Screening and Gas Production Capacity Analysis of a Halotolerant Zygosaccharomyces rouxii Strain Isolated from the Swollen Packages of Pixian Broad Bean-chili Paste
FAN Zhiyi, WANG Zeliang, ZHANG Qisheng,2, CHEN Gong,2, DENG Weiqin, RAN Yalin, LI Heng,2*
(1.Sichuan Academy of Food and Fermentation Industries Co.Ltd., Chengdu 611130, China)
(2.SichuanDongpo Chinese Paocai Industrial Technology Research Institute, Meishan 620000, China)
Abstract: Pixian broad bean-chili paste (PBCP) is a traditional condiment in Southwest China, and package swelling is one of the major quality flaws in commercial PBCP.In this study, PBCP and its two semi-finished products, namely broad bean moromi (BM) and pickled chili (PC) paste were collected.Salt contents and water activities (aw) of the samples were determined and the halotolerant microorganisms were isolated.The results showed that the gas-producing samples had relatively lower salt contents and higher aw values.By inoculating the diluted sample into YM broth media containing 12% (m/m) of NaCl, Zygosaccharomyces rouxii, a halotolerant yeast, was isolated.The production of gas could be induced through inoculating the yeast in YM broth medium, or by re-inoculating the yeast in the autoclaved BM, PC and PBCP with salt contents lower than 16% (m/m).The optimal growth conditions for Z.rouxii were 4% (m/m) NaCl, pH 4~6 and 30 ℃, with the gas production being largest at 8% (m/m) NaCl and pH 5~7.Gas production by Z.rouxii was inhibited when the salt content increased in the culture medium or the sterilized samples.Z.rouxii can grow under acidic and high-salt conditions and can produce gas in PBCP, causing package swelling.
Key words: quality defect; salt content; gas production; halotolerant; control
Pixianbroad bean-chili paste (PBCP), also known as horse bean-chili-paste andPixiandouban, is a traditional fermented condiment originated fromPixiancounty.As an important spicy ingredient for Sichuan cuisine, recent years saw the rapid growth of PBCP industry due to the popularity of Sichuan restaurants among Chinese people.PBCP production consists of three solid-state fermentation phases:moromimaking, red chili pickling and post fermentation[1,2].Inmoromimaking,kojiis made by inoculatingAspergillus oryzaeon blanched broad bean (Vicia faba) cotyledons covered with flour and fermented for 3 months after being mixed with saline water.Meanwhile, dry pickled red chilis (Capsicum annuum) were cut into small pieces.Subsequently, chopped pickled chilis and broad beanmoromiwere mixed for post fermentation according to customers’ requirements before broad bean-chili paste was obtained.
Similar to other traditional fermented condiments such as soy paste,doenjangandsufu, high amount of salt is added in PBCP to prevent microbial spoilage and promote the growth of some halotolerant and halophilic microorganisms[2-4]that contribute to the formation of flavor.Generally, PBCP is not pasteurized in order to keep the intact shape of broad bean cotyledons as well as to prevent undesirable scent produced in heat treatment.In this occasion, microbial activities sometimes may cause gas production of PBCP.Although gas production is a long existing phenomenon that may happen in any of the three fermentation phases in PBCP making according to our experience, it usually leads to package swelling of vacuum sealed PBCP during storing and retailing when temperature is high.Swollen package of PBCP is generally considered as an unacceptable quality flaw, which has become the most frequent reason for rejection by retailers and caused economic loss.
Some previous studies have focused on the aerogenic microorganisms in traditional fermented condiments.For example, Liu, et al.[5]reported thatClostridium butyricumcaused the bottle leakage and explosion in packaged soy sauce.Cheng, et al.compared the microbial communities between normal and swollen canned soy sauce and concluded that contamination of several LABs and bacillus were involved in the swelling of cans[6].Niu, et al.screened aBacilluslicheniformisstrain that caused gas producing spoilage of broad bean paste after heat treatment[7].ForPBCP, however, microorganisms that are involved in gas production is still unknown.It is essential to study the microbial reason underlies the package swelling of PBCP before control measures were taken.
In this study, PBCP and its two major ingredients, broad beanmoromi(BM) and pickled chilis (PC) were investigated for their salt contents and water activities (aw).Meanwhile, an aerogenic yeast,Zygosaccharomyces rouxii,was isolated and gas production capacity of this strain was measured.
1 Materials and Methods
1.1 Sample collection
In a local production plant inPidudistrict, Chengdu, 13 bags (approximately 2 kg for each bag) of broad beanmoromi(BM), 26 bags of pickled chili (PC) and 34 bags ofPixianbroad bean-chili paste (PBCP) were sampled at several timepoints through the year and from different areas of fermentation pools.Samples were directly put into sterile polyethylene ziplock bags and stored at 4 ℃before analysis.
1.2 Determination of salt contents, aw and gas production
Salt contents in BM, PC and PBCP were determined according to the AgNO3titration method from Chinese National Standard GB/T 5009.44-2016[8].Water activities (aw) were measure by putting 5g of samples in a HD-3A water activity meter (Huake equipment Co., China) at 25 ℃ in a thermostatic chamber.Gas production was observed by vacuum sealing 300 g sample in a polyamine bag and incubated at 30 ℃ for 15 d.
1.3 Screening and identification of halotolerant microorganisms
Aerogenic samples from different groups (5 g) were suspended in 250 mL sterilized water followed by placing 1 mL of suspension on the sterile Petri dish.Melted agar culture was then poured into the plates and mixed with sample suspensions.Yeast-maltose (YM) medium and De Man, Rogosa and Sharpe (MRS) medium supplemented with 12% NaCl were used for screening of yeasts and lactic acid bacteria.Plates were incubated at 30 ℃ (for yeasts) or 37 ℃ (for lactic acid bacteria) for 7 d in a 2.5 L Anaerojar (Oxoid, UK) with AnaeroGen 2.5 L atmosphere generation system (Oxoid, UK) and anearobic indicator (Oxoid, UK).For screening of thermotolerant sporeformers, sample suspensions were heated to 85 ℃ for 5 min followed by inoculating into reinforced clostridium medium (RCM) agar plates (supplemented with 12% NaCl) and aerobically incubating at 37 ℃ for 7 d.After incubation, single colonies from agar plates were picked and inoculated in 20 mL corresponding media broth (containing 12% NaCl).Genomic DNA purification kits (Sangon Biotech, China) for yeasts and bacteria were used to extract genomic DNA according to the instructions.Samples were sent to TSINGKE Co.Ltd.for nucleotide sequencing of its ITS or 16s rRNA gene after being amplified in a T960 PCR machine (Heal Force, China).Sequences were compared in NCBI-BLAST to identify the species of microorganisms.
1.4 Aerogenic capacity evaluation of screened isolates in culture broth and autoclaved samples
Halotolerant microorganisms isolated from aerogenic samples were incubated in test tubes with 20 mL media broths (containing 12% NaCl) and inert Durham tubes for 7 d to test their aerogenic responses.Meanwhile, isolates were incubated in 500 mL media broths containing 12% (m/m) NaCl for 3 days before centrifugation at 4 000×gfor 5 min.Cell pellets were suspended with 0.8% sterile saline solution.3 mL of cell suspension with proper concentration was reinoculated in 300 g autoclaved samples to obtain initial inoculation concentration of 106CFU/g.Samples were vacuum packed in polyamide bags and incubated at 30 f℃or 5 d.Autoclaved samples without inoculation were incubated in the same environment as blank.
1.5 Growth curves of aerogenic yeast
Aerogenic yeast (about 104cell/mL) was inoculated into YM broth with different NaCl contents, different pH or incubated at different temperatures.2 mL of broth culture was taken out and measured for its optical density at 620 nm (OD620) at a certain time interval after shaking the broth thoroughly.
1.6 Volume measurement of gas produced by aerogenic yeast
Volume of gas produced by aerogenic yeast was measured according to the method previously reported[5]with some modifications.YM broth (100 mL) was inoculated with approximate 104cell/mL aerogenic yeast and mixed by shaking thoroughly.1 mL of broth media was immediately drew in a 5 mL sterile syringe with the luer slip sealed by Vaseline and the bevel needle was inserted in a rubber block.Syringes were placed upwardly in a sterile glass container and incubated at 30 ℃ for 72 h to observe the volume of gas produced.
1.7 Statistic analyses
All physiochemical analyses were triplicated for a single sample and the results were averaged to obtain the mean value.The data were visualized using Origin 2018 software.
2 Results and discussion
2.1 Salt contents, aw and aerogenic capacity evaluation of samples
Production of traditional fermented condiments requires salt as an essential ingredient to control the fermentation process as well as activities of microorganisms by loweringawvalue.According to our investigation in a fermentation plant, 14%, 16%~18% (m/m) salt is added in themoromifermentation and pickling of red chilis, respectively.Another 1% (m/m) salt is added at post fermentation stage whenmoromiand chopped chilis are mixing.Moreover, part of water in PBCP was evaporated during the post fermentation.For these reasons, PBCP is usually very high in salt content.In our study, salt contents andawvaried dramatically among three groups of samples (Fig.1a).The average salt contents for BM, PC and PBCP were 12.20%±1.24% (m/m), 18.99%±4.98% (m/m) and 18.90%±2.81% (m/m), respectively.This is generally higher than the salt contents in Chinese soybean paste (9.71%~16.71%) and Koreandoenjang(9.01%~18.78%) that are produced with similar processes[3,9].Inmoromimaking phase, BM was fermented indoors for 3 months with frequent stirring to guarantee the uniformity in the fermentation pools.Therefore, the salt content of BM was stable.Also, BM group showed lowest salt content and highestawvalue (0.795±0.008).Compared with BM, salt content andawvalue of PC and PBCP showed larger in-group variations.PC was made by covering salt directly on fresh or dried red chilis for months without any mixing.PBCP, on the other hand, was usually made through post fermentation in the open-air for more than 3 months after BM and CP being mixed.These are in fact very arbitrary operation processes that were influenced by many factors like weather condition or quality of ingredients[1], making salt contents varied greatly among different samples.
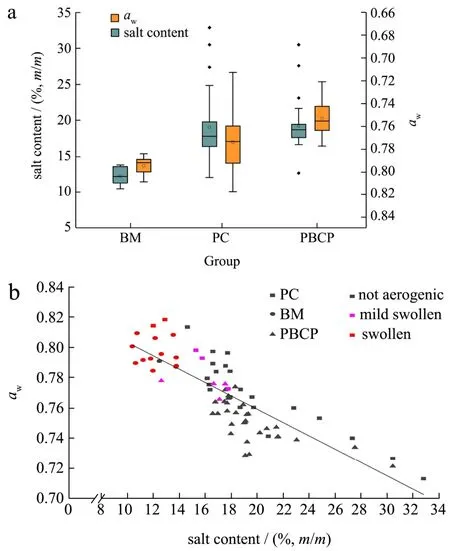
Fig.1 Salt contents, water activities (aw) and gas production of samples
Although there are various low molecular sugars, organic acids and amino acids exist in samples, salt contents may be the decisive factor toawvalues considering relatively low amounts of other components (data not shown).Fig.1b showed thatawwas negatively correlated with salt content (R=-0.83).BMs were distributed on top left in the plot, in which salt contents were lower than 14% (m/m) andawhigher than 0.78.Most samples in PBCP group were higher on salt content and lower onawand appeared on central and right bottom.As for PCs, they scattered on the whole plot from top left to right bottom since salt contents andawvalues were widely dispersed in this group.
Based on the amount of gas produced in vacuum sealed bag after 15 days of incubation, samples were divided into three types, namely, not aerogenic, mild swollen and swollen (Fig.1b).Almost all samples in BM group were “swollen”, which produced large amount of gas that made package bags expand significantly.However, only few samples produced gas in PC and PBCP groups.There were 4 “mild swollen” samples in PBCP group that only made package expand mildly.Interestingly, all aerogenic samples distributed on top left area in the scatter plot which stands for low salt content under 18% (m/m) and highawvalue above 0.76.This trend was seen particularly in BM group in which almost all samples scattered on top left corner while made vacuum bags swollen.For aerogenic samples in PC and PBCP groups, they were also lower on salt content and higher on awcompared with the average level in the group.It can be deduced that activity of aerogenic microorganisms were strongly related with highawvalue of samples that may be caused by low salt content.
2.2 Screening and identification of halotolerant microorganisms
Despite lower salt content of aerogenic samples compared with the average level in each group, they still contained over 10% (m/m) salt, which is not suitable for growth of most microorganisms.Some samples even produced gas with salt content higher than 16% (m/m), indicating strong halotolerance of aerogenic microorganisms in samples.Therefore, we used culture media supplemented with 12% (m/m) NaCl to screen halotolerant microorganisms from samples.According to NCBI-BLASTn comparison,Zygosaccharomyces rouxii,TetragenococcushalophilusandOceanobacillustimonensiswere identified.Z.rouxiiwere screened from aerogenic samples of all three groups.Colonies ofZ.rouxiiwere found to be round, yellowish white and smooth on the surface with size vary from 0.5~2.5 mm in diameter (Fig.2a).Light microscope observation showed that cells from colonies were spherical or ellipsoidal with buds attached to the parent cells.T.halophiluswas a kind of Gram-positive coccus isolated from BM and PBCP, which formed round yellowish white and smooth-surfaced colonies less than 1 mm on MRS plates (Fig.2b).O.timonensis, a Gram-positive bacillus that formed round and whitish colonies on RCM plates, was found in BM and PBCP (Fig.2c).All of 3 microbial species were halotolerant and could grow on media with 12% (m/m) NaCl.
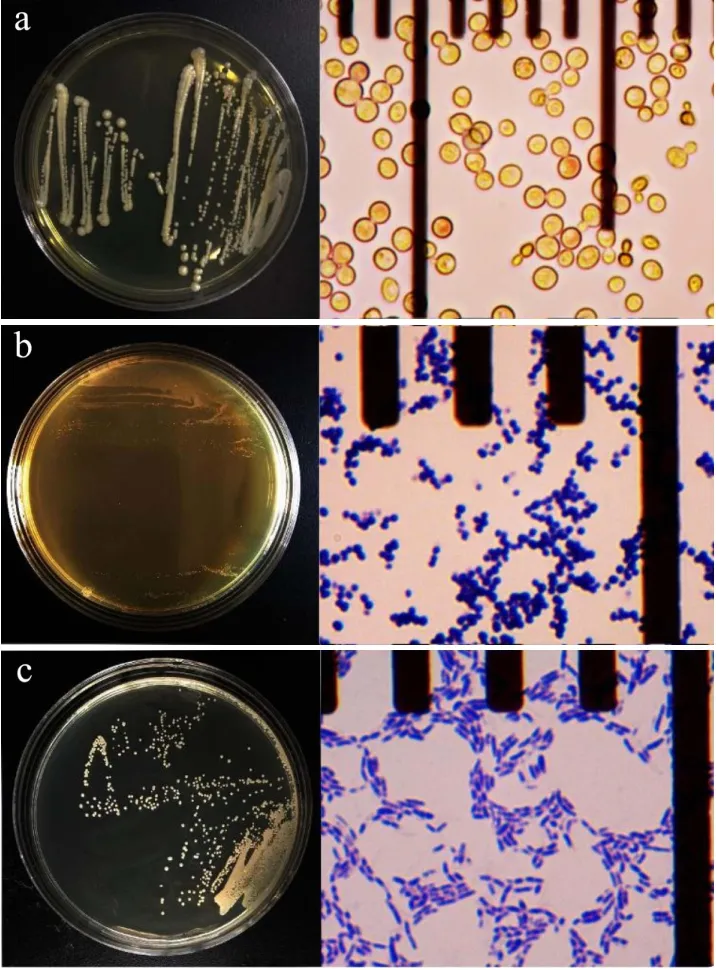
Fig.2 Colony and cell morphology of halotolerant microorganisms screened from aerogenic samples
According to previous studies,T.halophilusis a halotolerant lactic acid bacterium that produces proteinase and flavor compounds in traditional fermented condiments such as soy sauce,doenjangand fish[10-12].However,T.halophilusseems unlikely to induce package swelling since it is not able to produce CO2from glucose[13].Oceanobacillustimonensiswasfirst isolated by Senghor, et al.from human stool and reported as a Gram-positive, rod-shaped bacteria that was able to grow with 0.5% to 20% (m/V) NaCl[14].It was also screened by Lu, et al.from PBCP[15].As forZ.rouxii, it is strongly osmotolerant and can grow not only in high salt content but also in environment with sugar content as high as 70º Brix[16].Z.rouxiimetabolizes sugar and amino acids into various of volatile compounds such as fusel alcohols, esters, and furan derivatives[17-20], which contributes to the flavor formation of traditional fermented condiments.However,Z.rouxiiwas also frequently reported as a spoilage yeast in concentrated fruit juice, syrup, and bakery products[20-22], which usually lead to the alcohol or ester odors and evident gas production[21,22].Therefore, we suspected thatZ.rouxiicaused the package swelling of PBCP.
2.3 Aerogenic test of screened strains in culture broth and autoclaved samples
To test whether the isolated strains can produce gas,Z.rouxii,T.halophilusandO.timonensiswere inoculated into broth media and autoclaved samples.After inoculation ofZ.rouxiistrains in test tubes with Durham tubes, significant gas production was observed at 6th or 7th day of incubation.However,T.halophilusandO.timonensisdid not produce gas in corresponding media broths.Strains were then reinoculated into autoclaved samples with different salt contents adjusted by adding NaCl or sterile deionized water.It is showed that reinoculation ofZ.rouxiiinduced package swelling of BM, PC and PBCP.However, volume of gas produced byZ.rouxiidecreased with the increasing of salt content (Fig.3).Packages of BM and PBCP with 10% (m/m) salt expanded greatly after 5 d incubation, while there was only little expansion for samples with 16% (m/m) salt (Fig.3).For PC samples, only small bubbles were observed in packages after inoculation ofZ.rouxii, though amount of bubble was larger in low salt samples.There was no gas observed in samples of all three groups when salt content exceeded 16% (m/m).On the other hand,T.halophilusandO.timonensisstrains were not able to produce gas in samples of any salt content.Therefore,Z.rouxiiwas the aerogenic microorganism that induces gas production and swollen packages in PBCP with relatively lower salt contents.

Fig.3 Aerogenic test of Zygosaccharomyces rouxii in BM (a), PC (b) and PBCP (c) with different salt contents
Gas production in foods caused byZ.rouxiiwas previously known in bakery products and fruit juice[21,23].However,Z.rouxiiwas scarcely reported to be responsible for package swelling of fermented condiments.This may be due to the prevalent application of pasteurization and preservatives or the low content of substrates such as glucose in these products.Study by Liu, et al.demonstrated that bottle leakage and explosion of soy sauce was caused byClostridium butyricum, which was also isolated from PBCP in our lab by incubating sample dilutions inGifuanaerobic medium (GAM) agar plates in Anaerojar with AnaeroGen 2.5 L atmosphere generation system[5].However, aerogenesis ofC.butyricummay only occurs in sodium reduced products since in our studyC.butyricumwas totally inhibited when salt content of media higher than 5% (m/m), which was confirmed by previous study[24].This indicated thatC.butyricumis unable to induce package swelling in PBCP of which the salt content was over 16% (m/m).
2.4 Growth curves of aerogenic yeast in different salt contents, pH and temperatures
As shown in Fig.4a,Z.rouxiiwas strongly halotolerant and evidentincreasing of OD620values was observed in YM media with 0~14% (m/m) NaCl.Highest growth rate at exponential stage (Fig.4a) were found in 4% (m/m) NaCl although there was little difference on OD620values at stationary phase among groups between 0% and 12% (m/m) NaCl.The increasing of NaCl content prolonged time of lag phase and decreased OD620value in stationary phase.This is consistent with a systematic study by Yong, et al[25].In fixed NaCl content (12%,m/m) and incubation temperature (30 ℃), pH range of 4~6 was optimal for the growth ofZ.rouxiiwhile it could not multiply in media with pH of 2 or higher than 8 (Fig.4b).In some previous studies, pH range forZ.rouxiigrowth seemed to be wider.For example, Yong, et al.investigated dry cell weight ofZ.rouxiiincubated in YM broth containing 10% (m/m) NaCl and found that yeast was able to grow in pH of 2 and 9[25].Restaino, et al.also reported a wider pH range of 1.5~10.5 forZ.rouxiiincubated in 2×potato dextrose agar (PDA) media supplemented with 12% (m/m) glucose[20].The discrepancy may be due to the different culture media, pH regulator and incubation time.For yeast incubated in different temperatures, growth rate at exponential stage increased with the rise of incubation temperature under 35 ℃.However, OD620value at stationary stage seemed to decrease when the temperature was above 30 ℃ (Fig.4c).This was also in line with study by Yong, et al., in which showed that doubling time ofZ.rouxiiwas less at 37 ℃ during the exponential growth while total amount of growth was greater at room temperature (28 ℃)[25].In summary,Z.rouxiistrains screened from aerogenic samples were able to grow in wide ranges of salt contents, pH and temperatures.
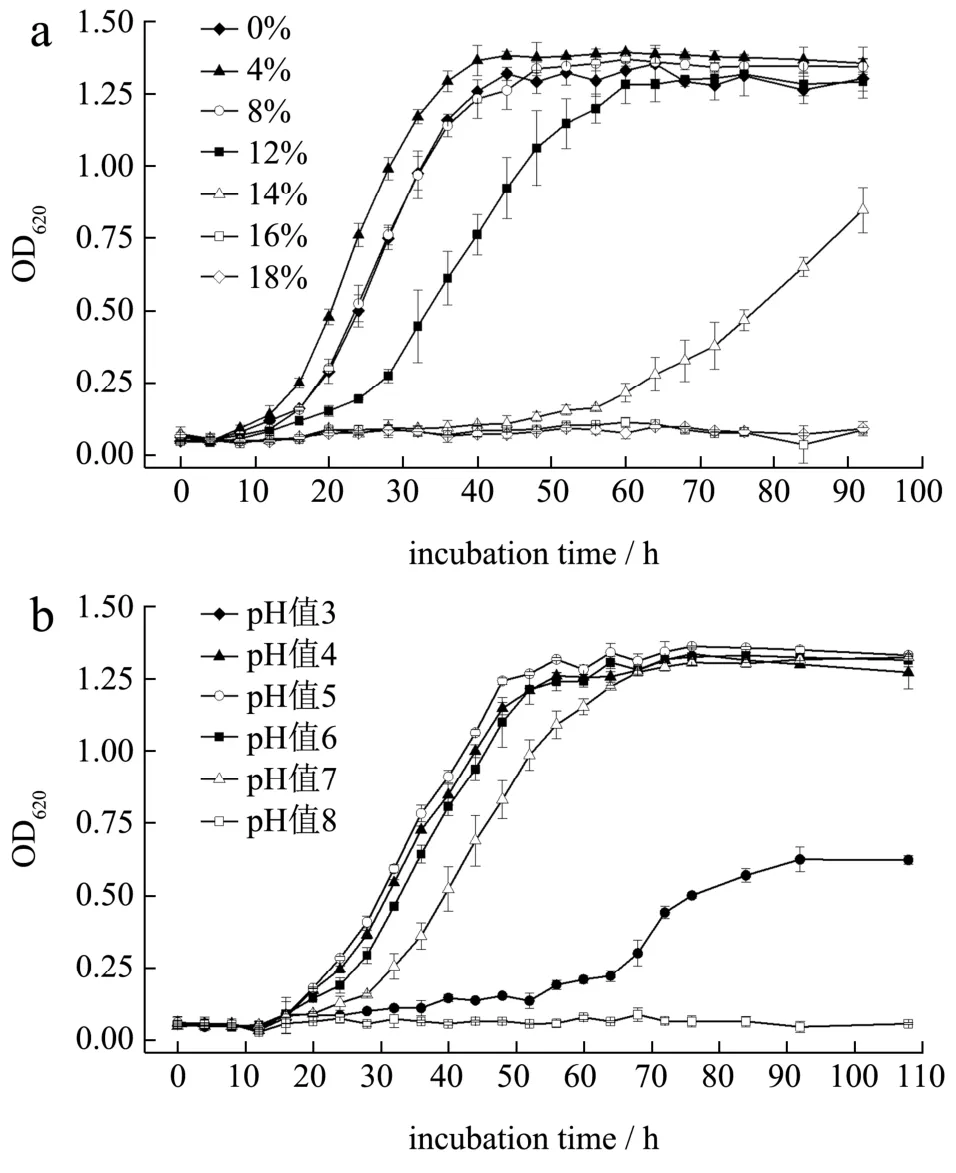
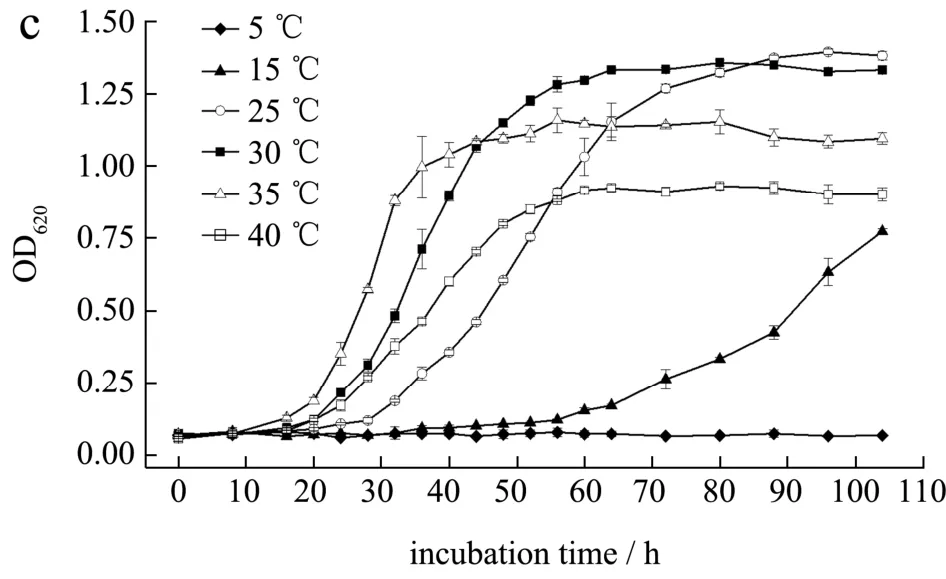
Fig.4 Growth curves of Zygosaccharomyces rouxii
2.5 Volume of gas produced by Z.rouxii
After 72 h incubation, volume of gas produced byZ.rouxiiwas measured.As shown in Fig.5, obvious gas production (gas volumes over 0.7 mL) was seen whenZ.rouxiiwas incubated in broth with 0~16% (m/m) NaCl and pH ranging from 3~8, while gas production was significantly inhibited when the pH of broth higher than 9 or lower than 2.Broths with 8% (m/m) NaCl and pH of 5~7 were optimal for gas production of yeast, in which volumes of gas produced were more than 1 mL.Although similar OD620values at stationary phase were observed at salt content of 0~12% and pH of 4~7 (Fig.4), volumes of gas produced by yeast were different, which may be related with their adaptive behaviors in different salt contents.Also, it is showed that sub acidity environment (pH value 4~5) promoted gas production of the yeast in higher salt contents.However, not like inoculation in the samples, gas production was completely inhibited when inoculatingZ.rouxiiin BM, PC and PBCP with salt content more than 16% (Fig.3).This is probably due to the different sugar contents in samples and broth media.Moreover, no changes of OD620values were detected after seedingZ.rouxiiin YM media with more than 16% (m/m) NaCl (Fig.4).It is indicated that in high salt environment, fermentation ofZ.rouxiisustained even though their proliferation was inhibited.According to our preliminary studies, pH value of BM, PC and PBCP were 4.67, 4.06 and 4.20, respectively (data not shown), making the package swelling induced byZ.rouxiihappen when there was enough substrate exists.

Fig.5 Volume of gas produced by Zygosaccharomyces rouxiiin YM broth media with different salt contents and pH
Traditionally, salt content in PBCP were not strictly controlled.Some previous studies have also described wide ranges of salt content in BM, PC and PBCP[1,26].Nevertheless, gas production byZ.rouxiiin PBCP was scarcely reported.It may be because the production of PBCP was used to comprise a longer fermentation time in which the activity ofZ.rouxiiwas inhibited when water was lost and salt content was increased.However, with the increasing demand in the market, post fermentation time of PBCP have been shortened greatly in order to reduce the lead time.In some production plants, post fermentation of PBCP was less than 2 months.Moreover, introduction of vacuum packaging highlighted the gas production and swollen packages that are considered as “spoilage” by customers.
A culture dependent and independent study by Lu, et al.on PBCP reported thatZ.rouxiiwas one of the dominant microorganisms in all three fermentation phases[1].In accordance with this result, our study isolatedZ.rouxiifrom BM, PC and PBCP samples despite their aerogenic properties.It seemed that unlike the accidental contamination in PBCP production,Z.rouxiiwas an inherent part of fermentation system and plays an important role in flavor formation.Therefore, activity ofZ.rouxiishould not be eliminated or totally inhibited.By reinoculatingZ.rouxiiin autoclaved samples, it was found that high salt contents significantly deterred gas production.This is also demonstrated by incubatingZ.rouxiiin YM media.However, salt content may not be the only factor that influence the activity ofZ.rouxii, since some samples did not produce gas even their salt contents were below 16% (m/m).Studies on other physiochemical properties (such as contents of fermentable sugar and protein) of samples and their relationships with aerogenic properties are needed for prediction of gas production.
3 Conclusions
The results from study indicated that activity of halotolerantZygosaccharomyces rouxiilead to the package swelling of Pixian broad bean-chili paste as well as pickled chili and broad beanmoromi.Aerogenic samples were lower on salt content (m/m) and higher on awvalue compared with the non-aerogenic ones.Optimal growth conditions forZ.rouxiiwere 4% (m/m) NaCl, pH value of 4~6 and 30 ℃, while largest volume of gas was produced in media with 8% (m/m) NaCl and pH of 5~7.Gas production byZ.rouxiiwas be inhibited when salt content increased both in autoclaved samples and in culture media.

