reported to be 15.58 (during June) % and 2.19%, respectively (Malla& Banik, 2015).The lowest GSI is observed from September to April,indicating pre- and postspawning periods (Malla & Banik, 2015).According to Mishra et al.(2013), the GSI value varies from 0.4 to 9.1 for females and 0.1 to 2.6 for males.Similarly, Sarkar et al.(2017) documented the average GSI of fully matured fish in most of the rivers of India.He reported the highest GSI of males (10.20%) and females(13.20%) during June–August in a wild riverine population.Males possess lower GSI than the female population in the wild population(Table 1) (Agnihotri et al., 2017).
Fecundity is a quantitative score of gamete production expressed as the quantity of eggs or sperm produced by females and males, respectively.Therefore, fecundity relates to “reproductive potential”, which are more general and qualitative terms expressing an individual’s reproductive success and its capacity to provide gametes and viable embryos, respectively.Fecundity measurements are of particular importance in fish biology since they are used for assessing population reproductive dynamics and energy and for estimating their annual reproductive output and to understand breeding success (Stearns, 1992).Under captive induced spawning, variation in the fecundity of the fish has been reported by several research groups (Table 1).Chakrabarti et al.(2012) reported a value of 1200 per g of ovary weight of females(gonadal weight of 25 g), whereas a relative fecundity between 18,000–22,000 eggs per 100 g fish body was reported by Biswas et al.(2018).Fecundity was also examined from wild spawning grounds by several authors.Sarkar et al.(2017) documented fecundity varying between 4,163.68 and 23,549.90 from different rivers in India.The relative fecundity was found to be 221 numbers per g body weight of femaleO.bimaculatus(Parameswaran et al., 1970), whereas Arthi et al.(2013) reported a fecundity of approximately 1,831 to 19,446 in the size range of 202–376 mm in total length and 63.80–328.10 g in weight.Malla and Banik (2015) documented that the absolute fecundity ofO.bimaculatusvaries from 2190 to 41552 eggs per fish.Agnihotri et al.(2017) documented that the mean absolute fecundity ofO.bimaculatusvaries from 4260 to 18382 for individuals with a total length of 189.9–267.5 mm.This observed variation in fecundity reported by these authors may arise due to the size of the fish and prevailing environment conditions, which influence the well-being of the fish and preparedness for spawning.In this regard, the correlation between ovarian protein and fecundity was reported to be highly significant in populations from major rivers and tributaries, wherein ovarian protein increases with increasing fecundity and oocyte weight (Mishra et al., 2018).Additionally, the average increase in egg size and egg number might decrease as environmental quality declines and environmental variability increases (Einum & Fleming, 2004; Smith & Fretwell, 1974).Sarkar et al.(2017) reported three reproductive patterns, viz.(i) high absolute fecundity, (ii) moderate absolute fecundity, and (iii) low absolute fecundity from 13 river populations of India.Factors such as nutritional status (Sarkar et al.2009; Gupta et al., 2014), time of sampling and maturation stage are known to affect fecundity (both within the species and between fish populations).
1.9.Gonadal maturity stages
Fishes generally spawn during a specified time of the year, which coincides with environmental factors during the season.The period during which the gonads attain full maturity and spawning occurs in the population is called the breeding season of the species.Thus, identi fication of the maturity stages of the gonads is important for breeders.Several research groups have observed and documented the gonadal maturity stages ofO.bimaculatuson the basis of external, microscopic and histological observations.Qayyum and Qasim (1964) classified five maturity stages for both sexes of fish, viz., immature, maturing virgin/recovered spent, ripening, ripe and spent.In contrast, Rao and Karamchandani (1986) classified eight stages inO.bimaculatusbased on microscopic observations.Recently, Arthi et al.(2013) reported four gonadal maturity stages inO.bimaculatus(immature, maturing, mature and spent in males and immature, maturing, mature and ripe stages in females).In line with this, Malla and Banik (2015) reported four and five maturity stages of testes and ovaries ofO.bimaculatus, respectively.The gonadal (testis and ovary) maturity stages along with the time of occurrence and the characteristic features are documented after Malla and Banik (2015), Mishra et al.(2016), Sarkar et al.(2017) and presented in Table 2.

Table 2Gonadal maturity stages of O.bimaculatus in Indian sub-continent.
The gonadal structure, including the average size and weight ofO.bimaculatushas been reported by various researchers (Table 1).Chakrabarti et al.(2012) reported that the mature ovary contains various sizes of eggs, wherein fully ripe ova are dull and yellowish in fresh conditions, with sizes ranging from 0.760 to 0.875 mm in diameter.The average weight of the ovary per 100 g fish was observed to be 25 g, and the number of eggs per gram of ovary was 1200.In another study, Malla and Banik (2015) reported that the diameter of immature eggs ranged from 0.218 to 0.543 mm, while the diameter of mature eggs ranged from 0.856 to 1.358 mm.Similarly, Sarkar et al.(2017) reported that the average weight of oocytes ranged from 0.28 mg to 0.58 mg.Biswas et al.(2018) observed ova diameters of 0.76–0.87 mm.In another study by Mishra et al.(2018), the diameter of the oocyte ranged from 0.21 to 0.34 mm (0.28–0.58 mg) in fishes collected from different wild populations of major rivers in India (Brahmaputra, Cauveri, Ganga:UP and WB, Godavari, Krishna, Mahanadi, Narmada, Subernrekha,Tapti) and their tributaries (Amravati, Betwa, Chambal, Ghaghra,Gomati, Hooghly, Ramganga, Sharda, Sone).
1.10.Sexual dimorphism
O.bimaculatusexhibits sexual dimorphism or secondary sexual characteristics that are distinguishable only during the spawning season.Sarkar et al.(2017) documented some sexual dimorphic characters ofO.bimaculatus.They reported that in females, the pectoral fin was smooth, and genital papilla were swollen and reddish in colour.They also reported that the abdomen was soft and bulging in appearance.The size of the female is generally thicker, broader and bigger than its male counterpart of the same age group.However, in males, pectoral fins become rough and serrated edge, the genital papilla pointed and narrow with freely oozing milt, while applying slight pressure on the abdomen.The paired mature ovaries ofO.bimaculatusare unequal in size, and the length of the right ovary is greater than that of the left ovary in all populations (Sarkar et al., 2017).Kurian and Inasu (1997) reported that females ofO.bimaculatusare nearly two times longer and five times heavier than males of the same age group.The Dorsal profile of the head of the male has an apparent downwards slope, whereas the dorsal profile of the head is more or less straight in the female.The lateral line in the female has a downwards bend rather at the middle of the body, while the lateral line is straight in males in the middle portion and sloped downwards only near the operculum (Gupta, 2015).The key sexual dimorphic characteristics of males and females as described by various authors(Banik et al., 2011; Biswas et al., 2018; Kurian & Inasu, 1997; Parameswaran et al., 1970; Rao & Karamchandani, 1986; Sarkar et al., 2017)are summarised in Table 3.

Table 3Sexual dimorphic characters of Butter catfish, O.bimaculatus.
1.11.Nutritional value
A brief account of the nutritional profile of the fish is depicted in Table 4.O.bimaculatusis an excellent source of protein, omega-3/omega-6 fatty acids, vitamins and minerals and represents an indispensable nutritional source, especially for growing children, pregnant females and elders (Alam et al., 2016; Beveridge et al., 2013; Dhar et al.,2019).Additionally,fish play an important role in avoiding diseases related to protein-calorie malnutrition (Beveridge et al., 2013).Furthermore,O.bimaculatusis rich in polyunsaturated fatty acids(PUFAs), with 27.49–40.92% and 22.97–26.54% polyunsaturated fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs), respectively.It also contains essential amino acids such as leucine, lysine, threonine,phenylalanine, valine and isoleucine in significant quantities.The ratio of essential to nonessential amino acids was 0.89.The fish is also rich in vitamins and minerals.Furthermore, fat soluble vitamins such as vitamins A, D, E and K are present in considerable amounts (especially vitamins A and D) (Paul et al., 2018).In a recent study, Paul et al.(2020)documented the proximate and fatty acid composition ofO.bimaculatusbrood fish, eggs and larvae to understand nutrient transformation.Their study revealed that the crude protein, fat and ash contents of brood fish, egg and larvae were 14.40, 25.90, 12.23 (%); 1.06, 0.64, 0.42 (%) and 2.33, 1.61 and 1.79 (%), respectively.Furthermore, the fatty acid composition reported that the saturated fatty acid (SFA) and polyunsaturated fatty acid (PUFA) contents (% of total fatty acid) of the brood fish, eggs and larvae were 88.48, 76.04 and 48.60 (%) and 4.52,10.05 and 33.35 (%), respectively.In summary, the study revealed that crude protein content was higher in eggs; crude fat and ash content were more elevated in brood fish.However, PUFA, EPA and DHA contents were higher in butter catfish larvae.Hence, the study of the nutrient profile of butter catfish reflects the nutrient abundance of the species.In addition, a comparative nutrient profile of a few freshwater fishes of importance is highlighted in Table 5.

Table 4Nutritional profile of butter catfish, O.bimaculatus.
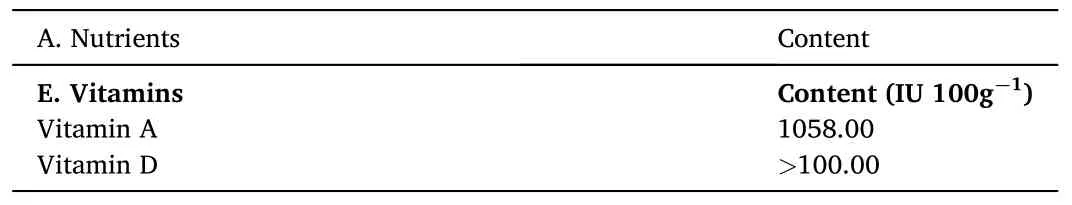
Table 4 (continued)
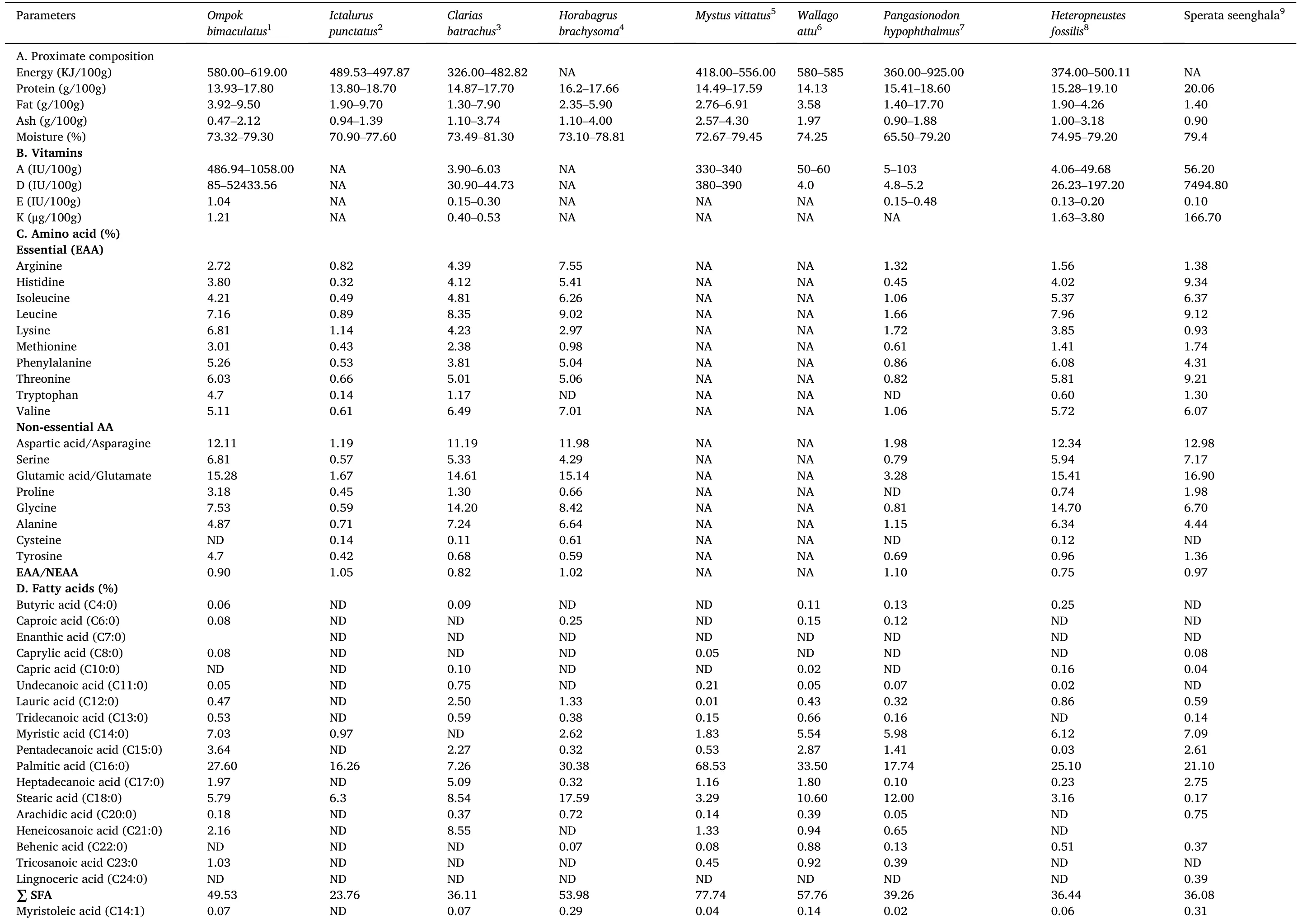
Table 5A comparative nutrient prof i les of few important commercial freshwater catf i shes.

Table 5(continued)
1.12.Present status of wild fishery
The majority ofO.bimaculatuscatches have been reported from the freshwater resources of Southeast Asian countries such as Bangladesh,Pakistan, Sri Lanka, Nepal and Myanmar, especially in India, where it forms as an extant species (Ng et al., 2010).Since the early 1970s,O.bimaculatus hassustained a minor fishery, especially in floodplain wetlands such as Beels, Jheels and flooded areas adjacent to rivers of the northeastern states, North Bihar and West Bengal, along with other species during monsoon and postmonsoon months.During the next decade, the population declined steadily (>50%), mostly due to alteration of habitat, indiscriminate use of pesticides and weedicides, loss of breeding habitats and over fishing of potential populations of mature fishes (Bhowmik et al., 2000; Lakra et al., 2010; Banik et al., 2012; Banik& Bhattacharya, 2012; Dhar et al., 2019).At present, the species is categorised as “Near threatened” by the International Union for Conservation of Nature (Ng et al., 2010), which calls for a need for sustainable management.Therefore, few efforts have been made to captive breed and include in the culture system to retrieve it from the edge of extinction.Consequently, the culture of this species has a double interest as a component for diversifying freshwater aquaculture in India and other neighbouring countries (NBFGR, 2011; Pradhan et al., 2014).
1.13.Genomic resources
The complete genome of a species will help better understand the genome organisation and develop strategies for conserving and managing endangered species (Dhar et al., 2019; Ryder, 2005).This also provides essential information to steepen up breeding processes and farming applications by identifying lineage-specific changes that are crucial for its adaptation in addition to knowledge about the risk factors and its immune system that helps in its survival in the wild or captivity(Ryder, 2005).Dhar et al.(2019) sequenced the genome of butter catfish from the Ganges River in India through a hybrid approach by Illumina short reads and PacBio long reads followed by structural annotations.They reported that the assembled genome size of butter catfish is 718 Mb and 21,371 genes.Moreover, the butter catfish genome was analogous toIctalurus punctatus(892 Mb genome and 27,156 genes) (Liu et al.,2016) and to the genome ofPangasianodon hypophthalmus(715 Mb genome and 24,083 genes) (Kim et al., 2018).Furthermore, considering its importance, systemic taxonomy and data validation through sequencing of mitochondrial DNA,O.bimaculatusis found to have a phylogenetic relationship with other closely related species (Barman et al.2017, 2018).
1.14.Artificial propagation
A successful breeding programme forO.bimaculatushas been reported by several research groups in India and Bangladesh.However,standardisation of a feasible technology oriented towards a farmerfriendly approach is needed for a larger expansion of the culture practice.Necessary information on various aspects of brood husbandry,nutritional requirements, compatible sex ratios and inducing agent/manipulation of the environment is very much useful in programming a better seed production program.
1.15.Broodstock management
In natural waters, spawning occurs once a year, especially during the monsoon season (June–August), with a peak in July.During the breeding season, males and females move in shoals and breeds in inundated shallow areas, mostly in the mouth of the Beels next to rivers(Biswas et al., 2018; Chakrabarty et al., 2007).However, these spawning habits are disrupted in captivity.Optimum culture conditions to relieve persistent stress, coupled with adequate nutrition as per demand during the reproductive growth period in terms of essential key nutrients, can improve the performance of the brood.Several authors have documented sets of practices with regard to husbandry practice as summarised below (Banik et al., 2011; Biswas et al., 2018; Chakrabarti et al.,2009, 2012; Chakrabarty et al., 2006, 2007; Debnath et al., 2011, 2016;Pradhan & Barman, 2014; Pradhan et al., 2012, 2014; Raizada et al.,2013; Sridharan et al., 2004).
1.16.Preparation of broodstock and maintenance
Broodstock for captive breeding can be collected from the wild;however, the declining population and subsequent timely unavailability of quality broods from natural waters constrain a timely breeding operation.Thus, one can raise broodstock from juveniles in the operation facilities (ponds/tanks) for mass scale propagation.Rectangular ponds with an area of 0.20–0.50 ha and an average water depth of 1.0–1.2 m are suitable for broodstock raising.In advanced systems,cemented tanks of 50–100 m2can be used for broodstock maintenance in intensive systems.Such systems should be free from aquatic weeds,predators and weed fishes.Standard manuring involves liming at a rate of 250 kg ha-1and thereafter the application of 10 tonnes cow dung ha-1year-1before the broodstock introduction.Broodstock ponds and tanks should be covered with netting to avoid bird predation.For the preparation of broodstock, fingerlings are reared at a density of 40000 fingerlings ha-1for 8–12 months (Debnath et al., 2016).In another study by Chakrabarti et al.(2012), the stocking density of fingerlings was 25000–30000 nos.ha-1for broodstock development.For broodstock development, fish can be fed a 30–35% (crude protein) supplementary diet daily at a rate of 3–5% of their body weight (Biswas et al.,2018; Chakrabarti et al., 2012).Traditionally, brood fishes can be fed daily with boiled chicken viscera and other fish wastes at a rate of 5% body weight for better gonadal maturation (Chakrabarti et al., 2009,2012).The health status of the brood fish should be examined periodically with minimal stress.Several studies have reported that the broodstock diet should have an optimum crude protein of 30–35% for better reproductive performance.In addition, Chakrabarti et al.(2009)emphasised the use of trash fish and boiled chicken viscera at approximately 5–10% of the total body weight per day in the brood fish diet.
Captive seed production techniques for mostO.bimaculatushave been developed with a considerable degree of success.Nevertheless, the inadequate and incoherent availability of healthy broodstock is the reason for the lower survival of this species in captivity.Thus, the availability of optimal broodstock nutrition is identified as a key factor for the sexual maturation and reproduction of catfishes in captivity(Rawat et al., 2018).There are still lacunae in the broodstock nutrition ofO.bimaculatusto a greater extent.
1.17.Artificial breeding
Under captive conditions,O.bimaculatuscan be induced to spawn using various synthetic hormones.Broods can be either obtained from farm broodstock facilities or natural waters.They are found to mature completely during the first week of July (Raizada et al., 2013).Ripe brooders weighing above 40 g and above (male and female) are most suitable for induced breeding and seed production (Chakrabarti et al.,2012; NFDB, 2018).Moreover, Singh (2019) reported that ripe brooders weighing more than 80 g males and 120 g females are generally appropriate for induced breeding programs.Such broods can be held in fibre reinforced plastic (FRP) tanks of the hatchery with a continuous water flow.Male and female fish are held in separate tanks and are not fed.Handling of fish should be done very carefully to avoid possible injury and secondary infection.Thereafter, a single injection of inducing hormone should be administered to all brood fish.Fish are injected intramuscularly above the lateral line towards the dorsal fin using a 1-ml syringe.The needle is inserted at an angle approximately 30°from the head.O.bimaculatusfish can be injected with Ovaprim @ 1–1.5 ml kg-1body weight of females and 0.5 ml kg-1body weight of males (Banik et al., 2011; Chakrabarti et al., 2012; Debnath et al., 2013; Pradhan &Barman, 2014; Pradhan et al., 2012, 2014).Injected spawners are kept separately in circular FRP containers with mild water flow or in a breeding hapa and then maintained in a cement cistern with mild water flow.Singh (2019) recommended a ratio of 1:2 females to males for better fertilisation during induced breeding.After a latency period of 9–10 h, the fish were removed.O.bimaculatusmales do not release milt upon induction; therefore, milt is obtained by surgically removing the testes and preparing a sperm suspension by macerating the testes in physiological saline using a mortar and pestle or directly used by squeezing the testis wrapped in a clean cloth and directly mixing with the eggs.The female fish are stripped by gently pressing the abdomen with a thumb from the pectoral fin towards the genital papilla, and eggs are collected in a clean enamel tray.The eggs are fertilised with sperm suspension by gentle mixing with a bird feather in the tray.After approximately 1 min, fertilisation occurred, and fertilised eggs were ready for incubation.However, several researchers have documented the successful induced breeding ofO.bimaculatususing ovaprim as an inducing agent.In parallel, Sridhar et al.(1998) successfully documented the induced breeding of this species using ovaprim at a dose of 0.5 ml kg-1body weight in both males and females.Their study also documented a fertilisation rate and hatchling survival rate of 75% and 55–60%, respectively, after a latency period of 5–6 h.Chaturvedi et al.(2013) have tried to induce the breeding ofO.bimacultususing ovaprims at a dose of 0.06 ml 100 g-1of body weight in males and 0.12 ml 100 g-1of body weight in females.In his study, they documented the successful breeding of the fish after a latency period of 10–12 h of intraperitoneal administration with a fertilisation rate of 70–80%.In line with this,Basavaraja et al.(2013) tried the induced spawning of this fishO.bimaculatususing a single dose of ovaprim at 0.8–0.9 ml kg-1in females and 0.4 ml kg- in males or ovatide at 0.9–1.0 ml kg-1in females and 0.3–0.4 ml kg-1males.Their study documented successful spawning at approximately 8–12 h after hormone injection, with fertilisation rates of 82.4–86.5 and 70–84% for ovaprim and ovatide, respectively.In another study, Singh (2019) reported the successful induced spawning ofO.bimaculatususing an Ovatide/Wova-FH inducing agent at doses of 1.5–2.0 and 0.5–1.0 ml kg-1body weight to females and males,respectively.On the other hand, Raizada et al.(2013) reported successful spawning of this fish using a sGnRH analog and dopamine antagonist at a dose of 0.7 ml kg-1of body weight in females and 0.5 ml kg-1body weight in males.Their study documented fertilisation and hatching rates of 75–90% and 80–90%, respectively.
1.18.Egg incubation and hatching
The fertilised eggs can be incubated using plastic tubs, aquaria or flow-through systems.The fertilised eggs were washed with clean water and kept in a flow-through system for hatching.Plastic tubs are cheap,portable and easy to clean.A framed fine-mesh nylon net screen is spread horizontally in the container, approximately 5–10 cm below the water surface, and fertilised eggs are evenly spread over the screen.Eggs are maintained under mild water flow and are well aerated, which gives them better survival.Water in hatching tubs or cement cisterns must be clean and free of chlorine.The eggs hatch after 20–22 h post-fertilisation(hpf).Eggs hatched within 24 h at a water temperature of 27–30 ℃.Hatchlings move to the bottom of the container through the nylon net.The screen and unfertilised eggs are removed from the hatching tub as soon as most eggs have hatched to avoid fouling of water by the unfertilised eggs.Larvae are reared in the hatching tubs themselves after removing the screen and unfertilised eggs with mild water flow.The newly hatched larvae are cylindrical, transparent, devoid of mouth, and have pectoral fins and body pigments.Larvae possess a large yolk sac(pale greenish) that is absorbed within 2–3 days.The rudiment of one pair of maxillary and two pairs of mandibular barbells appears.
1.19.Developmental stages
The embryonic development and hatching of eggs are similar to those of other cultivable catfishes, such asClarias magurandH.fossilis.Embryonic development begins 30 min post-fertilisation and hatches 22–24 h post-fertilisation at a prevailing temperature of 27–30 ℃ under captive breeding (Biswas et al., 2018; Chakrabarti et al., 2009, 2012;Debnath et al., 2013).The fertilised eggs are 1.2–1.4 mm in diameter and are pale yellow in colour and demersal and adhesive in nature(Raizada et al., 2013).The different developmental stages and characteristics ofO.bimaculatusare outlined in Fig.3.

Fig.3.Embryonic developmental stages of O.bimaculatus larvae (Adapted from Arambam et al., 2020).Changes during embryonic development at 0, 1,2,4,6,8,10, 12, 14 and 18 h post fertilization, hpf
1.20.Seed production and grow-out
Seed production and grow-out farming ofO.bimaculatusare summarised below after Sridharan et al.(2004); Chakrabarty et al.(2006);Chakrabarti et al.(2009, 2012); Banik et al.(2011), Debnath et al.(2011, 2016) and Biswas et al.(2018).Brief descriptions of the reported stocking densities ofO.bimaculatusin different rearing systems are given in Table 6.

Table 6A brief description of reported package of practices for the culture of O.bimaculatus in different rearing systems.
1.20.1.Larval rearing
The early nursery phase involves rearing 2 day-old hatchlings for 15 days (maximum 21 days) until they grow into the fry stage (15–20 mm)in an indoor rearing system.Larvae are usually raised in a plastic tub,cement tanks or aquaria for better survival and growth compared to earthen ponds.During the initial two days, newly hatched larvae use yolk-sac reserves as a food source.On the second day after hatching, the mouth opens, and therefore, small quantities of feed should be provided in the rearing system depending on the larval density and growth.The development of the larvae largely depends on the acceptability of feed.A stocking density of 1,000–2,000 larvae m-2is considered optimum for better growth and survival during indoor rearing (Biswas et al., 2018).A higher stocking density up to 10–20 larvae l-1of water is recommended with better management practices (Chakrabarti et al., 2012).In another study, Debnath et al.(2013) indicated a stocking density of 6 larvae l-1for better survival and growth.Maintaining a healthy environment is always a prerequisite for better performance of the larvae.Standard water quality parameters, including temperature (25–28 ℃), alkalinity(120–150 mg l-1) and dissolved oxygen (3–5 ppm), are optimal.Accordingly, indoor rearing tanks are provided with continuous aeration and water exchange periodically.Initially, the water level of the container should be 7–10 cm, which can be gradually raised to 15–20 cm after one week of raising.The water level is adjusted at various phases of larval development to minimise stress on the larvae.Since differential growth and cannibalism may occur during the early phase, segregation/grading of larvae based on size is highly essential and achieved by grading through nets of different mesh sizes.Hence, it is critical and advisable to keep a strict observation during this stage.Supplying larvae with live food organisms has always proven best and appropriate during early larval rearing (Debnath et al., 2013).First, feeding can be performed with mixed live zooplankton (8–10 cc.50 l-1water) up to 7 dph and then withArtemia naupliior chopped tubifex (25% body weight) or earthworms from 7 dph onwards at four times daily (Chakrabarti et al.,2009, 2012; Debnath et al., 2013).Among these items, chopped tubifex worms have proven to be best at controlling/minimising cannibalism during larval rearing (Rawat et al., 2019).The larvae can be fed ad libitum to avoid cannibalism and better growth.The environmental conditions of rearing systems also influence larval survival and growth.During larval rearing, the performance of butter catfish larvae in terms of survival and growth are found to be better under dark conditions (0 light: 24 dark) (Arambam, 2018), and therefore provision of hiding places should be given.Larvae grow to 15–20 mm fry during a period of 15–21 days (Biswas et al., 2018; Chakrabarti et al., 2009; Debnath et al.,2013).Following 21 days (maximum days) of indoor rearing, the fry can be transferred carefully to outdoor rearing tanks for fingerling production.
1.20.2.Effect of photoperiod and light intensity
Arambam et al.(2020) examined the effect of light intensity and photoperiod on embryonic development, survival and growth ofO.bimaculatuslarvae.They observed that early embryogenesis, hatching rate and time were better under dark conditions, whereas growth was higher under light intensity, although a reduction in survival was observed.This finding reflects that the advancement of embryo transformation is faster at lower light intensities, whereas photoperiodic rhythm (total darkness) lowers hatching time.Furthermore, the trade-off on growth and survival showed an increasing trend towards higher light intensity up to 900 lx.Thus, the reduced growth observed at higher intensities indicates that extreme bright light (1200 lx) influences the behavioural changes of both larvae and prey, resulting in higher encounters.
1.20.3.Nursery rearing
After 15 days (maximum of 21 days) of larval rearing, the fry (15–20 mm; 0.6–2.0 g in weight) of butter catfish are raised further for 40–45 days into fingerlings (Biswas et al., 2018; Chakrabarti et al., 2009; Chakrabarty et al., 2006; Debnath et al., 2013; Sridharan et al., 2004).The fry can be raised to fingerlings (5.0–6.0 cm and 3.0–4.5 g) using either a well-prepared nursery or outdoor ponds or concrete tanks.Delay in transferring the fry influences survival.Generally, fry reared in outdoor (open) pond conditions do not show good survival due to natural mortality or predation.Hence, small concrete tanks of 10–20 m2with a soil base of 5–8 cm are found to be effective for good survival and ease of management.The tanks were fertilised with a single superphosphate (100 g) and filtered cow dung (2 kg) one week before stocking.The tanks were further inoculated with plankton collected from nearby ponds, and then fry were introduced after a week.Small seasonal/perennial earthen ponds of 800 m2sizes can also be used for this purpose (Biswas et al., 2018).When practicing fingerling production in such large bodies, a series of prestocking management measures,including drying, liming, predator/weed fish control, insect control, etc.are essential to provide a suitable environment for the fishes.Additionally, other activities, such as intermittent fertilisation, supplementary feeding, health management and water quality, should be checked to a great extent for higher performances.It is important to protect the nursery pond from predators, and provisions for small-mesh netting around the pond area can be constructed.Fry are reared at a stocking density of 5–7 lakh ha-1(Chakrabarti et al., 2012).The tanks/ponds can be provided with floating weeds such as water hyacinth to provide shade(especially during sunny months) and shelter for the growing fry.Fry should be fed nutritious and balanced formulated diets containing 34% crude protein and 6.5% lipid, which can be availed using fish meal,silkworm pupae, corn powder, soybean oil and mineral-vitamin premix.They can also be fed with boiled and finely chopped chicken viscera, egg custard or any kind of animal protein, mostly low-cost trash fish daily at 2–3 times using a feeding tray @ 3–5% of the body weight.A feed of 50% rice bran and 50% dried fish powder can be mixed and used as nursery feed at 20% body weight per day (Biswas et al., 2018).Fry attains fingerling size (5.0–6.0 cm and 3–5 g) within a rearing period of 40–45 days (Biswas et al., 2018; Chakrabarti et al., 2012; Debnath et al., 2013).Thereafter, the fingerlings are suitable for stocking in well-prepared stocking ponds for grow-out culture.In an earlier study, Das (2017)evaluated the use of in-pond cages (0.8x0.8 ×0.8 m) forO.bimaculatus,with a target to improve the growth performance of larvae and fry.Larvae stocked at 5 larvae l-1had significantly higher mean weight gain than larvae stocked at 15 and 20 larvae l-1.Furthermore, during the fry stage, he performed a study to optimise fry stocking density using 50,100, 150 and 200 fry m-2and reported that a stocking density of 50 fry m-2showed higher growth and survivability.These results were better in terms of yield than those observed under the normal pond system.They speculated that such positive attributes in terms of higher yield are largely because butter catfish, being relatively small fish, may exhaust their energy supply in search of food in a larger pond system.Therefore,confining the fish to smaller cages will resolve the problem of food search and the effective use of water resources, even in culture along with incompatible species of interest.However, few farmers have started to use feeding trays in several parts of the pond as a strategy to minimise such loss.
1.20.4.Advances in larvae and fry rearing
Accessibility of quality, as well as quantity of seedstock, remains a bottleneck for the development of aquaculture of this species.Poor larval and fry survival duringO.bimaculatusseed rearing has been attributed to several factors, mainly heavy cannibalism and inadequate food.Chakrabarti et al.(2012) and Debnath et al.(2013) mentioned the carnivorous and cannibalistic mode inO.bimaculatusduring the early stage, which is due to intrinsic genetic effects ensuing in heterogeneous individual growth.Cannibalism results in high mortality during larval and fry rearing, even where surroundings conditions are ideal (Biswas et al., 2018; Parameswaran et al., 1970; Rawat et al., 2018), and it is typically more severe when food accessibility is restricted.To counter such impacts, several efforts have been made in line with nutritional studies using key nutrients by various research groups and are depicted in Table 7.Over the last few years, a number of studies have tried to reduce mortality during larval and fry rearing, and most of them have used dietary interventions.Biswas, Rawat, Jena, et al.(2019) and Biswas, Rawat, Patel, and Jena (2019) used dietary L-tryptophan for the fry rearing ofO.bimaculatusand documented better fry survivability(more than 50%).In another study, Rawat et al.(2019) reported that dietary supplementation with Tubifex at a rate of 5% was a promising seed rearing strategy for better fry survivability (42.66%).1.20.5.Nutrient requirement of O.bimaculatus
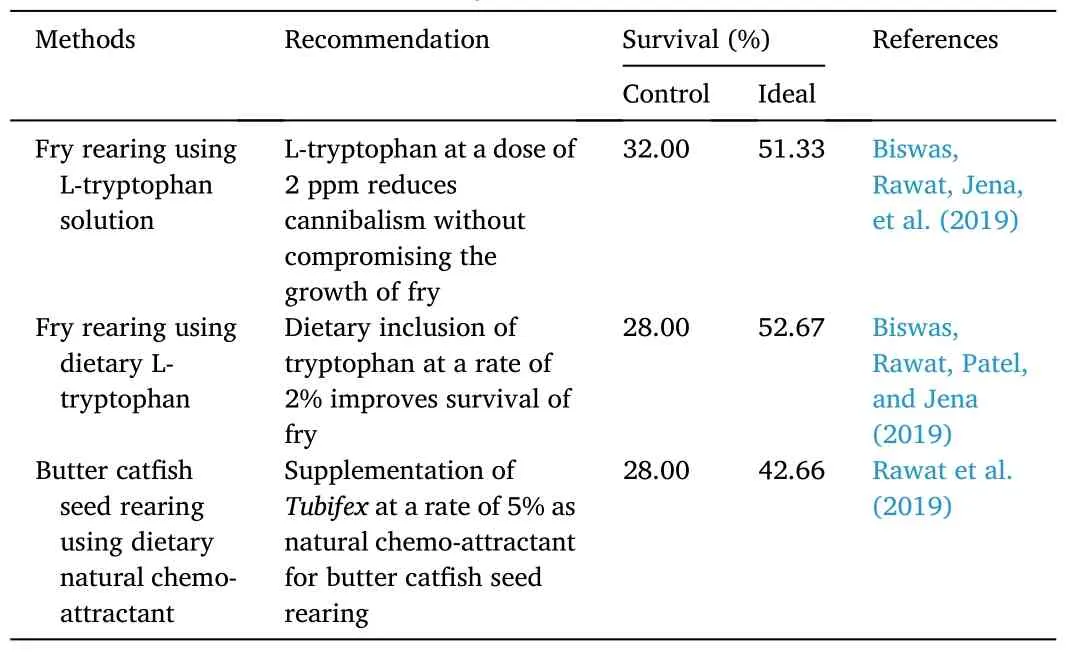
Table 7Recent advances in the seed rearing of butter catfish, O.bimaculatus.
Not much work has been initiated towards the nutritional requirement study ofO.bimaculatus.A recent study by Paul et al.(2020)revealed that a crude protein of 40% in the diet is sufficient for the optimum growth and survival of butter catfish larvae.Similarly, Biswas et al.(2020) studied the dietary protein requirement of butter catfish fingerlings.They observed the required protein to be 36.11% with a P/E ratio of 23.11 g protein MJ-1, as indicated by the optimum growth.However, inO.pabda, a related species, the values were estimated to be 33.2% during fry stages (Paul et al., 2012).
1.21.Grow-out farming
The diversification options in the midst of climate change adaptation and promotion of endemic fishes through aquaculture are key to sustaining the industry.Only a few limited studies have been carried out with regard to the grow-out culture ofO.bimaculatus.At present, pond culture ofO.bimaculatusis practised in eastern and northeastern India states, predominantly in Tripura, Assam and West Bengal (Banik et al.,2012; Debnath et al., 2016; Malla & Banik, 2015), apart from neighbouring Bangladesh.O.bimaculatus, being a new candidate species in aquaculture systems, is being used for culture in either mono- or polyculture systems.
1.21.1.Monoculture
Under monoculture practice, a stocking density of 40,000 fingerlings ha-1is reported to be optimum for growth (average harvesting weight:80 g), survival (70%) and better yield (2.2 tonnes ha-1year-1) in an eight-month crop period (Debnath et al., 2016).However, in well-maintained culture practices,O.bimaculatuscan grow to sizes of 85–120 g in 7–8 months of culture (Biswas et al., 2018), giving a higher yield of up to 2–3 tonnes ha-1year-1.Later, Jena (2018) documented a high stocking density rearing ofO.bimaculatusin a concrete tank under controlled management practices.Their study documented a stocking density of 80000 fingerlings ha-1with a total harvest of 2352.27 kg after 10 months of grow-out culturing (Fig.2).This study also reported a lower stocking density of 40000 fingerlings ha-1in the case of the necessity of larger individual sizes (Jena, 2018).In parallel, NFDB (2018)has reported a stocking density of 60000–80000 nos.fingerlings ha-1to be the suitable stocking density for culturingO.bimaculatusin a grow-out system.In a recent study, stunted fingerlings ofO.bimaculatus(8 g) were successfully cultured in varying stocking densities (15–35 no.m-3) in cages at Maithon Reservoir, Jharkhand, India.In this study, the fish reached an average size of 50–62 g (with more than 80% survival)with a total harvest of 150 kg after 7 months (ICAR-CIFRI, 2019).Additionally, seed unavailability in many areas of the country, where demand for seed to fulfil the higher stocking density needs under monoculture practice, is a common issue.According to Debnath et al.(2019), monocultures ofO.bimaculatusare profitable, but the bottleneck lies in the availability of sufficient seeds.In line with this, he suggested polyculture with carps for the efficient utilisation of the available aquatic niches by cospecies of butter catfish, which otherwise may remain unutilised.Recently, species suitability in biofloc systems has also been documented (Debbarma et al., 2021, 2022), providing a better horizon for the culture expansion of high-value species.
1.21.2.Polyculture with carps
In India, the demand for carp species under semi-intensive farming has been stable for many decades.However, in the wake of better returns with the optimal use of feed and fertiliser, research into the introduction of high value fishes is being prioritised.Such a combination of IMCs and other candidate cospecies may or may not give a better result, which mostly depends on the compatibility among the fish in terms of food and space.These needs have prompted the introduction of high-value fish such asOsteobrama belangeriandO.bimaculatusin carp polyculture practice.A preliminary study on the performance ofO.bimaculatusin polyculture with carp resulted in higher yield and income.This study encompassed a mixed culture of Indian major carp and butter catfish in composite fish culture and a total production of over 1,000 kg of fish ha-1in 180-day culture.A species composition of 40% catla, 30% rohu, 15% mrigal and 15% butter catfish at a stocking density of 4,000 fish ha-1was used for the experiment (Debnath et al., 2013).This study showed that Indian major carps are compatible withO.bimaculatusin composite fish culture (Debnath et al., 2013).Meanwhile, a stocking density of 25000–30000 fingerlings ha-1can be maintained in a mixed culture of butter catfish with carps (Chakrabarti et al., 2012).O.bimaculatusseeds stocked at a larger size (5–10 g)resulted in good survival and growth during grow-out culture.For polyculture ofO.bimaculatus, earthen ponds/stone-lined ponds/cemented tanks are suitable.The pond should be provided with floating aquatic macrophytes, at least 25% of the pond area, which serves as a hideout and shelter for the fish.These aquatic macrophytes can also harbour the aquatic insects and ideal natural food ofO.bimaculatus.Various studies have recommended the use of a 30–35% crude protein diet during the grow-out farming of this species both in mono- and polyculture practices(Biswas et al., 2018; Chakrabarti et al., 2012; Debnath et al., 2019).The fish are fed pelleted feed made of local animal and plant ingredients,such as rice bran, mustard oil cake, fish meal, etc., at 3–5% of their body weight twice daily that is placed in feeding baskets located in different places of the pond.In grow-out ponds, Chakrabarti et al.(2012) and Debnath et al.(2013) emphasised the better growth of butter catfish fed upon their natural diets.Recently, polyculture ofO.bimaculatuswith three IMCs has given a better yield when they are supplied a dual diet viz., 22% protein pelleted diet for carp and 35% protein for butter catfish and were fed at different times of the day (carp: day feeding;O.bimaculatus: night feeding).Such improvement is quite possible considering the feeding habits, with butter catfish obviously requiring more protein diet than carps, apart from the former being a nocturnal feeder (Meitei, 2020).Such an approach can be a good modified polyculture practice with minimum feed loss that is cleaner and more sustainable.However, few farmers have started to use feeding trays in several parts of the pond as a strategy to minimise such loss.Polyculture ofO.bimaculatuswithO.belangerihas also been attempted in the same institute, where a combination ofO.bimaculatus(30 000 ha-1) andO.belangeri(4000 ha-1) has the potential to significantly increase the net fish yield and economic performance of the culture system (Chandravanshi 2019).
1.22.Economic analysis
According to Debnath et al.(2019), monoculture ofO.bimaculatusis more profitable than polyculture.A comparative economic analysis of butter catfish in different stages of rearing is highlighted in Table 8.

Table 8Economical evaluation of butter catfish (Ompok bimaculatus) in different stages of rearing; Adapted and modified from NFDB (2018).
1.23.Future directions and focus
The current review comprises the existing information on the morphological characteristics, food and feeding habits, and reproductive biology as well as the early developmental ontogeny, breeding and culture potential ofO.bimaculatus.Looking at the importance and potential of this species for aquaculture, the following tasks/recommendations can be reverted to work for successful farming.
Task 1.Although artificial propagation of this catfish is successful in many countries, difficulties with regard to larval rearing remain a serious challenge.The need for a continuous supply of live food organisms, especially Tubifex and Artemia, during larviculture is indispensable.Considering that Artemia cysts are highly priced and imported from countries such as the USA, there is a need for alternative live prey production that can be easily practiced locally.Although Tubifex is sufficiently available from wastewater drains and canals around cities,its continuous supply in other parts of the country is still a major bottleneck.Many institutes have tried on-farm production of this live food using artificial conditions and inputs, but successful standardised protocols have not been reported.
Task 2.Another area that needs to be studied is the formulation of stage-specific microdiets, considering the digestive ontogeny of the fish,which was established earlier.The weaning strategies and digestive enzyme ontogeny of the fish were previously developed by Pradhan et al.(2013; 2014).They reported that weaning butter catfish larvae 7 dph is possible, considering the ontogenetic development of the digestive enzymes from the pancreas, stomach and intestine.Such useful research has to be taken forwards in developing age-specific microparticulate diets (MPD), microencapsulated diets (MED) or gel diets for early life stages for lowering early-stage mortalities.
Task 3.Few studies on the role of nutritional strategies to combat such scenarios have been initiated.Supplementation with dietary L-tryptophan has given promising results owing to its role in melatonin surge and subsequent reduction of larval cannibalism.Such findings need on-field trials to refine and promote the technology to end users.Subsequently, the results obtained from the possibilities of environmental manipulation strategies to lower cannibalism and improve survival of the larvae should be synergized with a dietary approach using L-tryptophan, Additionally, molecular-based studies on gene knockout/gene silencing technologies can be encouraged to minimise the cannibalism-based early mortalities of fish.
Task 4.Regarding brood-stock development, many farmers rely on natural riverine sources of broodstock, which is alarming, considering the present status of the fish listed in the IUCN list.To maintain a healthy natural population in the midst of conservation and rehabilitation, a suitable broodstock development programme is essential.Little effort has been put forwards towards quality broodstock production in captivity.Quality brood-stock development using a specially designed broodstock diet may improve the quality of larvae.Thus, the availability of an optimal brood stock diet is identified as a key factor for sexual maturation and reproduction of catfishes in captivity.To date, information regarding broodstock nutrition is scant and requires an urgent need to solve this issue.
Task 5.Moreover, investigations can be initiated on sex-specific feeding and digestion, especially on the nutritional requirements of gravid and spawning female fish to improve the quality of the eggs.Further investigation with regard to developing semi floating/sinking diets using natural attractants, feeding strategies and regimes is warranted.
Task 6.Studies have shown thatO.bimaculatuscan be reared successfully in confinements such as cages and fibreglass reinforced(FRP) tanks by using zooplankton, Tubifex, and artificial feed as diets.However, possibilities of rearing in advanced and efficient systems, such as recirculatory aquaculture systems (RASs), bioflocs and aquaponics systems, can be explored further.Additionally, earlier reported work on the successful rearing of larvae in smaller cages opens up the suitability of using cage-based grow-out culture practices.In their study, they assumed that butter catfish, being small fish, may spend their conserved energy in food searches if cultured in a larger water body.Therefore,using a confined cage structure in open water bodies can sustain energy loss, thereby improving growth.However, such possibilities can be assured through investigations on cage culture.In light of the preliminary success of grow-out farming of this species with carp species,studies pertaining to stocking density standardisation using other existing species of interest should be prioritised, looking to the fact that such a combination can generate higher profit.
Task 7.However, when using such a high stocking density intensive system, the functional immune system of the fish is often compromised.Therefore, improving the health of the fish through an enhanced immune status becomes quite necessary.Several immune-enhancing substances, such as probiotics, prebiotics, synbiotics, and herbal immunostimulants, which have shown promising results in several cultured fishes, can also be evaluated for this catfish.Such a dietary approach can counter the negative effect of disease occurrence in these systems to add to long-term sustainability.Additionally, studies on dietary approaches to enhance the quality of flesh for consumer-targeted marketing and postharvest preservation are also needed.
2.Conclusion
The present review describes the importance ofO.bimaculatusas a potential candidate species for aquaculture diversification program,amidst its importance from the conservation point of view.The detailed information compiled and synthesized in this review can invariably assist researchers in proceeding further on important research issues on breeding and culture aspects.Meanwhile, the species has not gained much momentum even after its breeding standardisation, and there seems to be a wide gap in highlighting the commercial importance in India and a few countries, such as Bangladesh.Serious efforts from scientists across the two nations need to be taken up for upscaling of the existing technologies, apart from implementing specific programs for their wide-scale diversification.Acting on this, the Department of Biotechnology, Govt.of India has already sanctioned the Centre of Excellence (COE) programme on upscaling the culture practice of prioritised endemic fishes, includingO.bimaculatus,in Tripura, India.Furthermore, targeted programme for its promotion and culture in other parts of the country (Northeast India) is underway.Such a programme also needs to be disseminated through collaborative effort in other parts of the country.
Conflict of interest
The authors declare that there are no conflicts of interest associated with this article.
Declaration of competing interest
Authors declare no conflict of any kind related to the work.
Acknowledgements
The present review is targeted to promote the wide-scale culture of this priced species in India.The work is supported by the Centre of Excellence in Fisheries and Aquaculture Biotechnology (COE-FAB)-Phase-II project funded by the Department of Biotechnology (DBT),Government of India.The financial support received from the DBT,Govt.of India under the project Technology scale-up and promotion ofOmpok bimaculatus(Pabda) as aqua-preneurship option and livelihood improvement vide No.BT/PR41713/NER/95/1861/2021 is also duly acknowledged.
 Aquaculture and Fisheries2023年1期
Aquaculture and Fisheries2023年1期
- Aquaculture and Fisheries的其它文章
- Cloning, prokaryotic expression, purification, and functional verification of the insulin gene in black carp (Mylopharyngodon piceus)
- Effective CRISPR/Cas9-based genome editing in large yellow croaker(Larimichthys crocea)
- Effects of BDE-209 exposure on growth performance, intestinal digestive enzymes, and intestinal microbiome in common carp (Cyprinus carpio L.)
- Expression of mapk1 and egr1 genes in Onchidium reevesii under tidal stimulation
- Assessment of bactericidal role of epidermal mucus of Heteropneustes fossilis and Clarias batrachus (Asian catfishes) against pathogenic microbial strains
- Mitochondrial cytochrome oxidase 1 reveals genetic diversity of the African Snakehead fish Parachanna obscura, Gunther, 1861 from Nigeria’s freshwater environment
