Regional chemotherapy for uveal melanoma liver metastases
Yang-Xu Tao, Hao-Wen Li, Jing-Ting Luo, Yang Li, Wen-Bin Wei
Beijing Tongren Eye Center, Beijing Key Laboratory of Intraocular Tumor Diagnosis and Treatment, Beijing Ophthalmology&Visual Sciences Key Lab, Medical Artificial Intelligence Research and Verification Key Laboratory of the
Ministry of Industry and Information Technology, Beijing Tongren Hospital, Capital Medical University, Beijing 100730,China
Abstract
● KEYWORDS: uveal melanoma; liver metastasis;regional chemotherapy
INTRODUCTION
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults[1]. About 50% of UM patients will develop metastases with a survival of 4-15mo from the onset of metastasis[2]. Liver is the most common site of metastasis. There have been considerable advances in recent years with regard to the management of hepatic metastasis in UM, including immunotherapy and targeted therapy, but with modest results similar to systemic chemotherapy[3-5].Tebentafusp, a novel form of immunotherapy, has shown promising results but is only indicated in patients who are positive for HLA-A*02:01[6-8]. It is not widely used currently and is not available outside the United States, Europe, and a few countries[9]. Thus, chemotherapy remains an important approach for the treatment of liver metastases from UM.
Regional chemotherapy for UM liver metastases includes hepatic artery infusion (HAI), transarterial chemoembolization(TACE), and isolated hepatic perfusion (IHP). Compared with systemic chemotherapy, regional chemotherapy has similar efficacy and fewer systemic adverse effects[10]. Regional chemotherapy is an ideal therapeutic option for metastasis confined exclusively to the liver. In this review, we aim to examine the efficacy of regional chemotherapy and compare HAI, TACE, and IHP in terms of overall survival (OS).
Hepatic Arterial InfusionHAI uses a surgically implanted pump or an arterial catheter connected to an external pump to deliver chemotherapy agents directly into the liver[11-12]. It is indicated for liver metastasis not amendable to complete surgical resection. The contraindications to HAI are liver involvement >70% or bilirubin >2-3 mg/dL. The common complications of HAI include gastrointestinal symptoms,chemical hepatitis, and bone marrow toxicity.
We searched PubMed with the search terms “hepatic artery infusion”, “intra-arterial chemotherapy”, “uveal melanoma”,and “ocular melanoma”. Ten studies investigated the efficacy of HAI in UM liver metastases (Table 1)[13-22]. There were six retrospective studies, three prospective studies, and one randomized trial. In total, 439 patients treated with HAI were described, ranging from 7 to 171 per study. The median OS ranged from 2.9 to 22mo. Fotemustine was the most commonly used drug. Other chemotherapeutic agents included carboplatin,cisplatin, vinblastine, dacarbazine, mitomycin, doxorubicin,and gemcitabine. Using carboplatin, Cantoreet al[13]reported an overall response rate (ORR) of 38% and a median OS of15mo with minor liver-related toxic effects. Leyvrazet al[14]conducted a prospective phase II trial with fotemustine, which showed the highest ORR of 40%. The study of Peterset al[16],also using fotemustine, reported the highest disease control rate (DCR) of 84.2%. The longest median OS was observed in the study by Siegelet al[17], reaching up to 22mo. On the other hand, the study by Booneet al[22]showed a median OS of 2.9mo with melphalan. The reported ORR and DCR were also lower than other studies, because the patients enrolled in this study had extremely advanced disease and were near the brink of liver failure. In the retrospective case series by Farolfiet al[19], no differences were found in toxicity or clinical outcome between fotemustine and carboplatin. Small sample size(n=18) might be the possible reason. The only randomized trial was conducted by Leyvrazet al[21], comparing hepatic intraarterial and intravenous fotemustine. They found no difference in OS, but intra-arterial showed better ORR and progression free survival (PFS).
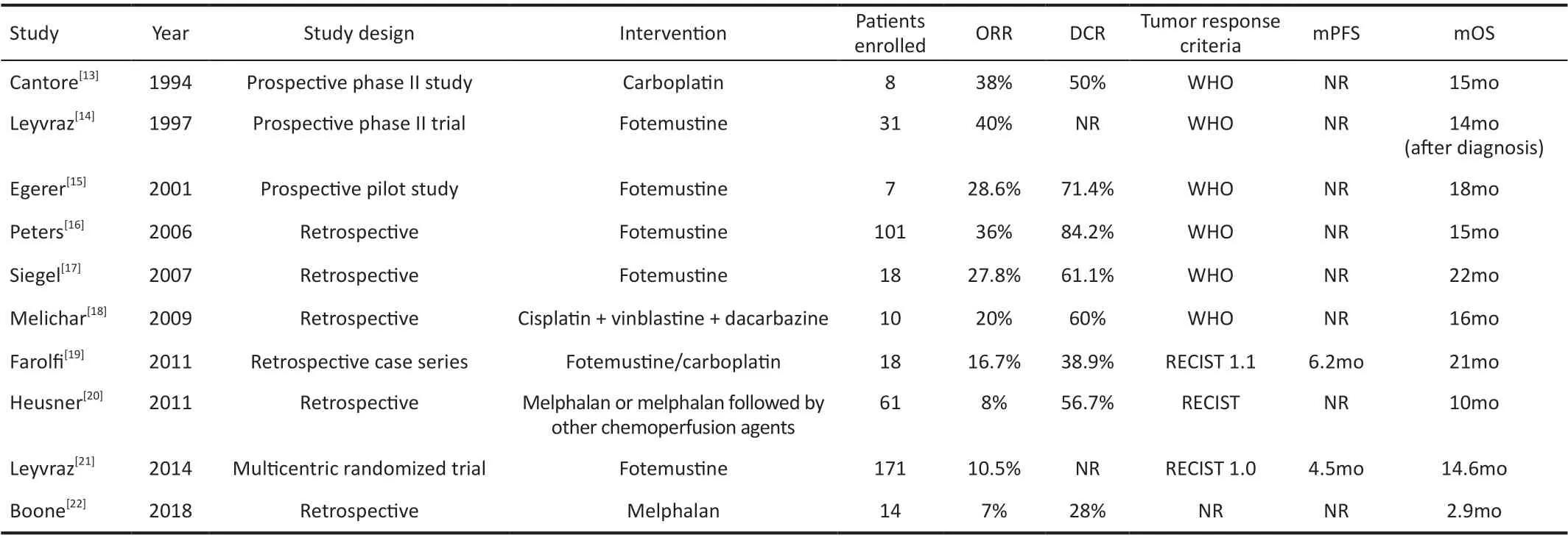
Table 1 Studies of HAI in UM patients with liver metastases
Transarterial ChemoembolizationTACE combines intraarterial chemotherapy with embolization of arterial supply to the tumor. The chemotherapeutic agents are directly delivered to the tumor in the liver through a catheter inserted into the femoral artery in the groin. The embolization can be accomplished using polyvinyl/gelatin sponges, polyvinyl alcohol particles, microspheres, and drug-eluting beads[23].This technique entraps a high concentration of chemotherapy to the tumor and also cuts off the tumor’s oxygen and nutrient supply[24]. Compared with other embolization approaches,drug-eluting beads can deliver stable therapy over time,achieving a higher DCR[25-28]. The contraindications to TACE are liver involvement >70%, main portal vein thrombosis,Child-Pugh C, and arterio-portal fistula. The common complications of TACE include decompensation with edema/ascites, acute cholecystitis, acute pancreatitis, liver rupture,liver abscess, renal failure, and postembolization syndrome[29].We searched PubMed with the search terms “transarterial chemoembolization”, “chemoembolization”, “uveal melanoma”, and “ocular melanoma”. Nineteen studies investigated the efficacy of TACE in UM liver metastases(Table 2)[30-48]. There were thirteen retrospective studies, five prospective studies, and one randomized trial. In total, 733 patients treated with TACE were described, ranging from 5 to 201 per study. The median OS ranged from 5.2mo to 23mo. Cisplatin was the most commonly used drug. Other chemotherapeutic agents included vinblastine, dacarbazine,vincristine, dactinomycin, carmustine, mitomycin C,doxorubicin, irinotecan, paclitaxel, carboplatin, and melphalan.Though fotemustine and carmustine have pharmacological advantages for local intra-arterial treatment of liver metastases,no comparative clinical trial has shown that they have better results than cisplatin or other agents. Mavligitet al[30]used cisplatin and polyvinyl sponge, achieving an ORR of 46% and a median OS after metastases diagnosis of 11mo. Bedikianet al[31]retrospectively reviewed 201 cases and compared the results of systemic therapies, hepatic intra-arterial chemotherapies, and chemoembolization. The study showed that chemoembolization was the most effective treatment, with an ORR of 36%. Agarwalaet al[32]conducted a randomized phase I/II trial evaluating intrahepatic chemotherapy with cisplatin with or without polyvinyl sponges. They reported an ORR of 16% and a median OS of 8.5mo. Besides, the results of this study indicate that the maximum tolerated dose of cisplatin for direct hepatic artery infusion is 125 mg/m2, given with or without embolization. At 125 mg/m2, half of the patients experienced grade 3 or higher toxicity (renal, hematological,and hepatic toxicity). Using carmustine, Patelet al[33]and Gonsalveset al[45]reported a median OS of 5.2mo and 7.1mo.There were four studies using irinotecan-charged beads:Fiorentiniet al[35]reported the highest ORR of 100%, never the less the study had a small sample size of 10 patients; Venturiniet al[40], reported an ORR of 60% and a DCR of 80%; Valpioneet al[46]reported a median OS of 15.5mo; However, the study of Carlinget al[44]showed that the effect of drug-eluting beads loaded with irinotecan alone on OS is questionable; Longest median OS was observed in the study by Shibayamaet al[47],reaching up to 23mo. Carleet al[48]reported a median OS of 16.5mo with melphalan.
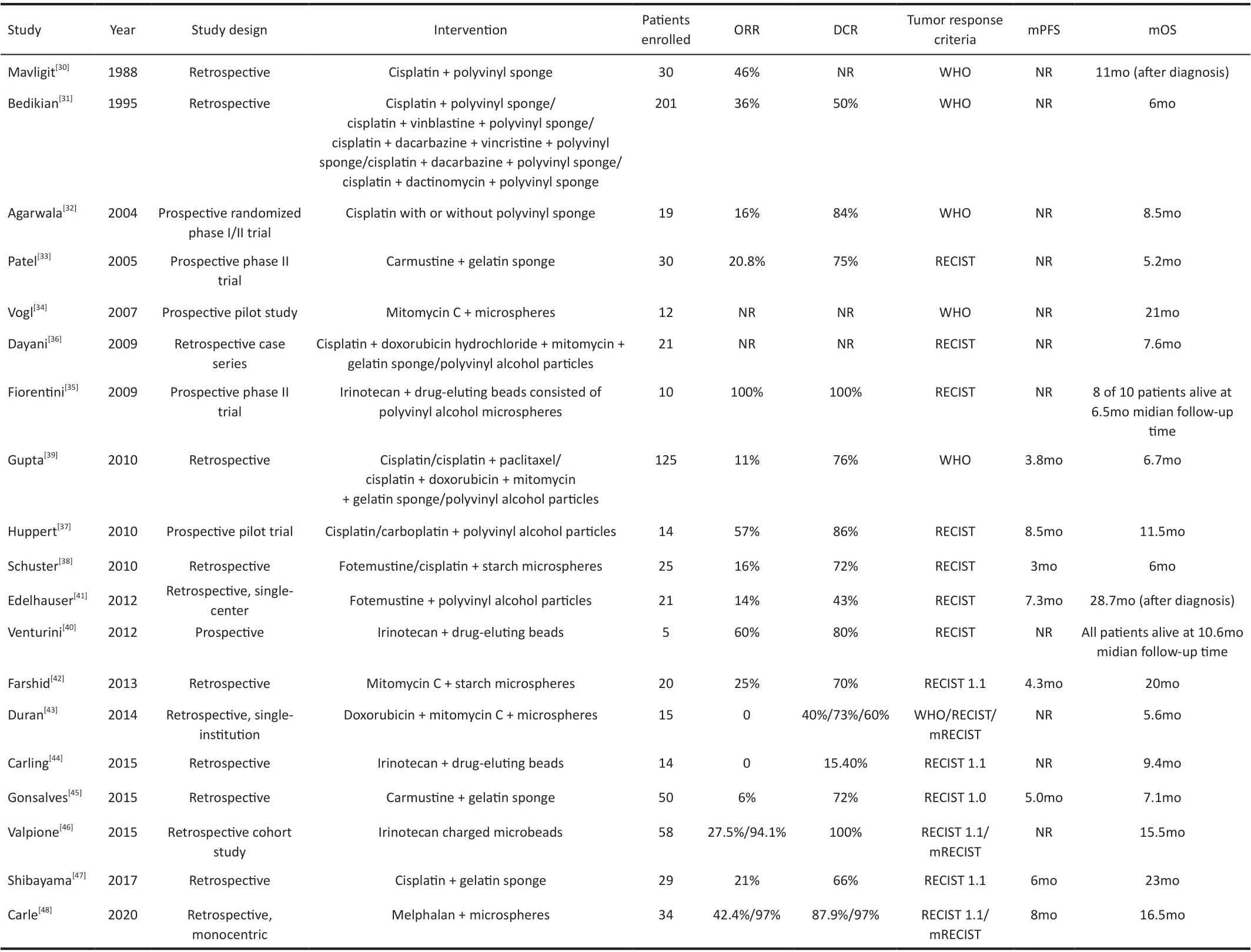
Table 2 Studies of TACE in UM patients with liver metastases
Isolated Hepatic PerfusionIHP involves temporary vascular isolation of the liver and perfusion of high-dose chemotherapeutic agents while limiting extrahepatic toxicity[49].It is used mainly for patients with surgically unresectable liver tumors. Percutaneous Isolated Hepatic Perfusion (PHP) is a minimally invasive alternative to IHP and is repeatable[50]. IHP/PHP is indicated for tumors totally or predominantly localized to the liver. The relative contraindications to IHP/PHP include insufficient liver function (bilirubin >1.5 mg/dL, elevated INR,platelet <100 000), portal hypertension, intolerance to heparin,and age >70[51]. The most common complication of IHP is bone marrow suppression. Complications related to vessel catheterization might occur as well.
We searched PubMed with the search terms “isolated hepatic perfusion”, “percutaneous hepatic perfusion”,“uveal melanoma”, and “ocular melanoma”. Twenty-eight studies investigated the efficacy of IHP and PHP in UM liver metastases (Table 3)[52-79]. There were seventeen retrospective studies and eleven prospective studies. In total, 814 patients treated with IHP or PHP were described, ranging from 3 to 101 per study. The median OS ranged from 4.5mo to 27.4mo.Melphalan was used in all studies. Hafströmet al[52]used melphalan and cisplatin as chemotherapeutic agents, achieving a median OS of 4.5mo. Alexanderet al[53]performed IHP using melphalan alone or with tumor necrosis factor (TNF),with a median OS of 11mo. The study reported a longer overall median duration of response in those treated withTNF than without TNF (14 versus 6mo;P=0.04). van Ierselet al[60]performed a phase I study to determine the optimal dose of oxaliplatin in combination with a fixed melphalan dose. They reported 100 mg as the maximal tolerated dose of oxaliplatin. However, the study indicated that applying this dose of oxaliplatin in IHP is not expected to improve results in patients with isolated hepatic metastases. Pingpanket al[56]performed PHP using melphalan and determined that 3 mg/kg is the maximum safe tolerated dose of melphalan administeredviaPHP. Ben-Shabatet al[66]retrospectively analyzed if adding a buffering agent to the perfusate during IHP would reduce toxicity and complication rates. The study showed that buffering would reduce the value of liver function tests,but could not reduce complication rates. In the study by Tonget al[76], positive predictors for survival in melphalan-PHP treated UM patients were identified, including primary tumor radiotherapy, normal baseline LDH, and PHP cycles.

Table 3 Studies of IHP/PHP in UM patients with liver metastases
DISCUSSION
The three regional chemotherapy approaches, HAI, TACE,and IHP, have their advantages and limitations. Compared with HAI, TACE has the benefit of trapping the chemotherapy in the tumor and also cutting off the supply of oxygen and nutrients to the tumor. Thus, TACE is more suitable for tumor with abundant blood supply. IHP is a more invasive treatment in comparison to HAI and TACE. Its alternative, PHP, is less invasive, but is still technically demanding[80-81].
The different growth patterns of hepatic metastases would result in different responses to regional chemotherapy.Hepatic metastatic UM has two radiographic growth patterns, namely nodular pattern and infiltrative pattern.Compared with infiltrative pattern, nodular growth pattern on angiography indicates the tumor to be more responsive to chemoembolization or radioembolization.
The efficacy of different chemotherapeutic agents in the treatment of UM liver metastases is uncertain, and there is a lack of unified criteria. Further clinical trials excluding confounding factors are needed to identify more effective chemotherapeutic drugs. Our review shows that none of the chemotherapeutic drugs can significantly prolong the survival time of patients, which merits more basic experiments looking for UM-sensitive drugs.
The three approaches showed no obvious difference in OS results. There exist variations in the survival data within each approach, which may be due to different inclusion criteria of different studies. The burden of liver tumor, previous treatment,and extrahepatic metastasis may hinder the fair comparison of survival data. Further randomized controlled trials are required to evaluate the efficacy of the three methods.
ACKNOWLEDGEMENTS
Foundations:Supported by National Natural Science Foundation of China (No.82141128); The Capital Health Research and Development of Special (No.2020-1-2052);Beijing Natural Science Foundation (No.7204245); Science& Technology Project of Beijing Municipal Science& Technology Commission (No.Z201100005520045;No.Z181100001818003); Scientific Research Common Program of Beijing Municipal Commission of Education (No.KM202010025018); Beijing Municipal Administration of Hospitals’ Youth Programme (No.QML20190202); Beijing Dongcheng District Outstanding Talents Cultivating Plan(No.2018).
Conflicts of Interest: Tao YX,None;Li HW,None;Luo JT,None;Li Y,None;Wei WB,None.
 International Journal of Ophthalmology2023年2期
International Journal of Ophthalmology2023年2期
- International Journal of Ophthalmology的其它文章
- Perspectives and clinical practices of optometrists in Saudi Arabia concerning myopia in children
- Progression of myopia among undergraduate students in central China
- Flipped classroom approach to global outreach: crosscultural teaching of horizontal strabismus to Chinese ophthalmology residents
- Topical ketotifen treatment for allergic conjunctivitis: a systematic review and Meta-analysis
- Pseudomembranous conjunctivitis in a patient with DRESS syndrome
- Two cases of persistent shallow anterior chamber after cataract surgery combined with goniosynechialysis
