Use of biopolymers in wastewater treatment: A brief review of current trends and prospects
Ahmed M.Elgarahy, M.G.Eloffy, Eric Guibal, Huda M.Alghamdi, Khalid Z.Elwakeel2,,
1 Egyptian Propylene and Polypropylene Company (EPPC), Port-Said, Egypt
2 Environmental Chemistry Division, Environmental Science Department, Faculty of Science, Port Said University, Port Said, Egypt
3 National Institute of Oceanography and Fisheries (NIOF), Cairo, Egypt
4 Polymers Composites and Hybrids (PCH), C2MA, IMT Mines Ales, Alès, France
5 University of Jeddah, College of Science, Department of Chemistry, Jeddah, Saudi Arabia
Keywords:Biopolymers Biomass Bioseparation Coagulation Photocatalysis Sustainability
ABSTRACT Indeed,polymeric materials have thrived in worldwide sectors over the last five decades due to their versatility and durability,to the point that we can no longer envisage a product that does not contain them.However, many synthetic polymers that have been produced are mostly sourced from petroleum and coal as raw materials, making them environmentally incompatible because they cannot be integrated with what is a natural recycling system.One of the most important aspects of the transition to a circular bioeconomy (CBE) is the provision of more sustainable strategies for resource and waste management.Considering the environmental consequences associated with petroleum-based polymers(PBPs),natural biopolymers,originating from biomass,can be conceived as a promising solution to gradually replace the PBPs, and address, and resolve the potential challenges and prevailing research gaps in the PBPs.The biopolymers have significant advantages over PBPs in terms of low-cost/zero-cost precursors, environmental friendliness, and user-friendliness.The present review dissects the sources, synthesis pathways,structures,characterization,and employment of biopolymers and their composites in water and wastewater treatment applications via different scenarios.Furthermore, the CBE model framework proposes potential approaches to applying CBE principles in the wastewater management sector, with a heavy emphasis on not only technology but also organizational and societal reforms.To sum up, the reliance on biopolymers can be considered a crucial tool for assessing the global progress toward CBE, as well as future environmental management and planning.
1.Introduction
Nowadays apart from energy, environmental issues are currently a major concern for humans and other species.Many petroleum-based polymers (PBPs) products have been developed over the years to meet human needs such as polypropylene (PP),polystyrene (PS), low-density polyethylene (LDPE), high-density polyethylene (HDPE), polyvinyl chloride (PVC), and polyethylene terephthalate (PET) [1].The great demand for conventional PBPs has dramatically expanded because of population growth, and technological advancement in our everyday lives.Typically, these materials are created by polymerizing low-molecular-weight organic molecules derived from fossil resources.The annual production of synthetic polymers has increased from 1.5 million metric tons in 1950 to 359.0 million metric tons in 2018 [2].These synthetic materials have severe impacts on the environment and human life by generating hazardous waste that negatively impacts land, water, and air.Unfortunately, the main issues related to the manufacturing processes of these non-biodegradable synthetic polymers are the release of greenhouse gases (GHGs).This is thought to be the primary source of the global average temperature rise[3].To address these life-threatening challenges,prospective solutions for environmental conservation must be created and implemented.Over the last several decades,there has been a surge in interest in bio-based materials derived from natural renewable resources and environmentally friendly products such as biopolymers,which will play an important role in the replacement of synthetic polymers [4,5].
Biopolymers are promising alternatives to petroleum-based polymer materials to release the burden of the environment and resources due to their diverse physical, chemical and biological properties.Biopolymers are natural polymers generated from renewable natural sources or biosynthesized by living organisms.As natural macromolecular substances produced in vivo, biopolymers mainly include exopolysaccharides, polyhydroxyalkanoates,and polypeptides.They are non-toxic and emerging competitors for synthetic polymers, with significant advantages such as ecofriendliness and biodegradability nature [6].Mainly, biopolymers can be categorized into(i)natural biopolymers,(ii)chemically generated biopolymers, and (iii) microbial biopolymers.Nowadays,the use of biopolymers is expanding in a broad range of industries[7].Interestingly, these biopolymers have remarkable mechanical,physical, chemical, and biological characteristics of renewability,environmental compatibility, antibacterial activity, biocompatibility,and biodegradability.These properties make them available for commercialization in various industries such as food, pharmaceutical, medicinal, and environmental fields [8].Furthermore, the presence of reactive functional groups in their skeleton(i.e.,amide,carbonyl,carboxyl,hydroxyl,etc.) qualifies them for application in wastewater treatment.Considering their outstanding qualities,there has been a tremendous amount of research interest in increasing the production of biopolymers.Nowadays, wastewater remediation using biopolymers and biopolymer composites is one of the most exciting research subjects.In order to compete with the non-biodegradable conventional polymers, there is increasing research focusing on the development of biodegradable polymers for global welfare and the reduction of environmental pollution [9-11].A key issue observed during the up scaling of biopolymers is the cost inefficiency through the synthesis and purification processes.To summarize,biopolymers and their composites have been widely employed as coagulants[12],flocculants[13], adsorbents [14], membranes [15], and photocatalytic agents[16] to remove heavy metals, dyes, natural organic matter, and other water pollutants [17-19].In this study, a brief review of the sources, synthesis pathways, structures, and characterization are given.Their cumulative roles in the treatment of wastewater will be explored utilizing several known methodologies.
2.Biopolymers
2.1.Bibliometric analysis
The ongoing global issue of water resource pollution is today’s most significant health concern.Thus, multidisciplinary biopolymers can be regarded as promising materials for water treatment emerging from the growing concerns on water protection and conservation.Fig.1 displays a bibliometric analysis for the synopsis of biopolymers harnessing for wastewater remediation highlighting various aspects(i.e.,countries of publications,number of published articles,and Bibliometric maps of several cited articles in different countries or regions).The data come from dimension, an available search, and metrics for more than 100 million publications and research information.The raw data were collected from the Web of Science database and then exported to VOS viewer software to minimize the gap in knowledge surrounding the utilization of biopolymers in wastewater treatment.Regarding the contribution of countries and regions toward these publications vs.citations,China had the highest number of published papers; 350 vs.4500 citations followed by Malaysia with 300 papers and 3750 citations,and the publications in the USA are 275 vs.3500 citations.The top 10 contributing countries or regions are presented in Fig.1(a).
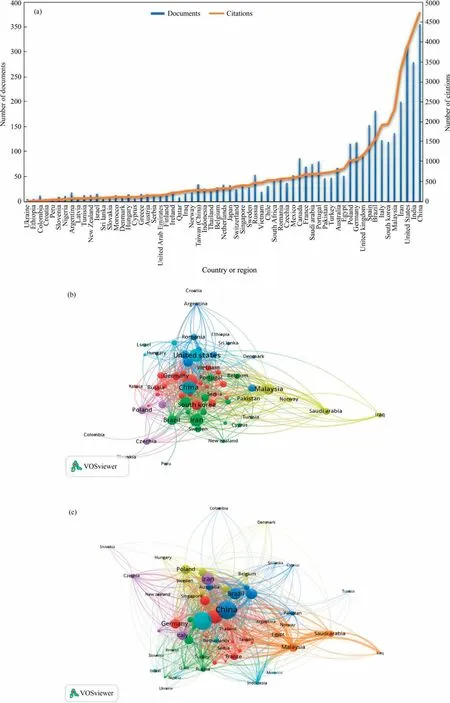
Fig.1.A bibliometric analysis for the synopsis of biopolymers harnessing for wastewater remediation highlighting various aspects: (a) countries or regions of publications and number of published articles; (b) bibliometric map for biopolymer applications in the world (2015-2022); (c) bibliometric map for biopolymer citations in the world(2020).
The use of biopolymer as desired material and environmentfriendly worldwide has been getting increasing attention in recent years.Fig.1(b)presents the most countries or regions in the world that select biopolymer as an ideal applied material for different applications.The research covered the period from 2015 to 2022.China comes in front of countries using biopolymers.Chinese manufacturers seek to increase their products of biodegradable polymers made from plants.While Fig.1(c) had been covered the average of citations in ~90 countries or regions around the world during 2020, the top five in the citation are India, China, Brazil,Iran, and Italy.India recorded the highest score in the citation(1054) followed by China (974).
2.2.Sources of biopolymers
In general, biopolymers are cheap materials, originally made from biological resources,and consist of monomeric units with linearly or branched-like organized molecules.The term‘‘monomeric unit” refers to molecules that contain nucleic acids, nucleotides,saccharides, or amino acids derived from protein sources.Structurally, biopolymers are made of carbon-neutral resources that are mostly made up of biopolymers like carbohydrates and proteins, which have a higher biodegradability than synthetic polymers [4].Polymers are created by repeating a building unit,especially a monomer composed of carbon(C), oxygen(O), hydrogen (H), and nitrogen (N).Fig.2 illustrates the major biopolymer resources.Biopolymers can be chemically synthesized from monomeric units of diverse biological sources such as animals (i.e.pig and cattle),agricultural sources(i.e.cassava,potatoes,and wheat),biomass wastes(i.e.paper waste,and wood wastes),vegetable oils(i.e.corn, castor, soybean, and sunflower), fish oils, microbial sources (i.e.algae, fungi, and yeasts), and marine sources (i.e.corals, shrimps, fishes, lobster, and sponges), which are considered as major substitutes for the production of natural biopolymers[7,20].The physicochemical characteristics of the as-produced biopolymers (i.e., color, transparency, resilience, stiffness, heat/-electricity conductivity, etc.) are largely affected by the constituents’ monomers type, polymerization degree extent, and bonding pattern/order.
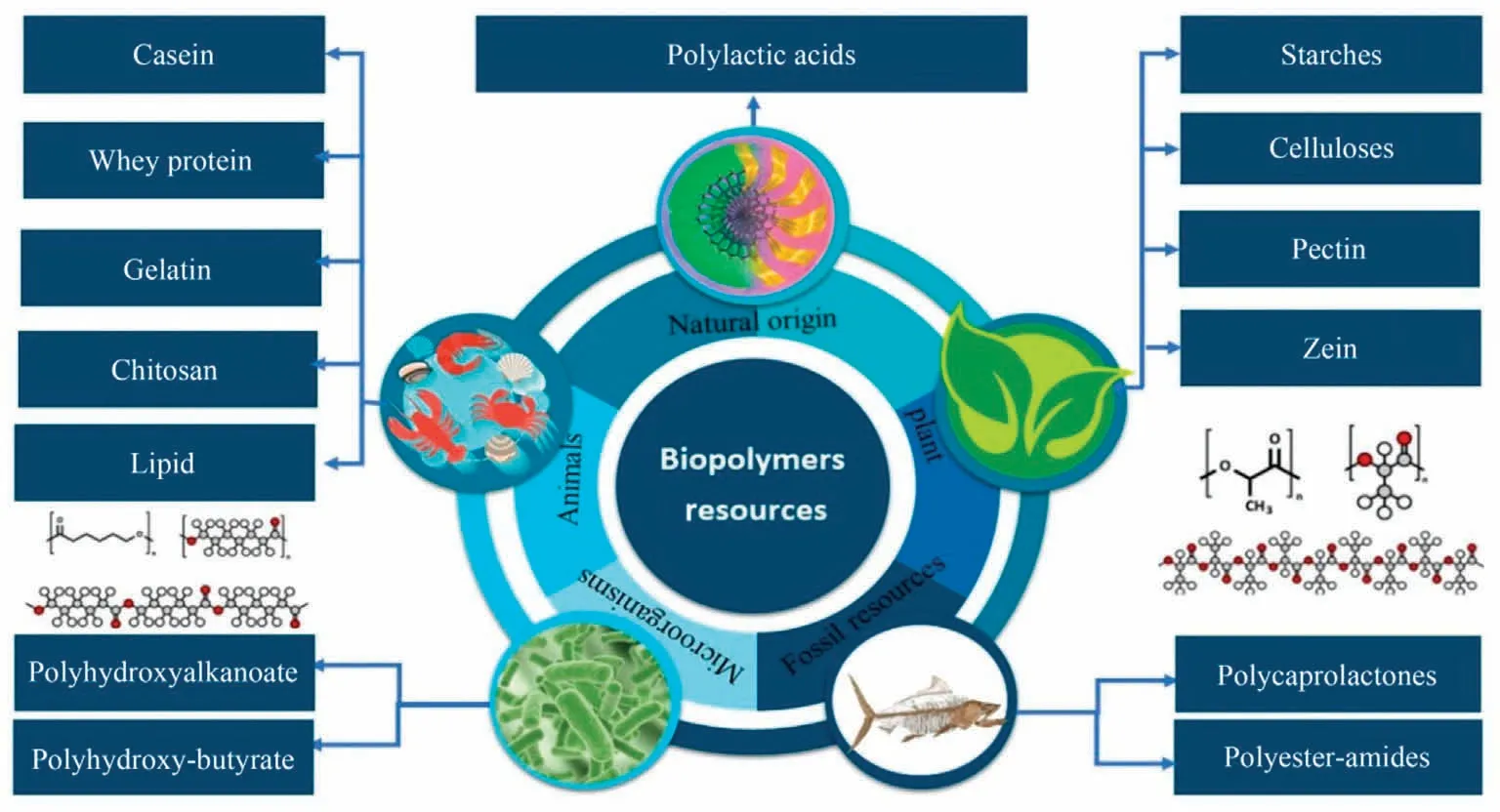
Fig.2.The major resources of biopolymer.
2.3.Classification of biopolymers
Inherently, biopolymers can be categorized based on different scales.They are divided into two types: biodegradable and nonbiodegradable [21,22].Moreover, they can be classified into two main categories based on their origin: natural sources and fossil fuels.Similarly, they are classified as elastomers, thermoplastics,and thermosets depending on their thermal condition reactions.Biopolymers are classified into three types based on their composition: blends, laminates, and composites [23].Natural, synthetic,and microbial biopolymers are the three categories of biopolymers based on their raw material source.Natural biopolymers based on biomass are classified as protein and carbohydrate-derived polymers that can be synthesized from natural resources comprising aerial, terrestrial, and marine living organisms of microorganisms,animals,and plants[24].Fig.3 shows the classification of biopolymers according to their origin.Microorganisms such as bacteria,fungi,yeasts,molds,smuts,and many other forms of primitive life,for example, have the potential to provide huge diversity of polymeric biomolecules with exceptional structural and biochemical properties.Alginate,collagen,egg protein,soy protein,gelatin,keratins, etc.are examples of protein-based biopolymers, whereas carbohydrate-derived polymers include agar, alginate, cellulose,lignin, chitosan, hyaluronic acid, gums, starch, and etc.[25,26].
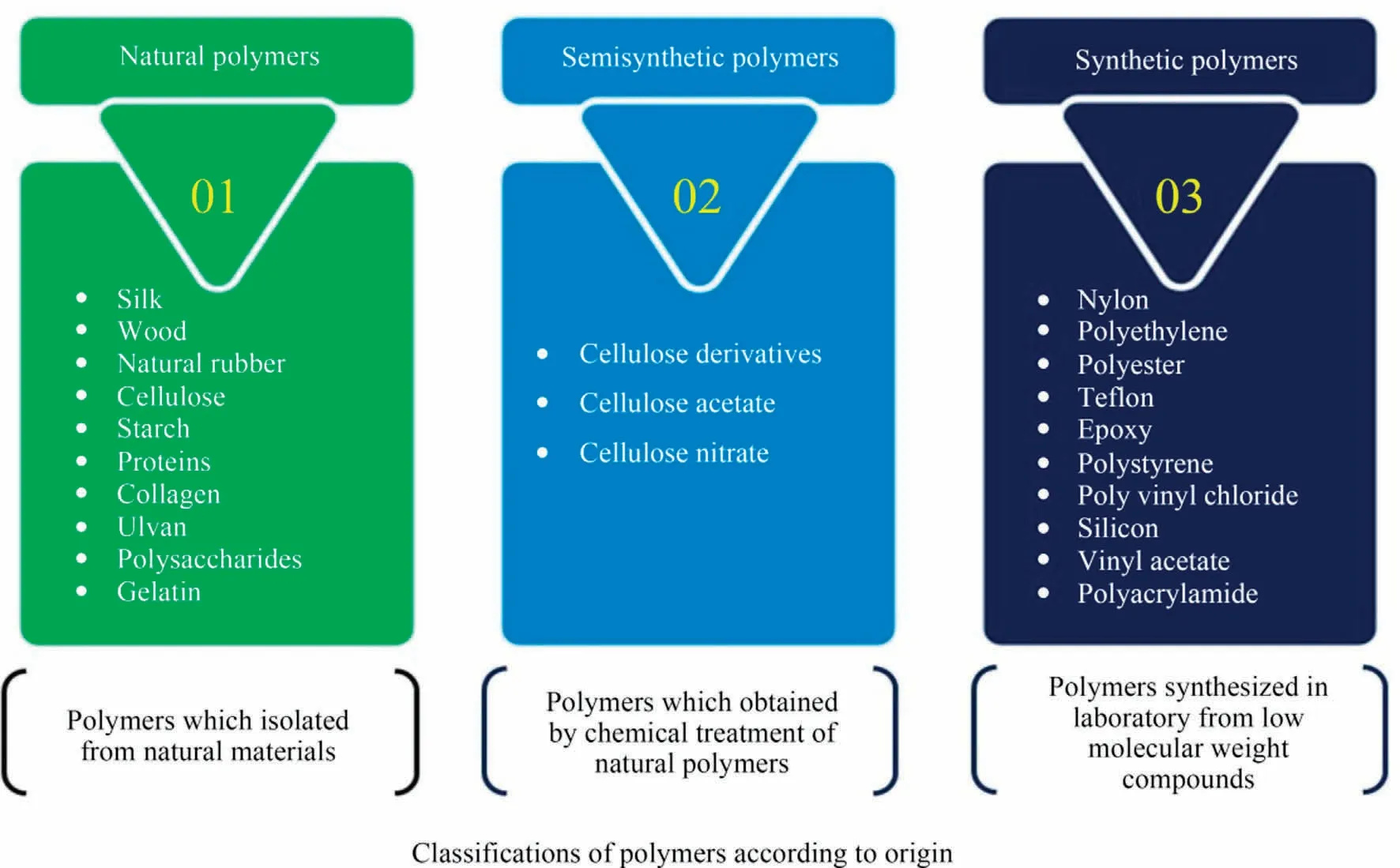
Fig.3.The classification of biopolymers according to their origin.
Natural biopolymers are categorized into three categories based on the monomeric unit present and the structure (i) polynucleotides, (ii) polypeptides (proteins), and (iii) polysaccharides[27,28].Nucleic acid is a generic biological material made up of a huge number of molecules in a certain order.These are polymers in which nucleotides serve as monomer units.Deoxyribonucleicacid(DNA)and ribonucleic acid(RNA)are the two types of nucleic acids.These are linear polymers with smaller molecules(monomer units) connected sequentially.Polynucleotides are made up of many nucleotides that are connected linearly by covalent bonds[29].A nucleotide molecule is made up of three separate molecules: a five-carbon sugar, a phosphate group, and a nitrogenous base.A nucleotide monomer in DNA and RNA polynucleotide biopolymers has a phosphate group, which is connected to the sugar of the next nucleotide monomer,forming a chain with a regular sugar-phosphate group.Proteins can be extracted from a wide range of materials such as wool,leather, silk, gelatin, and collagen[28,30].Proteins come in a variety of shapes and sizes due to differences in the sequence of amino acids.Immune responses, cell adhesion, and cell communication are all important functions of proteins in living beings.Polysaccharides are long chains (linear or branched) of monosaccharide units bound together by glycosidic bonds that, when hydrolyzed, produce monosaccharides or oligosaccharides.Polysaccharides include storage polysaccharides like starch and glycogen,as well as structural polysaccharides like cellulose and chitin [31].
Semi-synthetic polymers come from natural sources but are chemically altered to make them more useful.Natural polymers have greater benefits than synthetic ones due to their biodegradability, and lower toxicity.As a result, semi-synthetic polymers are those that are both produced synthetically and naturally.Typically, cellulose, a polymer found in nature, serves as the starting point for semi-synthetic polymers.Thermoplastic polymers are another name for semi-synthetic polymers.cellulose acetate can be produced through acetylation using acetic anhydride and sulfuric acid.
Synthetic polymers usually created chemically from biomass,such as polylactic acid (PLA), and PBPs such as polycaprolactone(PCL), polyglutamic acid (PGA), polyvinyl alcohol (PVA), polyamides, polyethylene, and polystyrene.Polyhydroxyalkanoates(PHAs), poly(hydroxybutyrate-co-valerate) (PHBV), polyhydroxybutyrate (PHB), curdlan, dextran, gellan, levan, and xanthan[32,33].
Biopolymers are mostly composed of polysaccharides,proteins,and nucleic acids derived from plants and bacteria.Collagen, elastin,albumin,fibrin,gluten,and soy proteins are all commonly utilized proteins.Alginic acid, chitin, chitosan, cellulose, starch,hyaluronic acid, and pectin are examples of polysaccharides.The most common synthetic biopolymers are polyethylene, polyamides,polylactic acid,polystyrene,polycaprolactone,and polyglutamic acid.Bacterial cellulose, bioplastics, dextran, pullulan,xanthan, and microbial polysaccharides (i.e., exopolysaccharides)are examples of biologically generated polymers employed in different environmental and industrial applications such as biosensors, wastewater remediation, cosmetics, clothing fabrics, food additives, packaging materials, industrial plastics, and in gas hydrate applications [34-37].
2.4.Structure of biopolymers
Biopolymers are remarkable in their unique properties such as degradability, hydrophilicity, chirality, and chemical modifying ability.The study of biopolymer chemistry and structure aids in the development of films, coatings, and membranes.Fig.4 reports the chemical structures of some common biopolymers.Comprehensively, the following section of this review will provide a systematic description of some examples of frequently used biopolymers in different applications as follow.
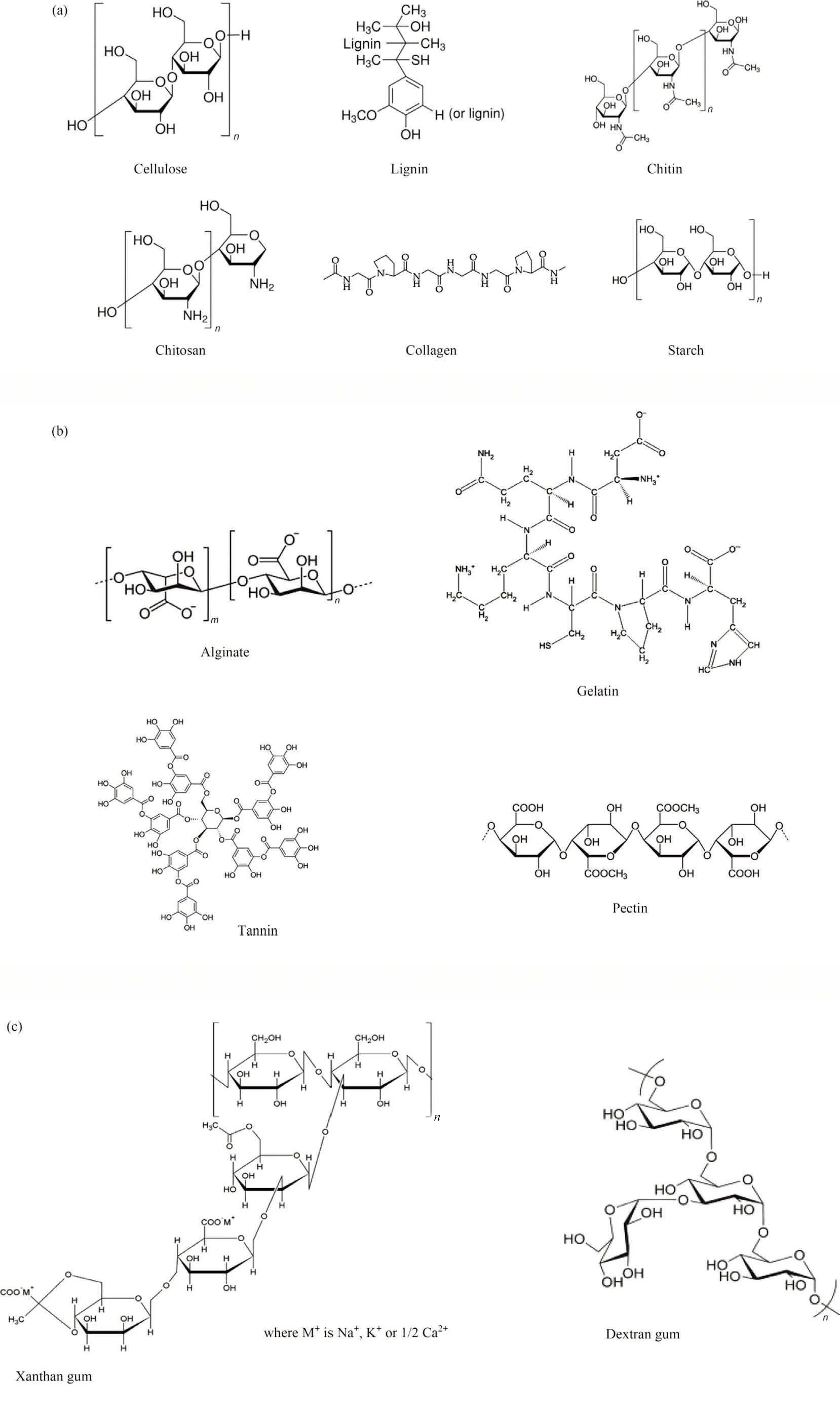
Fig.4.The chemical structures of some common biopolymers.
2.4.1.Cellulose
Cellulose (C6H10O5)n, is one of the most ubiquitous organic biopolymers in the world.It accounts for approximately 33% of all plant materials.Wood and cotton contain 50% and 90% of cellulosic-based matter,respectively[38].It is odorless,biodegradable, hydrophilic, and water insoluble.It is made up of glucose monomers linked together by β-(1 →4)glycosidic bonds.Glucopyranose rings are arranged in the chair conformation.The repeated β-(1 →4) D-glucose bonds are found in plant sources such as stalks, reeds, grasses, and woody sections of vegetables [39].The molecular weight of the cellulose unit is 162.14 g·mol-1.The degree of polymerization in cellulose ranges between 10000 and 15000 glucopyranose units.Cellulose and its derivatives can be used as re-generable, biodegradable, and low-cost adsorbents to remove various water pollutants from aqueous solutions [40].
2.4.2.Lignin
Lignin (C81H92O28), is a cross-linked phenolic natural carbohydrate biopolymer, mainly composed of three constituent monomers, p-hydroxyphenyl (4-hydroxyphenyl, H), guaiacyl (4-hydroxy-3-methoxyphenyl, G), and syringyl (4-hydroxy-3,5-dimethoxyphenyl, S), arranged in a hyper branched topology with no regular repeating structure[41].It is incorporated in lignocellulose biomass and occurs as a three-dimensional networking structure with functional groups such as carboxyl, phenolic hydroxyl,methoxy, and aliphatic groups.Lignin functions as a cellular glue substance, protecting and connecting cellulose and hemicellulose.It is a component of vascular plant tissue that provides mechanical support to plant tissue and individual fibers, as well as physical strength and stiffness to the biomass cell structure [42].The amount of lignin contained in pristine resources strongly varies with the origin of the material.For example,high relative fractions of lignin were reported in three types of biomasses: softwood(25%-30%, containing G units), hardwood (16%-24%, containing G&S units),and grasses(13%-18%,containing G,S,&H units).Lignin is typically derived from black liquor and paper mills [43].Based on its high fuel value,it has been reported that about 10%of lignin waste is used for fueling applications.Besides, it is utilized in a variety of applications such as dispersants, plasticizers, adhesives,and adsorbents [44,45].
2.4.3.Chitin
Chitin(C8H13O5N)n,is a linear chain polysaccharide made up of β-(1 →4)-2-acetamido-2-deoxy-D-glucose.Chitin, which is collected at an industrial scale from the shells of crustaceans, is the most abundant polysaccharide after cellulose [46].Depending on its origins, chitin can be found in three forms: α-chitin, β-chitin,and γ-chitin.In α-chitin, the sugar chains run antiparallel to each other with reducing groups on the opposite sides, whereas βchitin sugar chains run parallel with reducing groups on the same sides.Two sugar chains run parallel and one sugar chain runs antiparallel in γ-chitin[47].The chitin unit has a molecular weight of 203.19 g·mol-1.Chitin is reported as an adsorbent for the removal of different water pollutants.Differently, chitin is not as effective as chitosan as an adsorbent for water pollutants due to its poor solubility and dispersion in water and other solvents,and to the weaker reactivity of acetylated amine groups(compared with free amine groups).Different modification pathways with protonation, carboxylation, and grafting can be introduced to chitin for enhancing adsorption capacities [48-50].
2.4.4.Chitosan
Chitosan (C6H11NO4)n, is a natural amino-polysaccharide heteropolymer,that naturally occurs in crustaceans,insects,fungi,and arthropods, and it can be synthesized from fish waste.It is derived from the shell and cuttlebone of a lobster, shrimp, or crab skeleton.It is composed primarily of D-glucosamine and a trace of N-acetyl-D-glucosamine residues.Partial deacetylation of chitin yields this biopolymer[51].Because of its non-toxicity,biodegradability, biocompatibility, bioactivity, unique structure, and relatively good physical and mechanical performance, as well as high contents of hydroxyl and amine functional groups, it has been widely used in a wide range of applications in biomedical and industrial fields [52,53].These protonated amino groups have pKavalues ranging from 6.2 to 7.0 in their linear polymeric chains,affecting their solubility, reactivity, absorptivity, and biodegradability[54].Considering the plethora of amino and hydroxyl functional groups in the chitosan backbone,it has been widely used for adsorption as a cationic biopolymer [55].
2.4.5.Collagen
Collagen (C57H91N19O16), represents structural animal protein made up of amino acids that are linked together to form a collagen helix, It can be easily recovered from the skin, cartilage, tendon,and other connective tissues of domestic animals [56].It is the most prevalent protein in the body, accounting for approximately one-third of total protein content.It is a significant component of bones,skin,muscles,tendons,and ligaments and can also be found in many other regions of the body, such as blood vessels, corneas,and teeth [57]. Collagen has a molecular weight of ~300000 g·mol-1[58].Based on the profusion of amino groups in its structure,the material provides suitable binding sites,which work through the chelation mechanism; collagen-based materials are useful for removing pollutants from aqueous solutions [59].However, the weak physical characteristics of collagen as a biopolymer for pollutant removal from the aqueous phase limit its practical applicability [60].
2.4.6.Starch
Starch (C6H10O5)n, is a readily available, low-cost, and biodegradable biopolymer derived from renewable agriculture resources.It is found in nature and is frequently connected with plant-based biomass[61].This polysaccharide’s monomer is based on glucose,linked by α-(1 →4)links in amylose or α-(1 →6)links for branched structures (as in amylopectine).Amylose is a linear molecule containing glucopyranosyl units connected by α-(1 →4)glycosidic connections,whereas amylopectin has glucopyranosyl units connected by α-(1 →4) and α-(1 →6) glycosidic links.The starch unit has a molecular weight of 300 to 400 g·mol-1.Large amounts of starch are utilized in the textile sector,where it is used to strengthen the thread during the weaving process[62].As a natural derivative of plant-based biomass, starch has been studied for the production of bioplastics.Starch contains many hydroxyl groups, which can be used to make hydrogels.Etherification and grafting are two chemical alteration strategies that could be used to make hydrogels [63].
2.4.7.Alginate
Alginate (C6H8O6)n, is a linear copolymer composed of mannuronic acid (M) and guluronic acid (G) subunits that is abundant in brown algae and certain bacteria.It is negatively charged in aqueous conditions at pH >3.4 due to the presence of carboxyl functional groups in both G and M subunits, where the carboxyl functional groups tend to be deprotonated (alginic acid form prevailing at low pH).Kinetics studies revealed that dry alginate has a poorer adsorption capacity in general due to its low porosity,loose network, poor water resistance, and low diffusion of water pollutants[64].As a result,it is common to use the same material in a porous form(such as an aerogel)to boost its adsorption capacity.Adsorption and ion exchange are the two major ways by which water pollutants are bound by alginate.The porous structure of ionotropic gels of metal alginates is critical for pollutant adsorption from wastewater.Throughout the gelation process,calcium cations cooperatively interact with blocks of M and G residues,resulting in the production of ionic crosslinks between distinct polymer chains.Heavy metals such as copper can be used to replace calcium cations.In addition, alginate has mostly been employed as an immobilizing agent for microorganisms and enzyme systems [65].
2.4.8.Gelatin
Gelatin (C102H151N31O39), is a water-soluble protein formed by the hydrolysis of collagen,18 types of essential amino acids,which are part of a regular diet.It is a protein that has a lot of side chains and functional properties.It can be found in pork/cattle bones,bovine hide, and pigskin with extents as high as 23.1%, 29.4%,and 46%, respectively.Charged groups in the protein side chains,as well as specific sections of the collagen sequence containing either hydrophilic or hydrophobic amino acids, are present on the surface of collagen and gelatin [66].Based on compositions and synthesis methods, there are two forms of gelatin: Type-A and Type-B.The first type is generated via acidic hydrolysis of raw collagen in the presence of acid.It is made up of nitrogen(18.5%).Whereas amide groups (Type-B) are produced from the alkaline hydrolysis of collagen that includes 18% nitrogen atoms with no amide group (—CONH—).Simultaneously, it is a temperature-sensitive biopolymer at low and high-temperature ranges.When heated at high temperatures,it melts and coils.However, at lower temperatures, it forms a coil-to-helix structure.Because of the distinct sequence of amino acids, high contents of glycine, proline, and hydroxyproline have been widely employed in culinary and pharmaceutical industries,as well as in the manufacture of materials (cosmetics, laundry, and cleaning agents,papers, and match heads) [67].
2.4.9.Tannin
Tannin (C76H52O46) derivatives, also considered natural polyelectrolytes, represent important constituents of higher plants,with a high molecular weight of 1701.19 g·mol-1.This biopolymer is found in plant barks, roots, stalks, and fruits.It is a natural polyphenol that contains numerous phenolic hydroxyls, which have a high affinity for water pollutants.Tannins bear numerous aromatic rings that can serve as a matrix for grafting additional active groups through polymerization reactions [68].Vegetable tannins are made up of two types of chemical molecules, both of which are phenolic: condensed tannins and hydrolyzable tannins.It is a water-soluble substance and as a result, tannin separation following adsorption is a difficult process.One interesting approach to overcoming this limitation is to immobilize tannin on various matrices such as agarose and cellulose for the development of water-insoluble adsorbents[69].It is frequently utilized in water and wastewater treatment and is thought to have significant flocculant potential due to the presence of amino groups in its structure.Tannin-based flocculants were also found to be effective in marine microalgae harvesting for replacing inorganic flocculants[70,71].
2.4.10.Pectin
Pectin (C6H10O7), is a linear heteropolymer that is found in abundance in the cell walls of all land plants.It is a plant-derived anionic biopolymer with a variety of properties.It is extracted from plant cell walls to a range of 0.1%-30%depending on the method of extraction, the nature of the plant (and its growing stage).It is made up of α-(1 →4) linked D-galacturonic acid (GaIA) residues.The peel and pulp of various fruits have been used for pectin extraction, although passion fruit, grapefruit, soy husk, mango,citrus fruits, pumpkin, apple, and carrot show remarkably high contents [72].Pectin is characterized by exceptional properties,such as flexibility, biodegradability, non-toxicity, and availability of OH groups,that make it an attractive option for many scientific operations.However, when used in certain industries, pectin has some intrinsic limitations.Given the quick hydration, dissolving,and destructuration of pectin (due to its high-water solubility),and the ready inclination of the biopolymer to form lumps and to agglomerate,this limits the capacity to manage and operate pectin solutions.Pectin was extensively investigated for catalytic systems because it is a green and effective support for metal catalysts[73].Pectin’s backbone contains several single —OH and —COOH functional groups.Some single —COOH groups are naturally present as methyl esters,while others can be commercially processed with ammonia to form carboxamide groups.Because of the existence of these functional groups, pectin can form complexes with metal ions or reduce them to generate metal nanoparticles without the use of a chemical reductant or stabilizer [73].
2.4.11.Xanthan gum
Gums are hydrophilic polysaccharides, yet they rarely dissolve in organic solvents.In addition, they are non-toxic, odorless, and tasteless.Because of their intermolecular interactions and molecular weights, they cannot easily create a transparent solution [74].The gum biopolymer can be manufactured from different sources,including animals, plants, fungi, and other microbial sources.Nonetheless, plant-based ones are widely employed in all aspects of life.It is important to understand that the physicochemical properties of plant gums can be influenced by their chemical structure and extraction conditions.Microbial polysaccharides, also known as exo-polysaccharides, on the other hand, are made up of simple sugar units such as mannose, glucose, and fructose.Following dextran,xanthan gum(C35H49O29)with a molecular weight of 933.75 g·mol-1is the second microbial polysaccharide the most developed for commercial application.It is an extracellular heteropolysaccharide that is mostly generated by Xanthomonas campestris during the aerobic fermentation of corn[75,76].Because of its fantastic properties such as non-toxicity, cell development ability, and non-sensitizing effect, xanthan gum is regarded as an essentially natural and industrial biopolymer.The abundance of functional groups such as hydroxyl and carboxyl present on its skeleton, results in excellent adsorption of different water pollutants, via a chelation mechanism [77].This biopolymer can be associated with other polymers or biopolymers for preparing functionalized composite sorbents [78,79].
2.4.12.Keratin
Keratin is a plentiful inedible protein found in hair,horns,wool,nails, and feathers.Keratin’s polypeptide structure is made up of numerous amino acids,each having an identifiable side chain with a unique chemical structure, bonding capability, charge, and reactivity [80].These side chains are mostly involved in adsorption processes and do not contribute to polypeptide synthesis.Keratin can be utilized as an adsorbent because it contains several functional groups that can bind water pollutants.These chains can have diverse functions for eliminating impurities from wastewater by using different chemical treatments.One notable example is the occurrence of disulfide (—S—S—) bridges (cysteine-cysteine cross-links) from cysteine molecules,which account for 7% of keratin.Chemical treatment can break the cross-linked disulfide bonds,exposing free functional groups with a significant potential for trace element adsorption [8].
2.4.13.Dextran
Dextran (C18H32O16), is a water-soluble, biocompatible, and biodegradable biopolymer made up of α-(1 → 6)-linked D-glucopyranose residues with some α-(1 →2)-, α-(1 →3)-, or α-(1 →4)-linked side chains[81].Because of the presence of pendant hydroxyl functional groups in its backbone, dextran can be easily modified.The dextran-producing bacterial strain determines the degree and character of branching units in positions 2,3,and 4.Dextran is primarily synthesized extracellularly from sucrose by various lactic acid bacteria using dextransucrase, which catalyzes the transfer of D-glucopyranosyl residues from sucrose to dextran[82].
3.Biopolymer-based Composites
Because of their renewability and eco-friendliness in terms of biodegradability, biopolymers have sparked significant interest in the fabrication of products that have value in today’s globalizing market.Today’s research facilities have exhibited significant control in cultivating materials based on the aforementioned segments of qualities in order to generate innovative matter with the necessary degree and blend of functional characteristics [83].This rapid advancement has created opportunities for biopolymers to act as a viable alternative to PBPs,which are synthetic and damaging to the environment.However, this may not be the case in several manufacturing industries that require stronger mechanical and stability properties than those exhibited by natural biopolymers.To counteract this loss of usefulness while remaining environmentally friendly, a potential method for improving the quality and contribution of biopolymers has been developed by reinforcing the matrix of the biopolymer [4] through cross-linking.This results in a petroleum-based and environmentally friendly competitor that may be configured as biopolymer composites.
Polymer composites are composed of two or more materials in order to create a single component with the desired characteristics.In general, polymer composites are classified into three types: (i)particle-reinforced composites, which have a strong matrix relationship between two suspended materials, (ii) structural/sandwich composite structures of two distinct materials, whose layers are combined to form a resilient bond, and (iii) fiber-reinforced composites, which have long fibers with different materials are embedded to each other [84].
4.Synthesis and Characterization Techniques
Biopolymers are synthesized using a variety of technologies and techniques.Fermentation or polymerization of monomers can be used throughout their synthesis.Because the majority of these polymers either exist in nature or are created by natural organisms, these methods are frequently employed followed by chemical modifications [85].Microbial biopolymers are produced by fermentation employing microorganisms with specific C,N,minerals, and salts as sources.Their production is primarily associated with mechanisms of storage (energy, metabolites) and protection against external factors.Monomeric units, which can be sensitive to microbes or enzymes,etc.,can be chemically reinforced by polymerization.Polymerization of monomers, solvent-based extraction, fermentation, exo-biopolymer production, endo-biopolymer production, and bulk synthesis of biopolymers are the most common ways of the preparation of biopolymers [11].
During the production of biopolymer composites, diverse combinations of natural fibers and biopolymers are used.The resultant biopolymers were more potential than biopolymer composites incorporated with metal-based fillers or nanofillers.Because of the low interfacial adherence of the fiber and polymer matrix, a negative effect on the physical and mechanical properties may be observed;however,such limitations may be overcome by the addition of coupling agents or plasticizers,which improve the adhesion and other properties of the composites(stiffness,mechanical resistance, etc.) [11].Proper coupling agent and fiber treatment promote polymer adhesion and aim to improve overall fiberpolymer performance.Despite their high biological and biodegradable qualities, biopolymers lack some mechanical properties such as low chemical resistance, processing capacity, and storage duration[32].These issues are easily solved by combining biopolymers with natural or synthetic fillers of varying sizes in biopolymer composites.Some of the main polymer materials used in the production of biopolymer composites include cellulose esters,starch-based polymers, and polylactic acid [86].A variety of scenarios including grafting [87], molding [88], extrusion/injection[89], pultrusion [90], compression molding [91], infusion molding[92], intercalation [93], phase separation [85], filament winding[94], melt blending [95], solvent casting [96], electrospinning[97],and film stacking[98]have been conceived as the most prevalent ways for manufacturing biopolymers composites from natural and synthetic sources.
Characterization techniques are particularly important for biopolymers-related studies since they supply the information needed to understand and determine the physicochemical properties of produced products (i.e., mechanical, thermal, physical,chemical, and optical).Because numerous procedures provide us with diverse forms of information, relying on a single strategy would be ineffective [31].As a result, it is critical to characterize the resultant biopolymers with combined techniques.Once the characterization was employed, the characters were inspected in agreement with the American Society for Testing and Materials(ASTM) norms for their biodegradability, and eco-friendliness to meet the set quality standards.Some of the common characterization methods used widely for the characterization of the synthesized biopolymer and biopolymer composites are displayed as follows [99].Fourier transform infrared spectroscopy (FTIR) is utilized to detect the presence of various functional groups in a material [100].Dynamic light scattering (DLS) directly measures the translational diffusion coefficient from which the hydrodynamic radius of a molecule is derived [101].Scanning electron microscopy(SEM)is a form of electron microscope that generates images of a sample by scanning it with a focused stream of electrons[102].Electrons interact with atoms in the sample,producing a variety of signals that carry information about the surface topography and composition of the sample.Transmission electron microscopy(TEM)visualizes specimens and provides a highly magnified image by using an electron particle beam.TEM can magnify objects up to 2 million times.X-ray diffraction (XRD) is a nondestructive technique that provides detailed information about the crystallographic structure, chemical composition, and physical properties of materials [103].Energy-dispersive X-ray spectroscopy (EDX) is a surface analytical technique where an electron beam hits the sample, exciting an electron in an inner shell, causing its ejection and the formation of an electron-hole in the electronic structure of the element.Atomic absorption spectroscopy (AAS) is a spectro-analytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation(light)by free atoms in the gaseous state[104].X-ray fluorescence(XRF)is the emission of characteristic‘‘secondary”(or fluorescent)X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays.The phenomenon is widely used for elemental analysis and chemical analysis,particularly in the investigation of materials.Atomic force microscopy(AFM) is a very-high-resolution type, with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the optical diffraction limit [105].
5.Application of Biopolymers and Their Derivatives in Water and Wastewater Treatment
The demand for water, food and agricultural products, and other natural resources has expanded dramatically as a result of industrialization, urbanization, and population growth.Climate change, overuse and depletion of water supplies, and anthropogenic water pollution have prompted worries about the world’s ability to provide clean and useable water[106].Water contamination has become a public issue,threatening ecosystems and human life security.Dumping of wastewater from industrial, agricultural,and municipal sources has significant environmental consequences, including excessive nutrient and oxygen depletion in water, which can negatively affect human health, wildlife, and aquatic life.The sources of wastewater can be classified based on the point at which they are released.Domestic(including commercial institutions),industrial(i.e.pharmaceutical and petrochemical industries),infiltration,and storm waters are the four primary categories[107].According to the United Nations World Water Development Report (UNWW DR) published in 2017, about 56% of the freshwater used in municipal, industrial, and agricultural applications is discharged as wastewater and 80% of the global wastewater is dumped into the environment without proper treatment.Moreover, approximately 95% of the wastewater generated in some developing countries is released into the ecosystem with suitable remediation [108].Water quality is critical for human health, economic prosperity, and long-term environmental processes.Water availability is relied not only on the number of available water sources but also on the quality of the water.The contaminants in wastewater differ depending on the source from which it is released.Despite all of the concerns about wastewater,it can be converted into a safe and sustainable supply of not only water but also useful by-products if managed properly and intelligently[109,110].The important aspects that must be conducted in an enhanced wastewater management system are pollution prevention at the source,wastewater flow minimization,regeneration of useable water, and recovery of beneficial by-products such as organic matter, nutrients, and so on.To support management and investment in sophisticated pollution control and wastewater treatment technologies, an effective legislative and regulatory framework is also required.Appropriate social education and increased community knowledge are also essential in emphasizing the importance of a comprehensive wastewater management system.The development of sophisticated wastewater treatment systems contributes to long-term economic performance by boosting water reuse, decreasing water resource loss, and providing energy and other byproducts [111].Depending on the quality of the treated effluent,it is possible to direct the water flow to different uses,such as irrigation, and domestic [112].Effective wastewater treatment system management and water pollution prevention require an appropriate policy framework and cost-effective technologies.The volume of wastewater, the nature of pollution, and the planned application of clean water, all influence the choice of acceptable and inexpensive technology.The traditional method of water treatment entails a series of steps such as preliminary,primary, and secondary treatment.The key processes in this treatment process are coagulation and flocculation, which are used to remove big coarse solid contaminants such as suspended particles[113].Different traditional physicochemical methods such as chemical precipitation [114], simple/chemical oxidation [115],ozonation [116], adsorption [117], and ion exchange [118], have been employed for wastewater treatment.Recently, biopolymers have proven to be a promising practical option,as environmentally friendly, biocompatible, and sustainable materials.In the modern era,such materials are commonly used for environmental cleanup.They can be applied in different forms (i.e., powder, beads, and composites).They remarkably reduce alkalinity, chemical oxygen demand (COD), biochemical oxygen demand (BOD), total suspended solids (TSS), total dissolved solids (TDS), and Natural Organic Matter (NOM) up to 90% from the upstream effluents.Moreover, they may actively capture wide varieties of water contaminants such as inorganic and organic pollutants from aquatic systems[119,120].Effluent treatment is mostly employed to meet the environmental regulatory criteria for discharged wastewater from the industrial sector.The next section highlights the recent advances in the different applications of biopolymers and their composites for wastewater reclamation.
5.1.Application of biopolymers in the coagulation and flocculation process
Coagulation-flocculation is widely regarded as an efficient and cost-effective method for the removal of NOM compared with other alternative scenarios.Coagulation is a chemical water treatment technique that involves altering the electrostatic charges of particles suspended in water to remove solids.Fig.5 shows the electric double-layer structure of the coagulation process.Small,highly charged molecules are introduced into water to disrupt the charges on particles,colloids,or greasy materials in suspension[12].Choosing the correct coagulant for a system improves overall system performance and,in particular,solids removal efficiency by improving filter and clarifier performance.Coagulation reactions are used in a variety of wastewater treatment applications,including removing colloidal particles from water,demulsifying oil emulsions,and paint detackification.Coagulants of various varieties are also available to satisfy the unique needs of a treatment process.In a chemical water treatment procedure, coagulation usually comes before flocculation [121].The use of polymers in water treatment offers various advantages,including increased drinking water output, increased filter shelf life, easier removal of dissolved organic matter,and self-contained pH and conductivity that do not change during the water clarification process.Natural anionic polymers such as heparin can be used to substitute synthetic polymers by bridging the broken coagulant ions with the particles via the addition of primary coagulants.Some natural neutral polymers,such as starch,cellulose,gelatins,glues,and microbial polysaccharides,can be commercialized as coagulants to reduce sludge completely and improve phase separations[122-124].These natural polymers can be used with air (Fig.6(a)) or without air (Fig.6(b)).
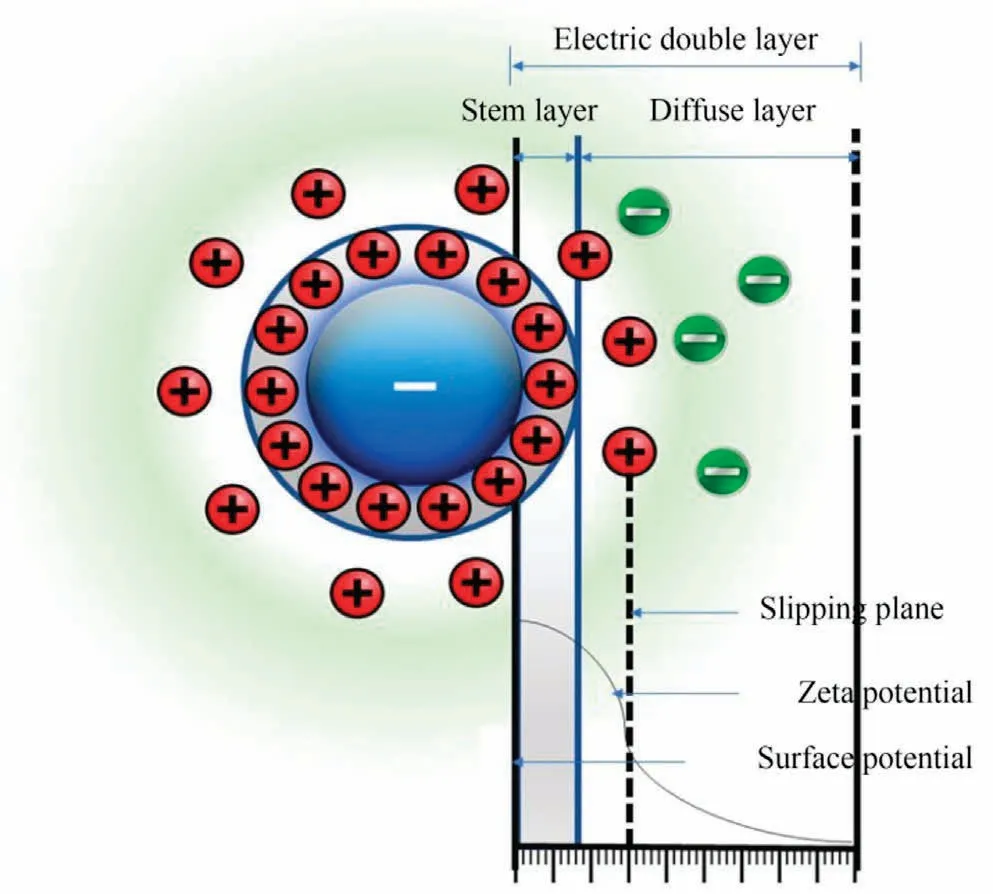
Fig.5.The electric double-layer structure of the coagulation process.
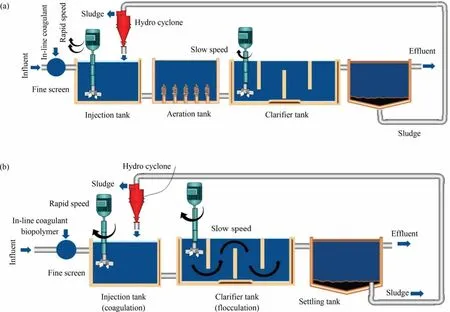
Fig.6.Coagulant use (a) with aeration, and (b) without aeration methods.
The flocculation process is used to promote colloidal suspension solid-liquid separation (Fig.7).It has the advantages of a low investment cost, high treatment efficiency, and ease of use.It can efficiently treat a variety of water pollution issues, including turbidity,dyes,heavy metal ions,other industrial/agricultural wastes,algae, and sludge dewatering.As a result, it can be described as a very cost-effective and efficient water treatment process [125].A flocculant is a water treatment agent.It can combine colloidal and particulate materials suspended in liquid to create bigger flocs,and then encourage the settlement of these particles from the stable suspension.Flocculants are classified into two types based on their chemical composition: inorganic coagulants and organic flocculants.Inorganic coagulants,such as iron salts and aluminum salts, are inexpensive, but their effectiveness is highly reliant on different operational parameters such as temperature, pH, and other factors [126].Furthermore, for flocculation based on inorganic coagulants,the dosage is enormous,and the types of sewage that are appropriate are limited.Moreover, they may result in the occurrence of elevated metal content in the treated water body,endangering human health.The features of flocculants, which are the core of the flocculation process,directly influence the efficiency of the water treatment pathway.Traditional chemical flocculants such as polyaluminium-chloride (PAC) have been employed to remove COD, SS, and aluminium (Al3+) from polluted water.Besides,organic flocculants are classified as synthetic organic flocculants and biopolymer-based flocculants.Synthetic organic flocculants have high effectiveness at low dosages but have low biodegradability[127].Biopolymer-based flocculants are materials derived from long-chain molecules produced by living creatures’cells,as well as materials created from these long-chain molecules(bio-based monomers).They present several excellent qualities,including a wide range of adjustable pH, ease of modification and processing, environmental friendliness (low toxicity/non-toxicity,simple biodegradability), minimal dose requirements, and good flocculation efficacy [128,129].The physicochemical properties of biopolymer-based flocculants influence their flocculation efficiency.These properties are obtained through the diverse implemented modification scenarios of graft copolymerization(i.e.,thermal initiation, plasma initiation, microwave initiation, and radiation initiation),etherification,amination,esterification,acylation, oxidation, and cross-linking [130,131].As a result,biopolymer-based flocculants have the potential for economic scale-up, making them likely to be widely used in the future.Table 1 displays different biopolymers and its synthesized composites as coagulants and flocculants for water treatment.
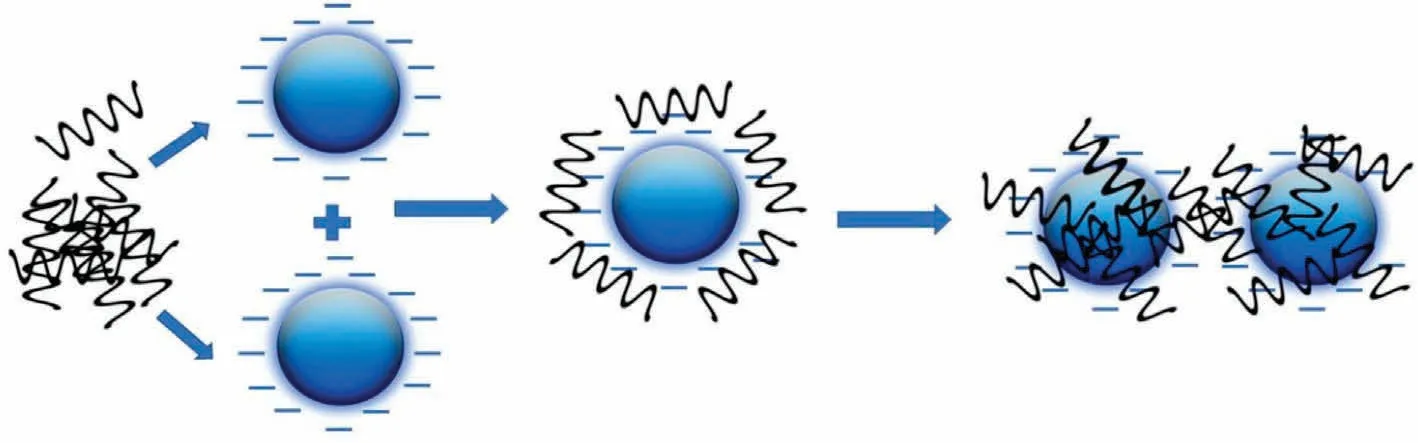
Fig.7.The flocculation process using biopolymers.
Hou et al.[144] designed a coagulant by functionalization of chitosan (grafting poly(N-n-propylacrylamide), PNNPAM).They characterized its thermoresponsive properties in terms of simultaneous coagulation-flocculation of heavy metal ions and antibiotics.They observed the remarkable correlation between the efficiency of the combined flocculation and the strength of the interaction between the antibiotic and the metal.They also designed a treatment strategy for efficient decontamination with a two-step procedure playing with the optimization of parameters such as the length of the PNNPAM branch,flocculant dosage(and storing temperature of the flocculant).They also demonstrated the feasibility of flocculant regeneration for improved competitiveness of the process.
Another strategy suggested for improving the removal of antibiotics by coagulation-flocculation (as a pre-treatment before ultrafiltration) consisted of converting chitosan into hydrophobic derivatives [145].The grafting of polyacrylpiperidine (PNAPD)branches of different chain lengths demonstrated that the positive charge of the polymer derivative increases with the length of PNAPD chain, which, in turn, facilitated the charge neutralization effect of the flocculant.This length also affected positively the hydrophobic nature of the derivative (as measured by the contact angle).In river water, which contains also kaolin and humic acid,the efficiency in the removal of antibiotics was enhanced due to bridging between kaolin and/or humic acid and antibiotics.Notably, the post-treatment by ultrafiltration was facilitated in terms of fouling and permeation rate through the anchoring of PNAPD on the membrane while hydrophilic chitosan chains developed hydrophilic steric repulsive layers (which, in turn, inhibited fouling, and changed the hydraulic resistance through the formation of a structured porous cake barrier).This concept was extrapolated to other types of chitosan derivatives (a series of propylacrylamide-based compounds)[146].The synergistic combination of these hydrophobic derivatives of chitosan with alum improved the coagulation-flocculation of pharmaceuticals and allowed reducing the dosage of the modified biopolymer [147].These properties were modulated by the presence of dissolved organic matter (DOM): the surface charge, the hydrophilic/hydrophobic characteristics, and the molecular weight of these DOM influence the interactions with chitosan derivative.However,in terms of pharmaceutical removal the effect of DOM was only beneficial when hydrophobic groups of DOM reacted with hydrophobic groups of the pharmaceuticals (contrary to the neutral effect of hydrophilic groups).
The quaternization of starch(operated by 3-bromopropyl triphenyl phosphonium bromide, BTP) allowed synthesizing a series of flocculants (with different substitution degrees, DS) developing multi-functionalities[148].Indeed,in addition to the conventional flocculation of microorganisms (E.coli is used as a test) through mechanisms such as charge attraction and polymer bridging, the optimized derivative (DS ≈19%), brought outstanding bactericidal properties (>99.4% for E.coli) with limited membrane fouling(when the treatment was coupled with ultrafiltration process),and lower cytotoxicity (phosphonium-based vs.ammoniumbased compounds).
5.2.Application in the synthesis of materials
Zhao et al.[149]documented an original application of amphoteric starch derivative (bearing cationic quaternary ammonium groups and anionic carboxyl groups) for preparing building materials (ceramsite).After using the starch derivative for dewatering sludge(issued from lake sediment and fresh algal sludge),the combination of flocculation with pressurized filtration allowed the collection of a cake that was further mixed with CaO, fly ash, and kaolin, prilled, and finally sintered to produce highly microporous ceramsite beads.This production is effective (filling the national requirements for ceramsite) and cost-effective.
5.3.Application of biopolymers in the adsorption process
Adsorption is a metabolically inactive process (surface phenomenon), in which the adsorbate species (solute) migrate from the bulk liquid phase to the adsorbent surface.This procedure can be used efficiently to translocate contaminants from wastewater and concentrate them on the adsorbent surface [19].On the other hand, adsorption can be thought of as one of the concentration/enrichment strategies,because contaminants translocate from the bulk liquid phase and concentrate on the adsorbent surface.The concentration gradient between the bulk liquid and solid adsorbent majorly drives the adsorption process and the transport of adsorbate onto adsorbent is entirely mediated by molecular diffusion.The suitability of adsorbents is recognized as the first and most significant step in controlling the selection and preparation of the adsorbent itself [150,151].The cost-effectiveness of assynthesized adsorbent is conceived as one of the most important criteria to be considered during the fabrication process of the adsorbent.Basically, the adsorption process of adsorbate (pollutant)onto the adsorbent’s surface is based on a variety of physicochemical parameters that influence the whole process pathway.The feasibility of adsorbent can be interpreted by using either batch(Fig.8)or dynamic approaches(Fig.9).Most researchers prefer the batch technique of adsorption because it is more efficient as it can be operated in small volumes and simply performed with a constant volume [152].On the other hand, the continuous flow of adsorption necessitates the use of a fixed-bed column (filled with sorbent particles); the design of sorbent particles (size, mor-phology, chemical stability, etc.) may strongly influence hydrodynamic properties and column clogging.Table 2 compares the operational parameters of batch and continuous flow systems throughout the adsorption process [153].
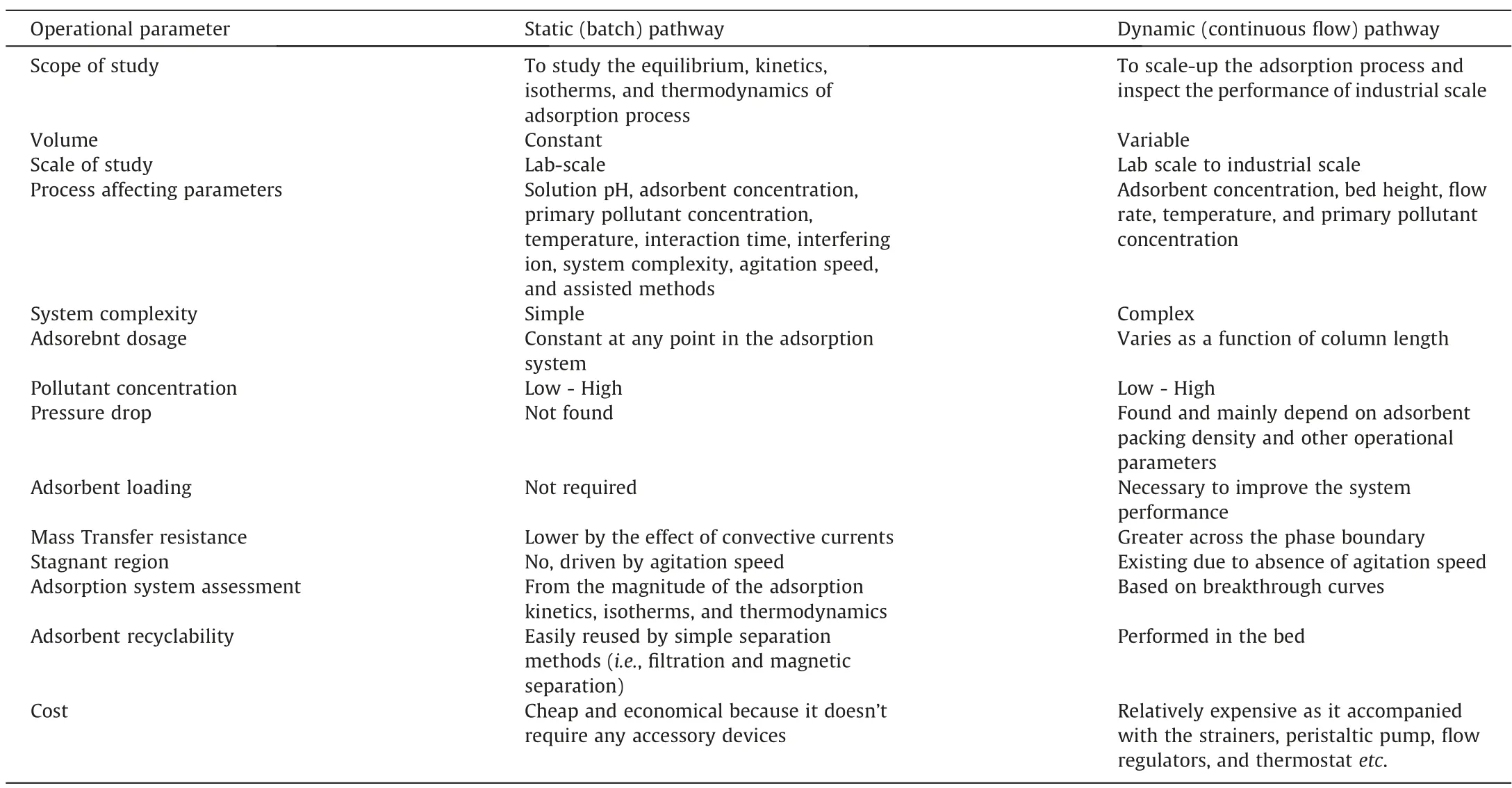
Table 2 Comparison between operational parameters characterized to static (batch) and dynamic (continuous flow) through adsorption process
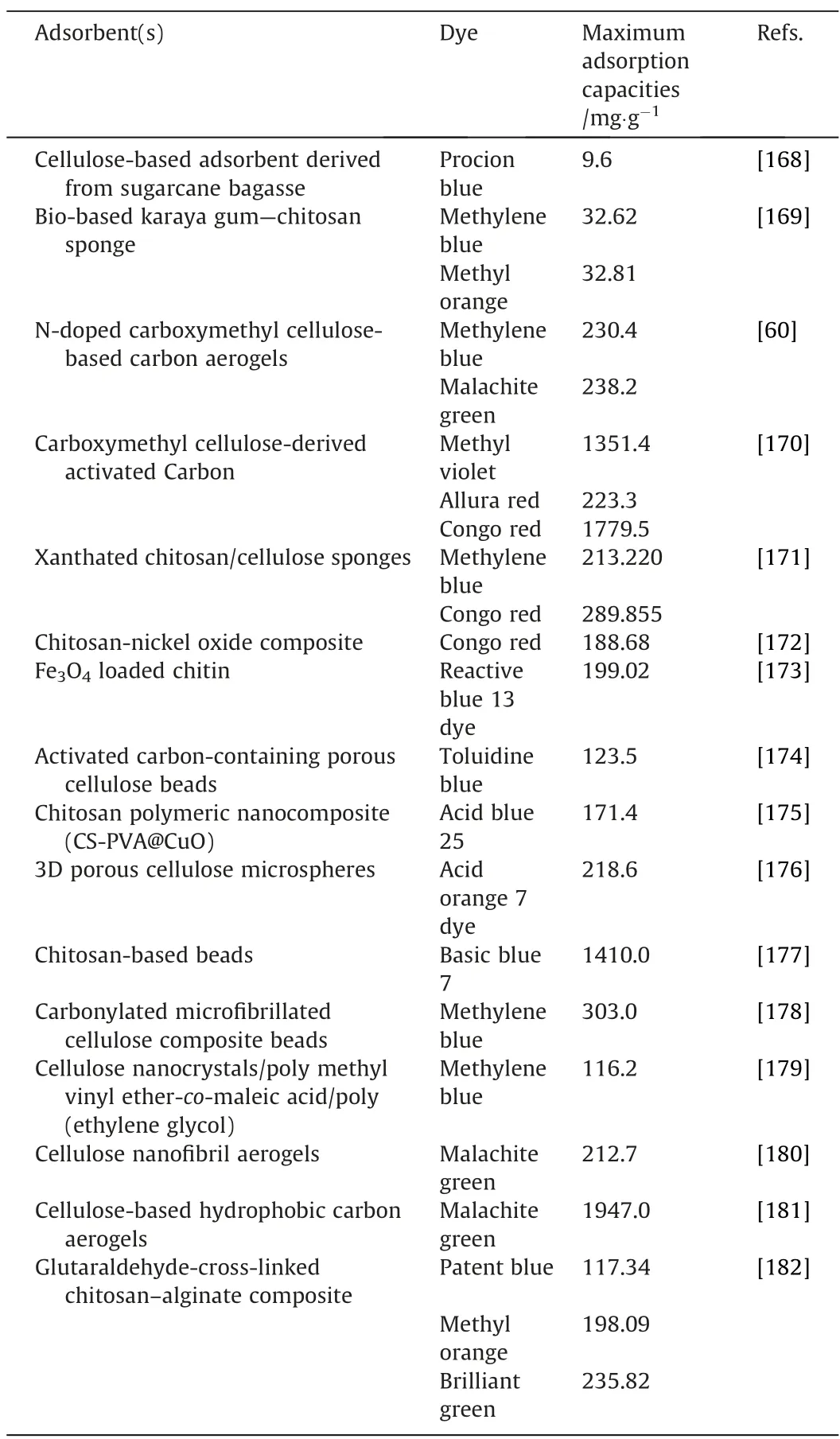
Table 3 Adsorption of organic dyes using biopolymer-based adsorbents
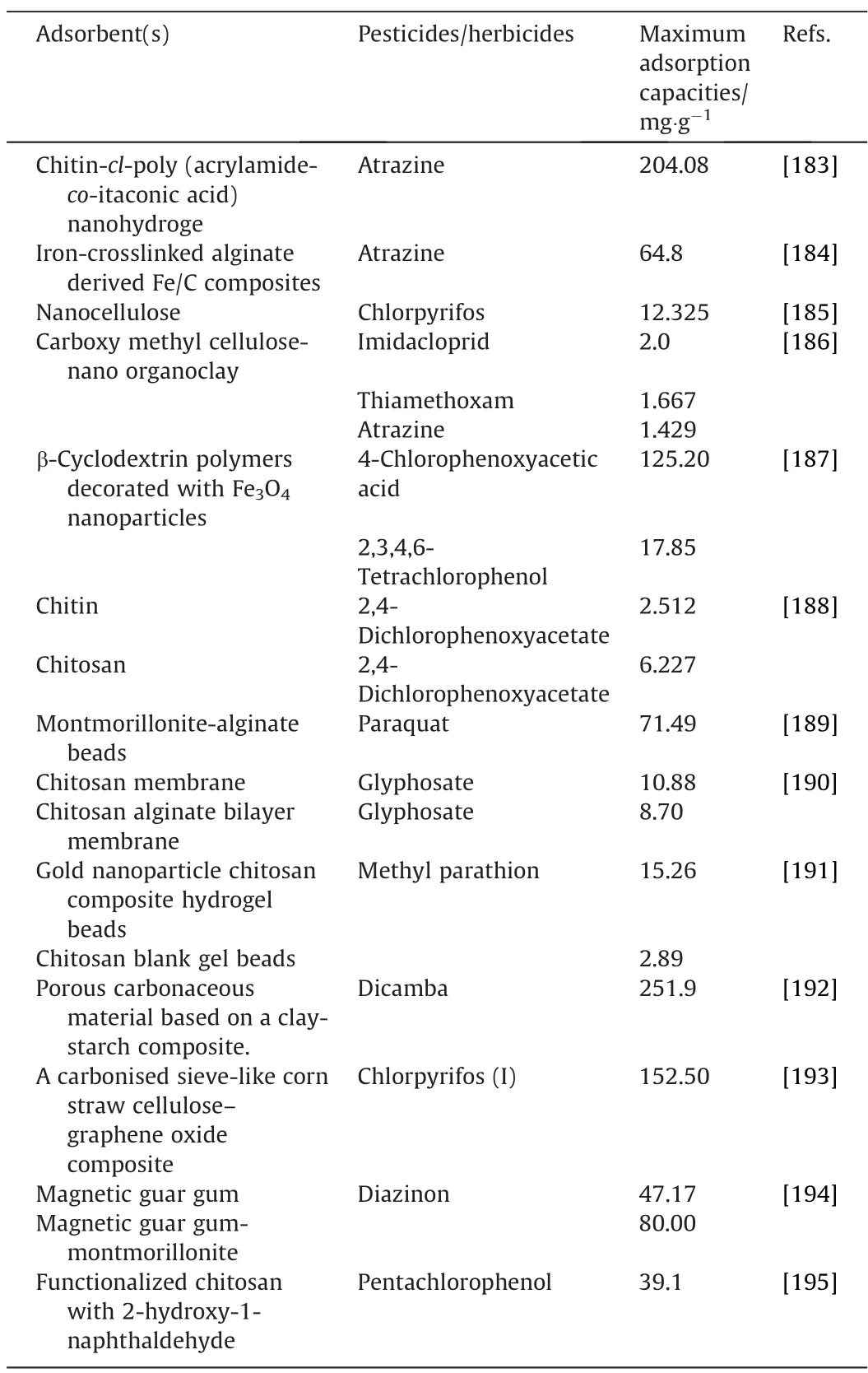
Table 4 Adsorption of pesticides/herbicides using biopolymer-based adsorbents
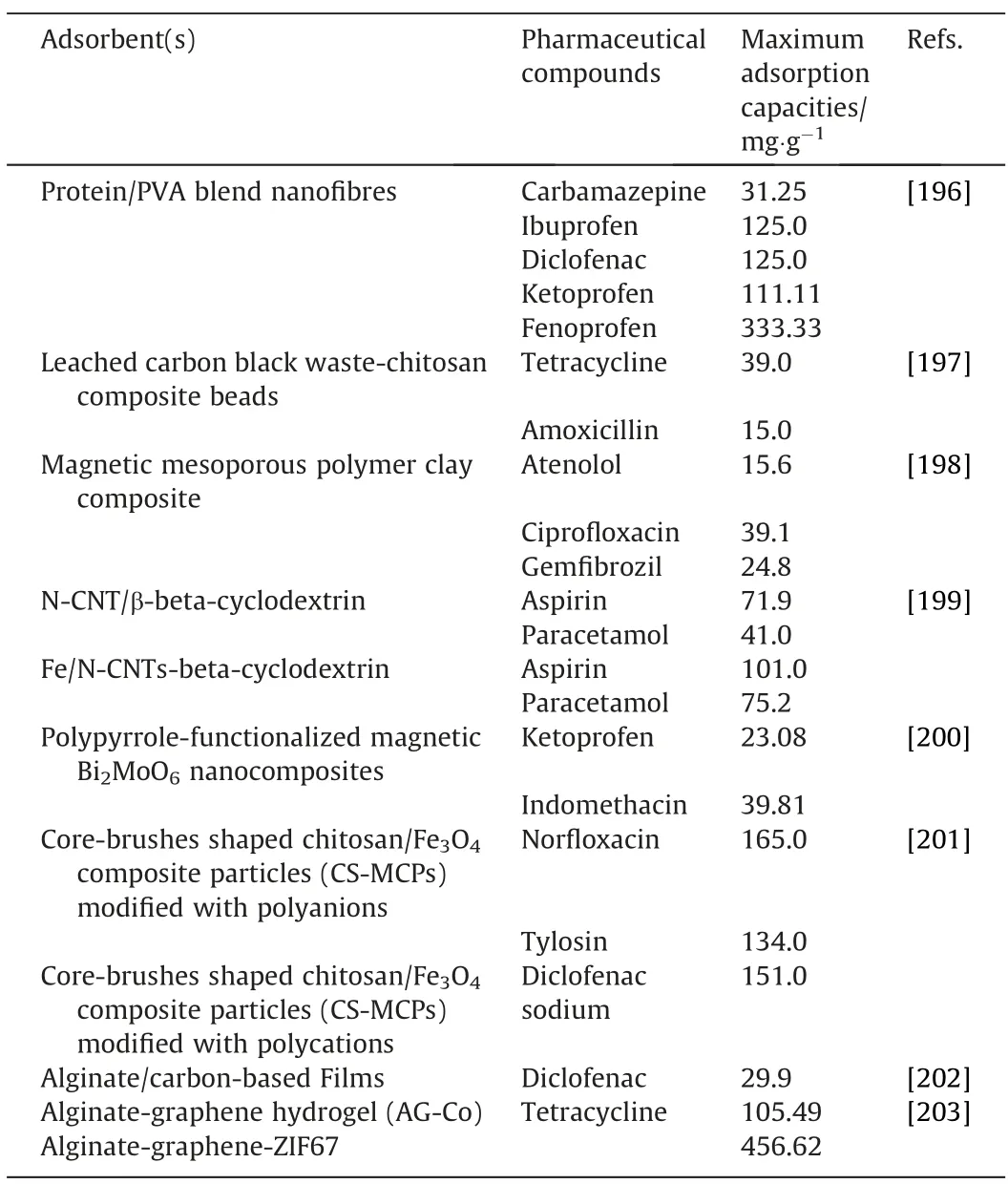
Table 5 Adsorption of pharmaceuticals using biopolymer-based adsorbents
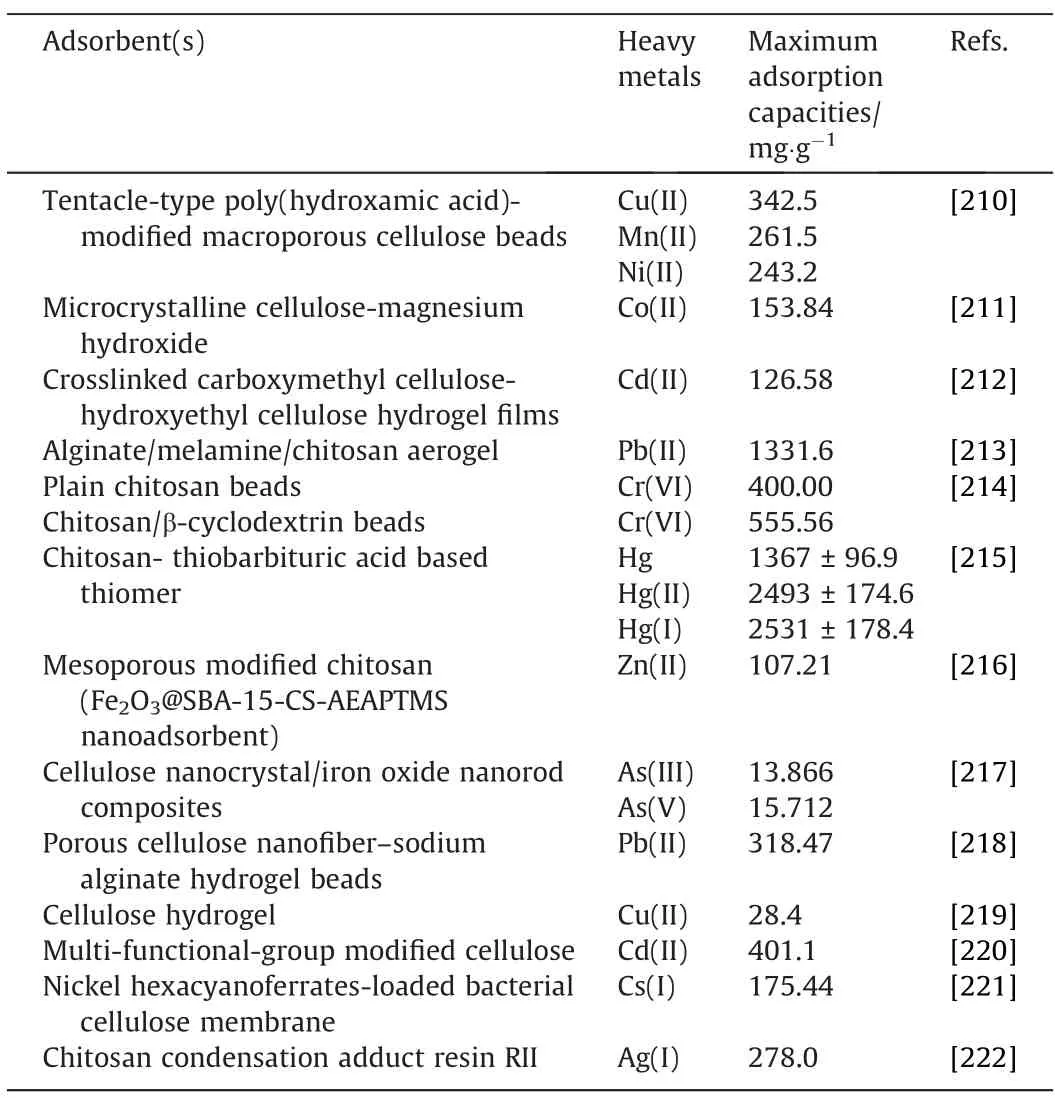
Table 7 Adsorption of heavy metals using biopolymer-based adsorbents
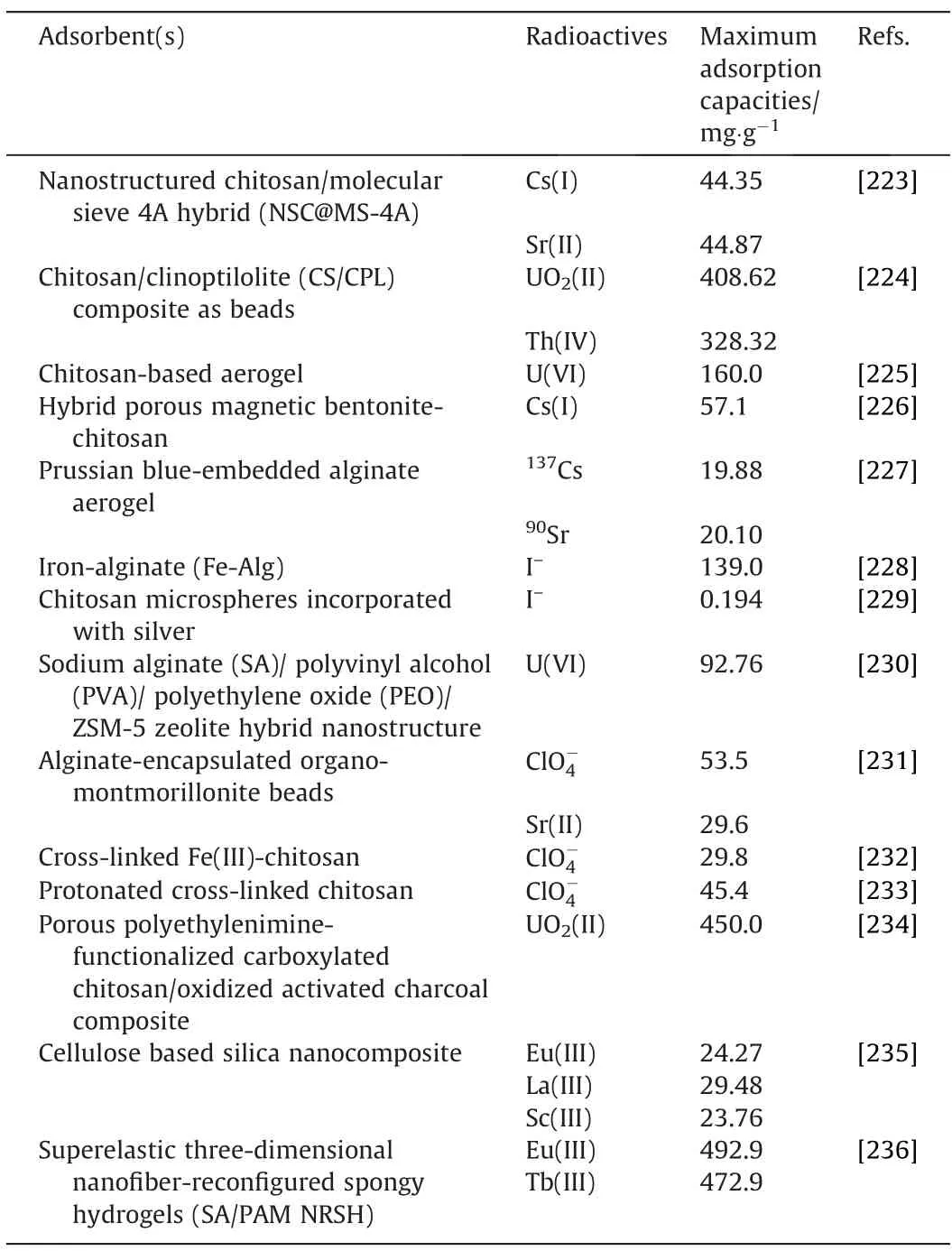
Table 8 Adsorption of radioactive substances using biopolymer-based adsorbents
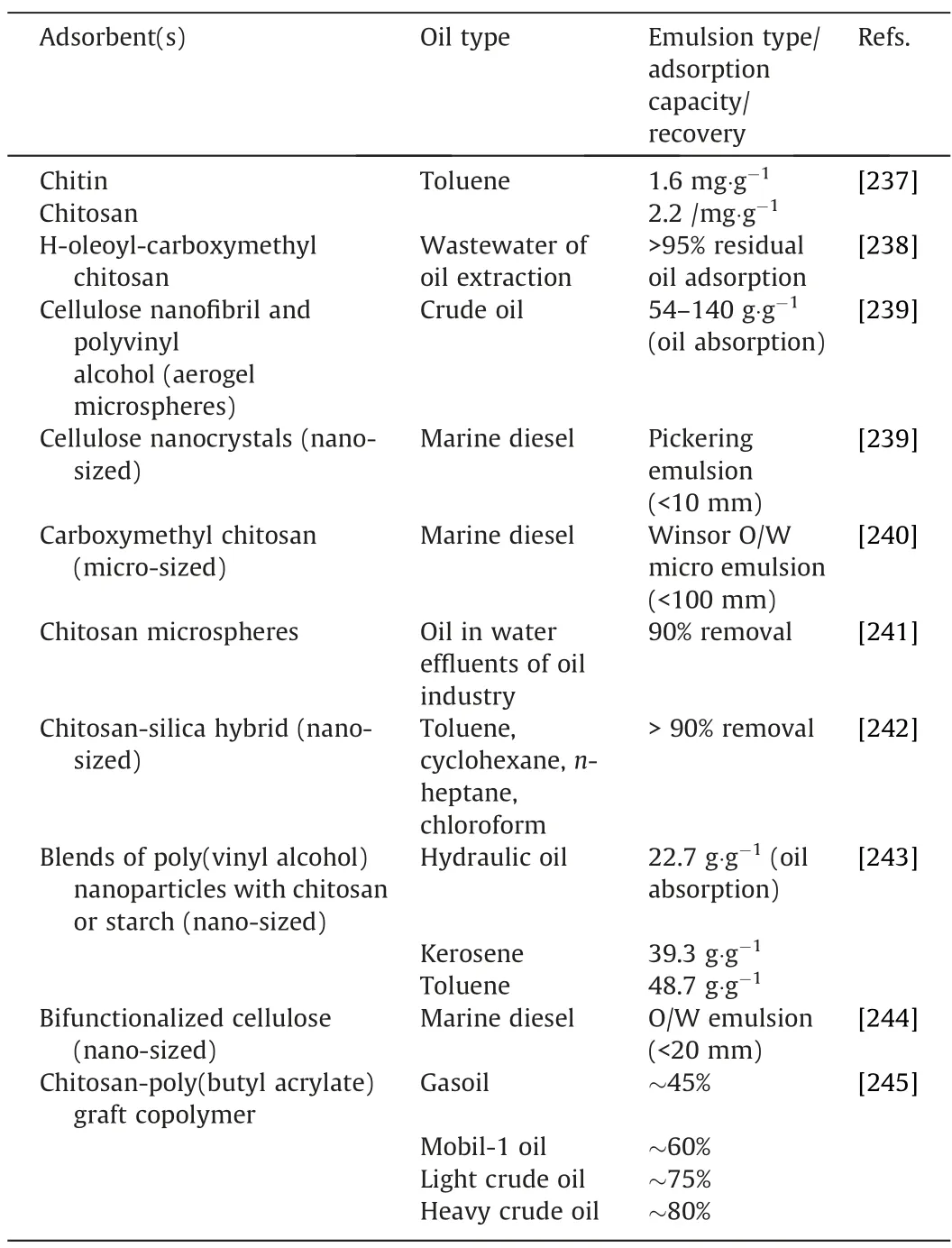
Table 9 Adsorption of oil spills using biopolymer-based adsorbents
Fig.8.Batch adsorption method.
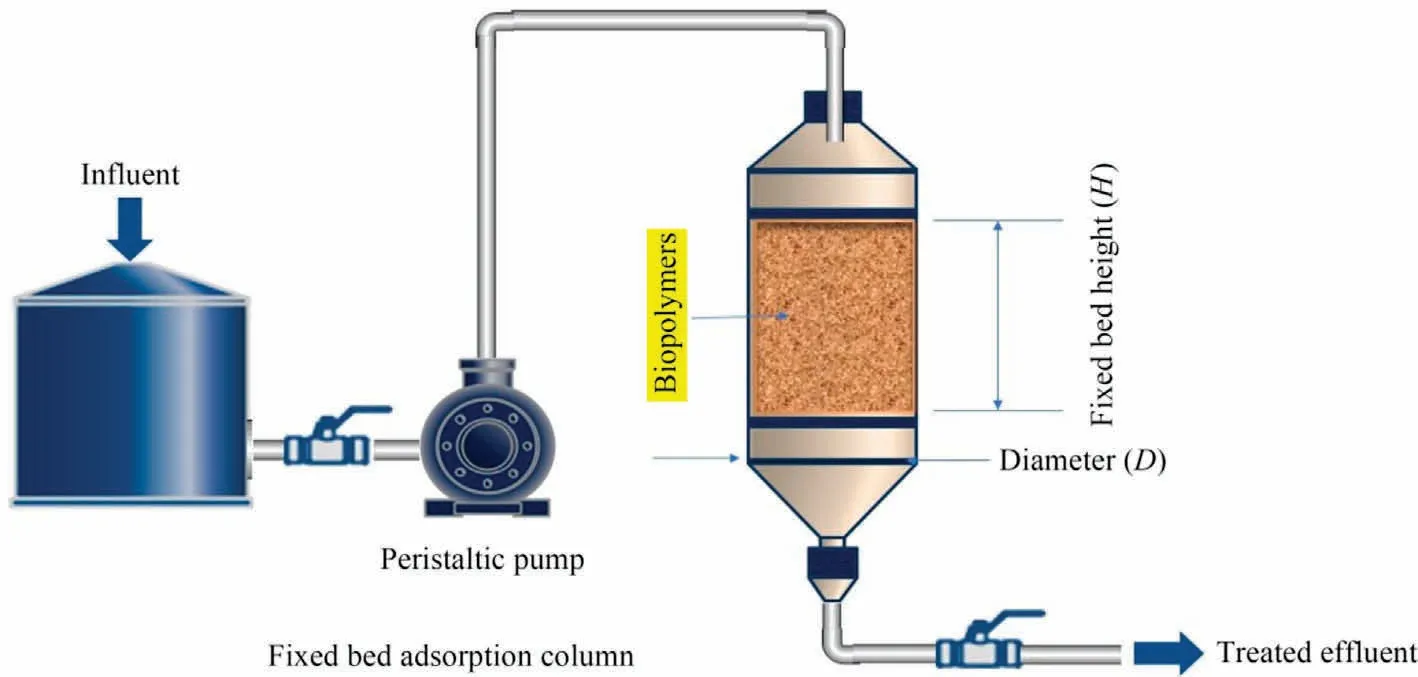
Fig.9.Column adsorption method.
Traditionally,a batch scenario is employed as a preliminary step to assess the potency of adsorbents to remove contaminants from their matrices.Throughout the solid-liquid interface,spontaneous molecular diffusion can be improved with the advection currents to form convective mass transfer.These convective currents cause the adsorbate enriched bulk liquid phase to move in a circular motion, increasing the average collision frequency of the diffusing molecules [154].This resulted in a decrease in diffusional resistances and, as a result, an increase in mass transfer flux across the solid-liquid interface.Once, the adsorbate reaches the adsorbent surface, a variation in the molecular dynamic occurs at the interface zone.The presence of various functional groups on the adsorbent surface largely controls the interactive nature between the adsorbate and adsorbent surface.Several parameters can be studied during the static scenario including pH of the solution,adsorbent concentration,adsorbent particle size,primary pollutant concentration, interaction time, agitation speed, and agitator’s type, assisted techniques (i.e., microwave radiation, ultraviolet radiation, and ultrasonication), temperature, interfering (coexisting)ions,and systems complexity(i.e.,binary or tertiary solution) [155,156].The loading capacity of the utilized adsorbent at time t (qt, mg·g-1) equilibrium time (qe, mg·g-1) and the removal percent (R, %) can be calculated from Eq.(1), Eq.(2), and Eq.(3),respectively.in which C0is initial pollutant concentration,mg·L-1,Ctis pollutant concentration at time,t·mg·L-1,and Ceis equilibrium pollutant concentration, mg·L-1, V is the volume of the solution, L, m is mass of the adsorbent, g.
Moreover, the outlined data derived from the adsorption system are employed to inspect the adsorption kinetics, isotherms,and thermodynamics.Kinetics modeling is crucial to elucidate the adsorption rate, equilibrium time, and mechanism of the adsorption process.It is considered to simulate the experimental data, understand the mechanisms involved in the adsorption process, and thereafter be useful during scaling up of the effluents treatment process at the industrial level (after experimenting when possible in fixed-bed systems,if the condition of the sorbent is compatible with) [157].Otherwise, isothermal modeling is a very significant aspect to extrapolate a more detailed analysis of the adsorbate-adsorbent relationship at the interface.It gives insights into how adsorbate molecules interact with adsorptive sites and hence optimizes the outlines for the adsorbent synthesis process [158].Besides, thermodynamic studies have a vital role in deducing the nature of the adsorption process.They help in understanding the influence of temperature on the mechanism of the adsorption process.In fact, in addition to the adsorbent’s loading capacity, other features such as stability and recyclability are extremely important from an economic standpoint for the asused adsorbent [159].The study of the effect of competitor metal ions and anions is also an important step before transferring the process to the treatment of real effluents.
From a different perspective, in the fixed-bed column study, a finite amount of native or pre-treated adsorbent is packed as a solid matrix in a column of defined length.The strainers are put at the bottom of the column to prevent adsorbent and liquid phase run-off.To ensure temperature consistency, the adsorbent-loaded column is kept in a thermostat and is coupled to a positive displacement pump with a flow regulator,such as a peristaltic pump.The adsorbate-rich liquid phase is allowed to travel through the fixed-bed column at the predetermined flow rate.Because of its relative affinity,the adsorbate from the liquid phase is translocated and binds on the surface of the stationary matrix during this wetting of the solid bed [160].The adsorbate molecule translocation and surface binding proceed until the pores or functional moieties of the adsorbent reach equilibrium.After this point of saturation,the adsorbate transfer process ceases at the solid-liquid interface and further adsorption is not practically feasible.Furthermore,because the transfer of adsorbate across the phase boundary is being hampered by a series of diffusional resistances produced by the surrounding film and intraparticle, sufficient time must be provided for the adsorbate molecules to remain inside the column[161].Because the residence or retention time is a function of the volumetric flow rate of the liquid samples, it is one of the critical parameters in the optimal design of the continuous adsorption process.Additionally, other operational variables such as bed length, temperature, contact time, packing volume and density,and so on have a significant impact on the adsorbate removal process.The primary benefit of dynamic adsorption is utilizing data collected from continuous operation with a high degree of reliability.Scaling up the adsorption process for the treatment of industrial effluents can be achieved effectively using dimensional similarity methodologies,either geometric or kinematic similitude[162].However,due to many obstacles such as significant pressure drop, non-ideal flow behavior, channeling effects, and diffusional resistances, among others, this mode of operation is only used in a restricted number of wastewater treatment systems.The performance of the adsorption process can be evaluated using breakthrough curves (Fig.10), which can be drawn by plotting the ratio of adsorbate concentration at time t over the initial concentration C0, (C/C0), and time (t).It will provide critical information about the breakthrough and saturation points.Moreover,the curve can be used to anticipate the length of the used and unused portion of the bed by considering the area under the curve [163].Many important definitions strongly depend on variable reaction conditions such as flow rate, bed height, influent concentration, and chemical composition of adsorbent material,which may be derived from mathematical calculations of fixed-bed column performance as follow.Empty bed contact time (EBCT) is a measure of interaction between sorbate and sorbent.Mass transfer zone (MTZ) is a function of hydraulic loading rate and is defined as the length of the fixed bed where adsorptions of solutes take place, and the number of bed volume(NBV) is defined as the ratio of the volume of water treated till breakthrough point to the volume of the packed bed.Better and more efficient adsorption is indicated by a lower value of adsorbent exhaustion rate (AER).They can be investigated from Eqs.(4)-(7), respectively [164].
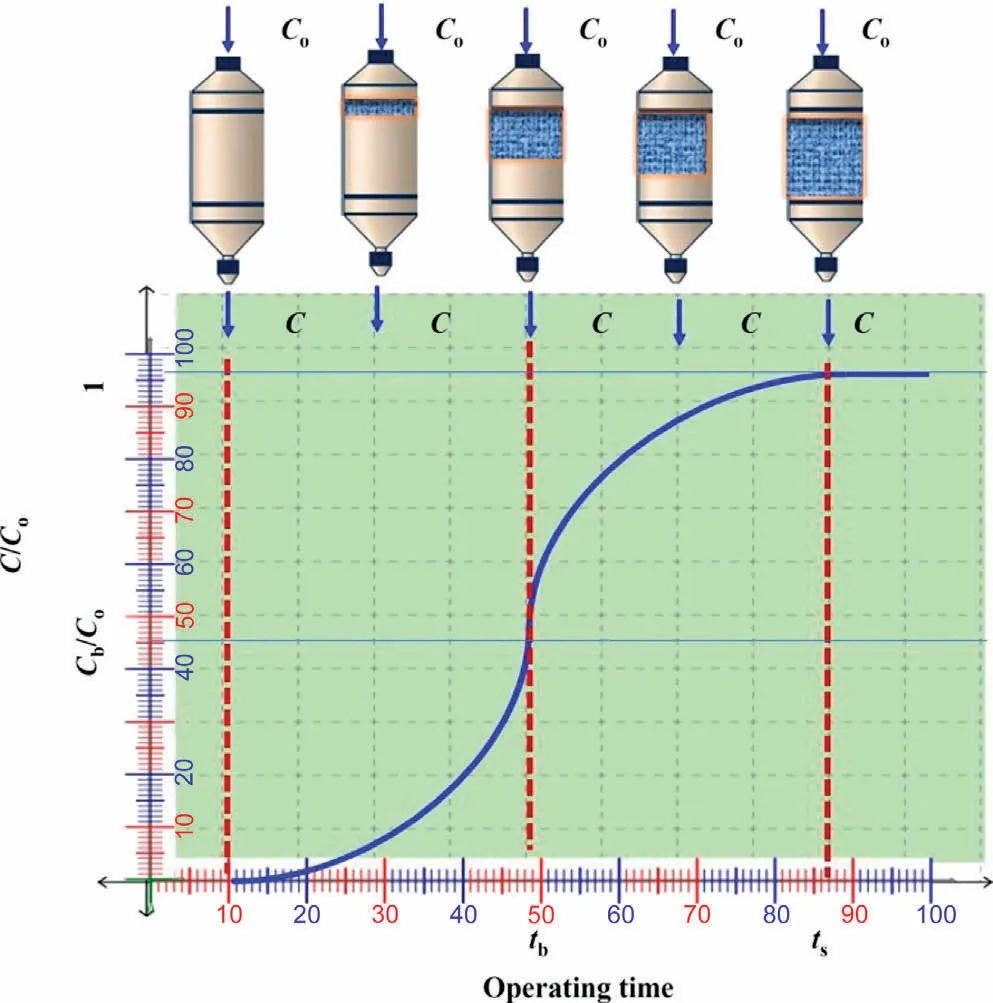
Fig.10.Typical breakthrough curve.
which Z is the bed depth,S is the cross-sectional area of the adsorption bed,and Q is the flow rate.Bed depth is the height of the resin or filter media in a fixed-bed column after it has been properly conditioned for effective operation, usually expressed in inches:
in which tband teare breakthrough and exhaustion time,respectively.
Models of the continuous fixed bed mode can be defined as mathematical formulas that connect the desired output as a function of the input variable(s) in terms of linear, quadratic, logarithmic, or exponential relationships.Because adsorption is a physicochemical process,the model development approach is very beneficial in predicting critical characteristics such as loading capacity, rate-related parameters, service time, usable bed length or capacity, and so on [165].Furthermore, the generated models can provide more light on the numerous diffusional resistances found in continuous flow research.From the literature, different mathematical models such as the Thomas model, Bohart-Adams model, bed depth service time (BDST) model, Yoon-Nelson model,Clark model, Wolborska model, and modified dose-response model were frequently employed to evaluate the adsorption sys-tem and determine the vital variables [166,167].The adsorptive capacities of different effective biopolymers and their synthesized composites used in the removal of diverse contaminants (i.e.,organic dyes, pesticides/herbicides, pharmaceuticals, polycyclic aromatic hydrocarbons, heavy metals, radioactive substances,and oil spills)via the adsorption process are tabulated from Tables 3-9, respectively.
5.3.1.Regeneration of exhausted biopolymer-based adsorbents
Regeneration is the process of recovering and reusing used biopolymer-based biosorbents to evaluate the effectiveness and reusability of the biosorbent.Wasted biopolymers can be renewed through chemical, physical, or biological techniques.The best regeneration method for adsorbent regeneration is determined by the type, varieties, cost, and processing of biosorbent and sorbate.In addition,the choice of procedure is influenced by effectiveness, ecological friendliness, cost-effectiveness, and operational efficiency.The majority of biopolymer sorbents can be used with chemical regeneration because of their low cost and ease of use.biosorbent regeneration techniques are determined by the nature,types,cost,and processing of adsorbent and adsorbate.Aside fromthat, efficiency, environmental friendliness, economic effectiveness, and operational efficiency all have an impact on process choices.Because of its low cost and ease of use,the chemical regeneration process is appropriate for most biopolymer sorbents.Several eluents are used to desorb various biosorbents.For example,in the case of lignin-based biosorbents, research has been conducted using various desorbing agents such as sodium carbonate,nitric acid, sodium nitrate, and EDTA.With lignin-based porous carbon on Cd(II)and Pb(II)solutions,the maximum desorption efficiency was 92%and 96%,respectively[246].Hamza et al.[247]prepared functionalized cross-linked chitosan (Chito) by an original sulfonating process to synthesize a highly efficient sorbent(Sulfo-C) for Li(I) sorption.Using 0.2 mol·L-1HCl solutions, metal desorption is highly effective, and Sulfo-C sorption efficiency is maintained at a high level for at least 18 cycles of sorption and desorption.
In another study,according to the findings of Brião et al.(2018)[248], the regeneration capacity of zeolite manufactured from chitin biopolymer can be used effectively for up to 15 cycles.The authors carried out the regeneration experiment with sodium hydroxide for 15 consecutive cycles of cationic dye adsorption.The adsorption ability of crystal violet, basic fuchsin, and methylene blue dyes remained nearly the same [248].Under acidic con-ditions, cellulose degradation in crosslinked biopolymer resin resulted in a decrease in biosorbent mass [249].
Also, the biological regeneration method was employed to regenerate the magnetic amine-crosslinked biopolymer adsorbent prepared from corn straw [250].Perchlorate-reducing bacteria have been tested for biological regeneration methods.Better regeneration was achieved with biological compared to the chemical regeneration process.However, in most circumstances, spent biopolymer adsorbents are reused in the adsorption process, but in other cases, they can also be used as catalysts in certain processes or as soil fertilizers [10].
5.4.Application of biopolymers in membrane-based technology
Water purification through separation techniques can be accomplished physically through adsorption, chemically through degradation, and biologically through other oxygen-based therapies [251].These approaches have a lower efficiency when used solely,but when integrated,they provide higher efficiency in water treatment by successfully removing different water pollutants.Among them, membrane technology has arisen as promising way to address these issues due to its superior qualities such as high separation efficiency,enhanced selectivity,increased life span,outstanding mechanical,thermal,and chemical durability,cheap cost,and low maintenance [252].Furthermore, membrane purification technology allows for better industrial design by eliminating the need for any additional chemicals or toxic by-products, polymeric membranes are regarded as the most suitable membranes for water purification applications.More crucially, by altering membrane architectures and pore sizes, membrane separation may selectively remove pollutants[253].Fig.11 shows types of applied membrane surfaces along with their use according to pore sizes.The key parameter in membrane separation technology is pressure.In general, considering the membrane pore size and operating mechanisms, the pressure-driven membranes techniques are classified into microfiltration (MF), ultrafiltration (UF), nanofiltration(NF), reverse osmosis (RO), and forward osmosis (FO) [254].MF pathway is commonly used to remove suspended particles,prokaryotes, yeasts, and fungi, UF strategy is frequently used to remove viruses, colloids, and macromolecules, NF scenario is mostly used to remove hardness, heavy metals, and dissolved organic matter, whereas RO is utilized for desalination, water reuse,and the manufacture of ultrapure water[255].Inorganic(ceramic) materials and organic (polymeric) materials are the two fundamentally diverse groups of membrane materials.Both types of membranes have been thoroughly investigated for water filtration.Inorganic membranes are very resistant to corrosive chemicals such as strong acids, bases, and oxidants, as well as having superior mechanical strength and temperature tolerance[256,257].Inorganic membranes outperform polymeric membranes in terms of maintenance because they are less susceptible to bacterial deterioration.To minimize biofouling and achieve high flux recovery, high-temperature chemical cleaning can be used.In many applications where extreme environmental conditions are required, inorganic membranes are the only option.Inorganic membranes, on the other hand, are less frequent in water treatment due to their high manufacturing costs, difficulties in handling, and relatively poor control over pore size distribution.Contrarily, polymeric membranes are extremely adaptable.Polymers can be divided into two types based on their origin: nonsynthesized (natural) polymers which can be obtained from sources such as plants and animals,and synthetic PBPs[258].Their pore diameters can be narrowed to a specific range.The properties of the membrane can be altered by modifying the casting circumstances, monomer molecules and concentrations, additives, and coagulation bath conditions.For more than two decades,researchers have been exploring the membranes synthesis process based on naturally occurring biopolymers such as cellulose, chitosan,and starch, or by the microbial fermentation of renewable raw materials, such as PLA and PHAs attributing to their accessibility,abundance, and their outstanding characteristics [259,260].The key elements of any membrane process are related to membrane flux, permeability, operational driving force per unit membrane area (trans membrane pressure, TMP), rejection percent of pollutants, and fouling phenomenon.Overall, the membrane flux is the amount of permeate produced per unit area of membrane surface per unit of time.The permeability of a membrane is the rate of passive diffusion of molecules through the membrane.These molecules are known as permeant molecules.It largely depends mainly on the electric charge and polarity of the molecule and to a lesser extent the molar mass of the molecule [261].The rejection (R)could be defined as the number of particles that have been removed from the feed water.The corresponding mass balance equations are to control the operation of a membrane process,two modes, concerning the flux and the TMP, can be used.
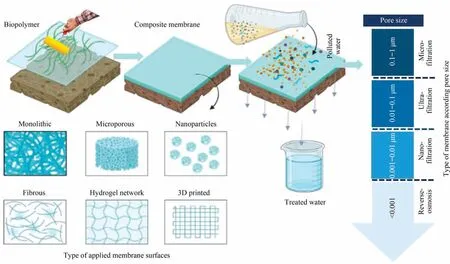
Fig.11.Types of applied membrane surfaces along with their use according to pore sizes.
The flux (J) of membranes can be measured in the unit of L·m-2·h-1by the equation:
where V, A, and t are the volume(L) of the solution passed through the membrane,the area of the membrane,m2,and the time interval,h, respectively.Moreover, the rejection percent of pollutants (R, %)may be calculated by the equation:
where Ciand Cfare the initial and final concentrations of pollutants before and after the filtration process, respectively.
Fouling is the most serious issue connected with the industrial usage of membranes, and it has been a major focus of membrane research for decades.Membrane fouling can be induced by a variety of solutes or particles.Fouling diminishes the separator’s permeate flow rate, shortens the membrane’s life, and raises operating expenses.An upgrading of membrane-processing conditions by modifying its surfaces via grafting components and producing membrane materials to prevent membrane fouling is of great interest [262,263].The wide application of bio-based polymeric membranes in wastewater treatment is demonstrated in Table 10.
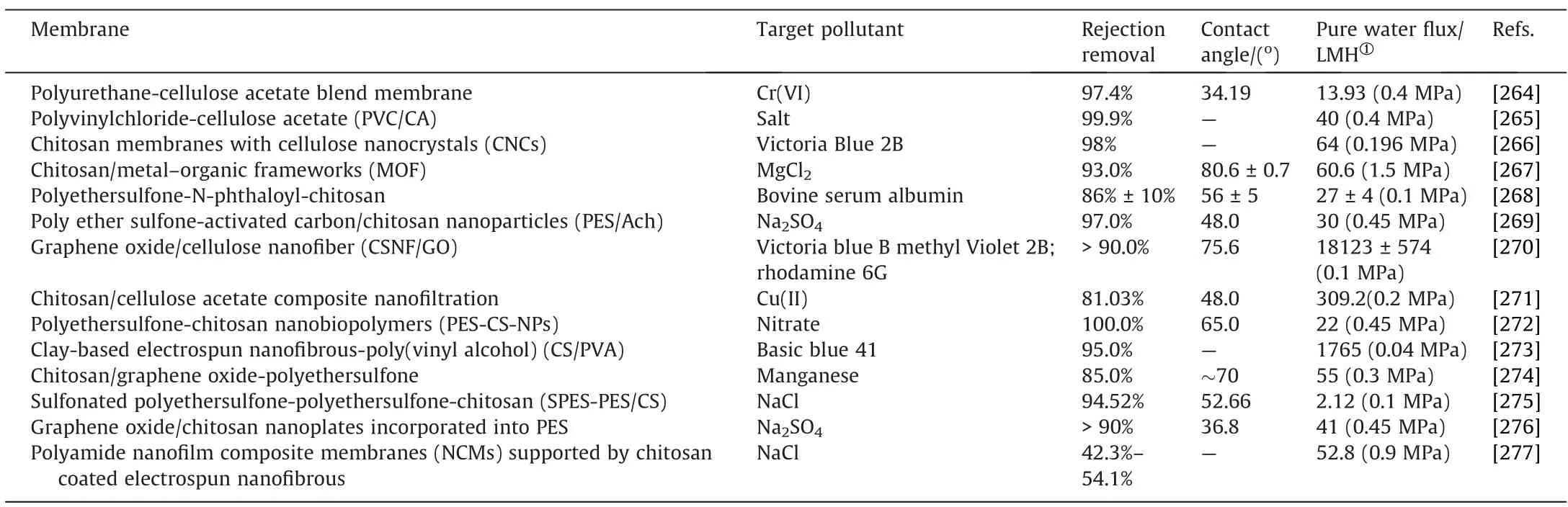
Table 10 Performance evaluation and characteristics of different bio-based polymeric membranes for wastewater treatment
Recently, Russo et al.[278] extensively reviewed the applications of biopolymers in CO2separation.They considered natural biopolymers such as cellulose, starch, and chitosan, but also bioproduced polymers obtained by fermentation of biomass such as polyhydroxyalkanoate (PHA), or chemically synthesized such as polylactic acid(PLA)[279-281],polyvinyl alcohol(PVA)or polyurethane (PU).Comparing the properties of these alternative membranes with the performances of reference materials (such as polysulfone, polyethersulfone, or polyimide), they concluded that the biopolymers have promising perspectives providing some intrinsic weaknesses can be overcome.Hence, special attention must be paid to mechanical resistance (solved using blends with synthetic polymers,or incorporation of nanoparticles),water solubility (overcome with crosslinking), crystallinity (adding plasticizers), thermal stability, or physicochemical stability (to biodegradation or UV irradiation).Apart from gas separation,these membranes can be used for removing water from the organic solvent by pervaporation [282], and the separation of oil from aqueous media [283,284].
Polylactic acid (PLA) is economically obtained by fermentation of renewable resources such as starch.Vatanpour et al.[281]reviewed a series of PLA-based composites and highlighted the impact of the blending polymers and/or fillers on their specific properties (resistance, biodegradability, crystallization conductivity,etc.).An emblematic example of bioplastic,PLA may be readily prepared by phase inversion technique as membranes [279].For example, PLA may be dissolved in chloroform (at a concentration close to 10%, mass ratio) and casted (after degassing) over a glass plate(using a casting knife).After complete evaporation of the solvent,the membrane is peeled off and dried.Applied in gas separation the PLA membrane showed high efficiency for separating hydrogen from methane (ideal selectivity H2/CO2≈25) with good H2permeability characteristics(≈25 barrer,1 barrer=7.52×10-18m3·m-2·m·S-1·Pa-1).Iulianelli et al.[285] designed a derivative of PLA(PLA Easy Fil-White,trademark)that was used for preparing membranes by the phase inversion method according to the same method (described above).The produced membrane exhibited strong performance for separating carbon dioxide from other gases.While other gases(such as H2,He,and CH4)followed the linear correlation between permeability and critical volume, CO2left the trend.The ability of the membrane to separate CO2sounds to be controlled by the solubility properties of the gas (confirmed by much higher critical temperature) rather than by critical volume.The highest performances of selectivity were obtained for the system CO2/CH4(selectivity: 284, with permeability: 70 barrer).Alruwaill et al.[280] prepared PLA/polybutylene succinate blend membrane (with incorporation of multiwalled carbon nanotubes); they ranked the gas permeance (at 25 °C and under 4 bar)according to:H2>CO2>Ar >N2.Permeance increased with the pressure applied and the highest ideal selectivity (slightly higher than 25) was obtained for hydrogen separation from nitrogen.
In oil separation, PLA fibrous membranes were produced by blow spinning; their oil-saturated adsorption capacities were 2-to 3-fold the levels reached under similar conditions with commercially-available nonwoven polypropylene [283].Li et al.[284] preconized the melt blending of PLA with PBS (poly(butylenes succinate);leading to enhanced movement ability of PLA(plasticizing effect of PBS), and lower crystallinity.Under the most favorable conditions (i.e., PBS content ≈20% (mass); and foaming temperature at 115 °C), the adsorption capacity reached a maximum adsorption capacity higher close to 22 g oil·g-1.Tomietto et al.[282] promoted the combination of two bio-based materials(PHA and CyreneTM, a polar aprotic bio-based solvent with properties similar to dimethylformaide, for example) for the synthesis of membranes.The porous(and hydrophilic)properties have been controlled by the incorporation of a series of pore-producing substances before the pre-evaporation step (evaporation-induced phase separation process); which was followed by immersion in a non-solvent bath.After a thorough optimization study, they obtained highly promising pervaporative membranes(with porous characteristics suitable for ultrafiltration-microfiltration operations): the asymmetric membranes showed water permeability close to 3.50 L·m-2·h-1·MPa-1, while the membranes selectively separated methanol from methyl tert-butyl ether.
Thomas et al.[286] blended electrospun PLA membranes with chitosan nanoparticles; the blending increased the mechanical resistance of the fibers, their hydrophilicity, and their reactivity for cadmium binding in aqueous solutions.This enhancement was also explained by lowering the size of PLA fibers, and the decrease in the pre-size of the secondary porous structure.
Polyhydroxyalkanoates(PHA)may be obtained by fermentation processes of waste products, including wastewater streams [287-289]: through carbon recovery [290] with a controlled nitrate cycle.The advantages of PHA consist of the long-term release of organic material (which may stimulate reductive biological conversions) and the slow-release electron donor that can contribute to nitrate removal in mixed microbial cultures[291].These synthesized polyesters produced from natural resources have broad applications such as cosmetics,medical devices,packaging,or disposable tools [291].The process contributes to valorizing waste and reducing environmental impact.Santorio et al.[292] investigated the contribution of PHA(as a source of carbon)for improving nitrogen removal (through specific endogenous denitrifying activity).They optimized the experimental conditions for denitrifying effect and observed that under these conditions the process did not generate noxious N2O gas.Tu et al.[293] demonstrated that the selection of appropriate conditions (playing with aerobic feast/famine phases for PHA production and aerobic feast phase/anoxic famine phase for nitrate removal) made feasible the integration of the combined ‘‘synthesis PHA /denitrification” in global wastewater treatment.
Chang et al.[294]reported the use of PHA as a biofilm carrier in a moving bed biofilm reactor which was applied for the decolorization process.PHA film prepared by casting and evaporation was decorated with zinc oxide microparticles by deposition of ZnO/methanol suspension followed by fast evaporation [295].Another variant was prepared by the same method of ZnO deposition onto PHA-g-PMA membranes.The copolymerization between PHA and methyl acrylate was processed under ultrasound irradiation(using hydrogen peroxide as the radical initiator);the copolymer was precipitated by methanol and followed a series of purification steps(with methanol).These materials were comparatively tested for the photodegradation of methylene blue under UV light (at 254 nm).The UV activation of ZnO microparticles produced hydroxyl radical(i.e.,OH*)and superoxide anion(i.e.,O2-),which,in turn,reduced methylene blue.It is noteworthy that the composite PHAg-PMA membrane is more efficient than the pristine PHA membrane; Ishak et al.[295] suggested that the copolymerization allowed a more homogeneous dispersion of ZnO microparticles that strongly enhances photocatalytic activity.
Recently Zhu and Wei [296] used microbial engineering (cloning and gene encoding) for the synthesis of two materials: PHA bio-beads and tyrosinase-functionalized PHA beads.They used the tyrosinase-decorated beads for the biocatalytic degradation of bisphenol (and analogues).The biocatalysts exhibited high stability in terms of storage and reuse with good catalytic efficiency under environmental conditions; these results prove the concept to be promising for bioremediation.
5.5.Application of biopolymers in the photocatalysis process
Photocatalysis is regarded as a promising and long-term approach, can be utilized for water purification, and has potential environmental uses in the near future.In this context,light energy sources (sunlight, an ultraviolet source, etc.) for photons in conjunction with adequate semiconducting material have been used for degrading water contaminants (Fig.12).When a photocatalyst suspension containing aquatic contaminants is illuminated with adequate light energy, the photons collide with the surface of the semiconductor catalyst, they specifically cause electrons to move from the valence band(VB)to the conduction band(CB)in addition to the generation of hydroxyl and superoxide radicals [297].Considering thermodynamics concepts, the difference between CB and VB potentials determines the possibility of a reaction occurring[298].According to the International Union of Pure and Applied Chemistry (IUPAC), photocatalysis can be categorized into homogeneous or heterogeneous based on the phase of the photocatalyst and reactants.Homogenous catalysis occurs when the reactants and the photocatalyst are in the same phase.Whereas reactants and the photocatalyst are at various phases in imputed to heterogeneous photocatalysis.It is well understood that photocatalysts play an important role in photocatalytic degradation events[299].Numerous synthesis methods like polyol synthesis, sonochemical,spray pyrolysis,ball-milling,chemical vapors deposition,solvothermal, microemulsion, solution combustion, microwave irradiation,laser ablation, sol-gel method, etc.have been reported to modify the physicochemical properties (i.e., magnetic, catalytic,and optical) of photocatalysts [300].However, many of these synthesis processes necessitate high temperatures and pressures,extended reaction times, expensive instruments and accessories,and a large number of dangerous and expensive compounds.In reality, there are some drawbacks associated with homogeneous photocatalysis pathway.The homogeneous photocatalysts can’t be recovered, the low acidic pH value through Fenton reaction,and the production of a great amount of sludge (secondary pollution) [301].Contrarily, heterogeneous photocatalysis may offer a remedy to these drawbacks.Apart from wastewater purification,heterogeneous photocatalysis is also used in organic transformation and hydrogen generation,because it has the following advantages.It only requires ambient environmental conditions,photocatalyst recovery is possible, available dissolved oxygen in water can be directly used for photocatalytic degradation reactions, and complete mineralization of organic pollutants [302-304].To conclude,selecting appropriate components for the manufacturing of photocatalysts is a critical step.Nonetheless, some drawbacks characterized to conventional catalysts such as limited dispersion in water, rapid recombination of electron-hole pairs,mass transfer limits, and toxicity, make them unsuitable for practical usage in water treatment plants[304].Thence,scientists have worked tirelessly to improve the qualities of photocatalytic technology in order to efficiently employ it in wastewater treatment.Lately, different biopolymers (i.e., alginate, chitin, chitosan, cellulose,pectin,and etc.)have been utilized as a part(support)of photocatalytic materials during the catalytic oxidative degradation of organic pollution because of their fantastic features[305].Table 11 displays a list of recently published biopolymers and their composites based photocatalysts employed for the removal of different water pollutants.The preceding underlines the significance of photo catalysis as another technique that can be totally dedicated to the water treatment process,which involves the use of energy to separate pollutants such as dyes,dissolved metals,and ions in polluted water.Fig.13 summarizes the mechanism of biopolymer incorporating nanomaterial in photocatalysis process.
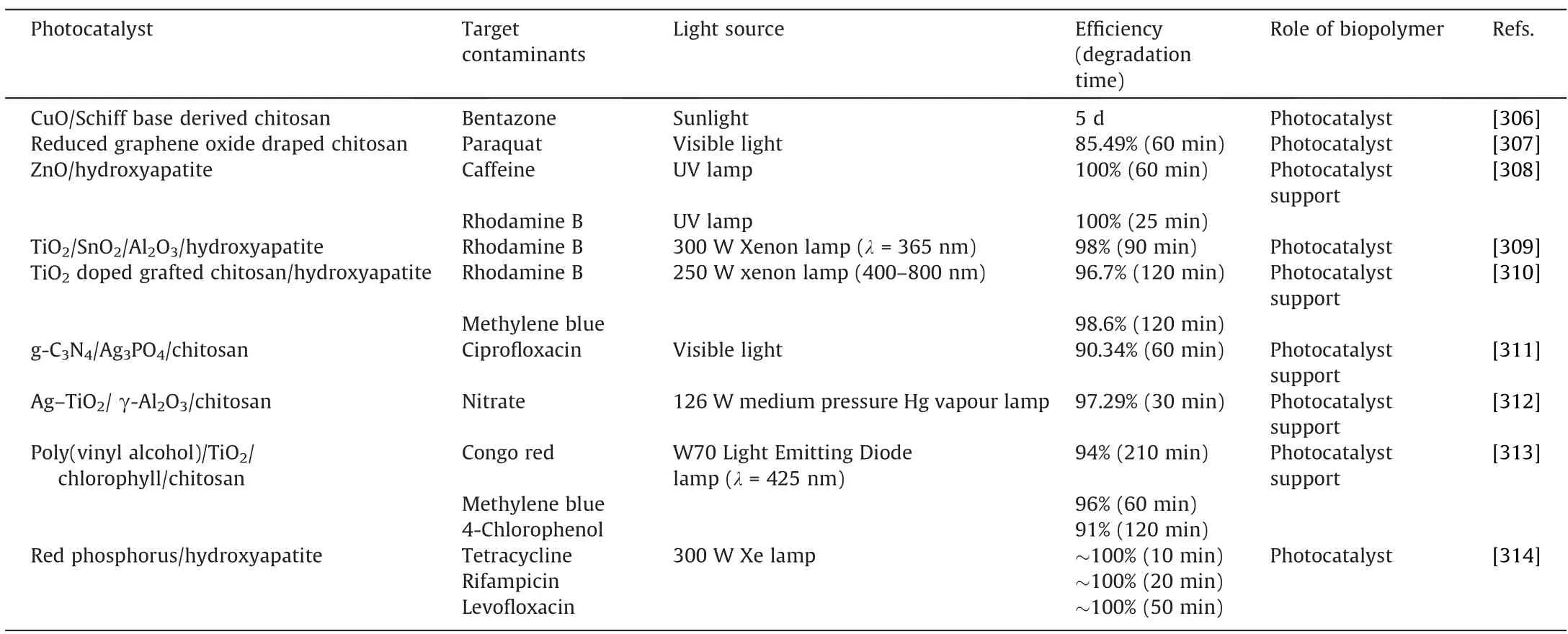
Table 11 List of recently reported biopolymers supported photocatalysts and their photocatalytic performances
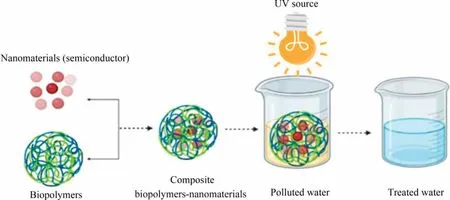
Fig.12.Use of biopolymer incorporating semiconducting material in photocatalysis process.
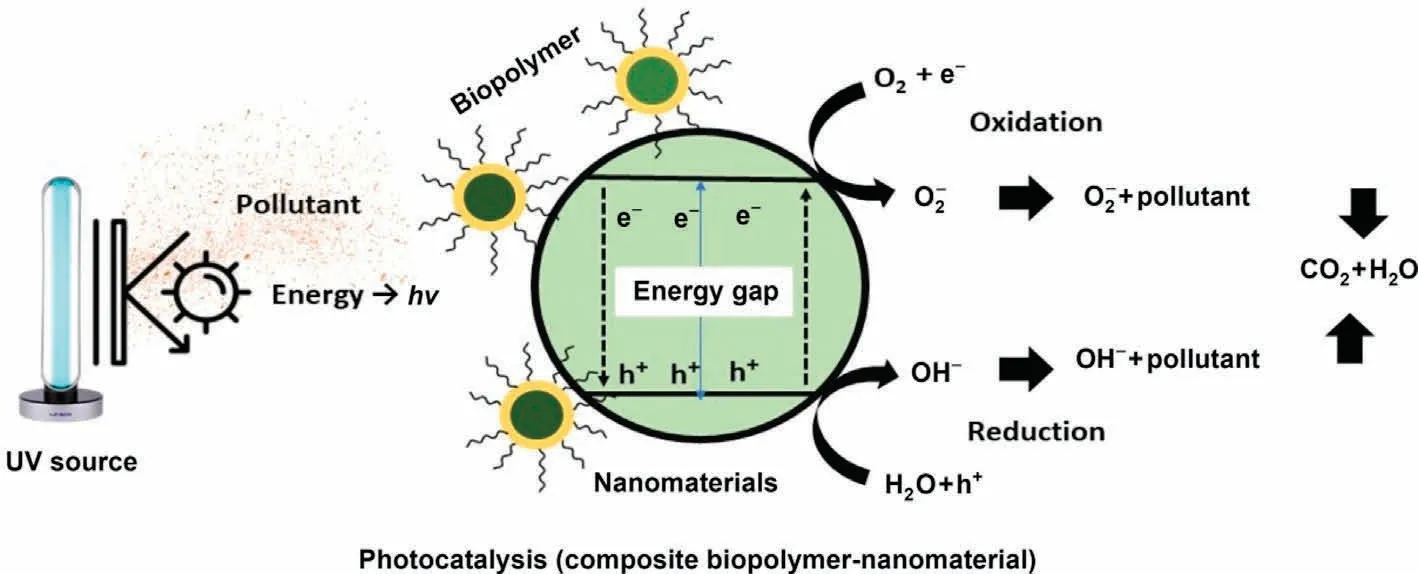
Fig.13.Mechanism of biopolymer incorporating semiconducting nano-material in photocatalysis process.
6.Biopolymers and Circular Bioeconomy
The current worldwide scenario of population growth increased industrial activity,high waste creation,limited resources,and insa-tiable demand for energy and goods has prompted the adoption of a sustainable solution known as a ‘‘circular bio-economy”.The phrase ‘‘circular bioeconomy” (CBE) refers to the convergence of the circular economy and bio-economy agendas, with varying degrees of emphasis on biopolymers.It is a parallel economy that includes the creation of value-added resources using technoeconomic biotechnologies without the use of non-renewable resources,hence increasing the carrying capacity of the ecosystem[315].The viability of the circular economy has gained significant regional and global traction considering its social,ethical,and ecological aspects.With growing awareness of the possible dangers posed by chemical and synthetic materials (i.e., flocculants and adsorbents), most governments have begun to strictly regulate their use in drinking water and wastewater treatment.Globally,innumerable studies have been conducted to replace the mentioned traditional materials with highly efficient and environmentally friendly biomaterials.Researchers have shown a strong interest in biopolymers for the creation of products due to their biodegradability, non-toxicity, cost-effectiveness, and easy availability from different resources.Manufacturing biopolymers from repeatable biomass (i.e., agricultural, industrial, and residential waste) can aid in the development of valuable byproducts [316].These biopolymers can be used to replace synthetic polymers derived from petroleum products,thereby lowering industrial carbon footprints.Many different types of biopolymers exist, each with a unique set of thermal and mechanical properties, and they have found use in a wide range of industries,including biomedical engineering, food industries, pharmaceuticals, and human and dietary requirements.Process integration with zero liquid and solid discharge can accomplish the CBE for biopolymers manufacturing.The following are the major barriers:(i)the significant environmental and social impacts of substituting the PBPs with biopolymers, (ii) the great reliance of national economies on conventional polymers resources, and (iii) the rapid development of business models for the urban population that compete with the recycling concepts [317].
Several biopolymers have been studied in wastewater treatment,including alginates,starch,cellulose,chitosan,tannin,gums,and mucilage, and they display remarkable selectivity towards hazardous chemicals and metals, making them useful in the removal of contaminants from wastewater.However, it has been noted that their usefulness and feasibility are limited due to their moderately short shelf life.This issue is addressed by creating modified biopolymers through crosslinking or grafting, and such modified biopolymers are reported to have exceptional pollution removal characteristics and biodegradability.Numerous factors associated with the biopolymer’s synthesis processes hinder their development on the industrial scale such as the as-used chemicals that may cause health and safety issues, the complexity of the operational systems and the requirement of high energy input,and the relatively high-cost equipment.To solve all of these issues,extensive research is needed to show the validity of modified biopolymers and promote their commercial applications.
7.Market Analysis
The evaluation of the cost of biopolymers reveals difficulty since the variability in their characteristics (source, molecular weight,purity, degree of deacetylation, degree of esterification, guluronnic/mannuronic acid ratio, etc.) strongly impact the production costs.The cost of the primary resource may affect the final cost,but other important criteria are taking more and more importance such as energy items or sustainability issues (with the increasing regulations).The specificity of these characteristics obviously drives the field of application:biomedical,pharmaceutical,or food applications require high grades,while industrial applications such as wastewater treatment do not need high-purity materials.In any case, the variability of the physicochemical may represent an important hindrance to developing commercial applications: in many cases, this criterion limits the transfer of innovative processes from the laboratory to real life.
In the case of chitosan, the expansion of the market (CAGR,compound annual growth rate, reaches currently about 14%; it is expected to increase to more than 20% within the next 5 years[318])is driven by high-value applications in cosmetics,biological and medical uses [319].This means that this strong demand supports the increase in the cost of biopolymer for the last decade(and the near future).The importance of shrimp in the economy of Ecuador motivated Riofrio el al.[320] to extensively evaluating of the global environmental and economic performance of the process of chitosan extraction.In their study, they attached great importance to the life cycle analysis (LCA) compared with parallel analyses performed in Spain and India.The global cost evaluation reached a value of 8.39 USD·kg-1in Ecuador, while they report a higher cost for production in Spain(i.e.,close to 14 USD·kg-1).They evaluated the net present value to 10.38 million USD for processing 5000 tons per year;with a rate of return close to 67%and a payback period slightly longer than 3 years.Obviously, the increase in the cost of energy may have a dramatic impact on the cost and profitability;their study published in 2021 is probably not representative of the current situation.
Saji et al.[321] published a comprehensive review on the extraction of alginate in link with sustainability criteria.Several methods have been investigated involving ultrasound,microwave,or enzymatic processes; however, the financial analysis did notshow any breakthrough compared with conventional techniques.Their main recommendations for decreasing sustainability issues(and following cost analysis) hold on developing algal cultivation(resource), reducing water footprint and diminishing the use of an organic solvent (environmental impact), and valorizing subproducts (economic income).Alginate business strongly develops(CAGR exceeds 3.3%, [322,323]) especially with high-added value applications; therefore, the market of wastewater treatment(lower added value)faces strong competition.This is another motivation for the valorization low grade products derived from algal industry[324].However,the strong growth of Sargassum biomass,which is frequently associated with contamination effects [325],represents an opportunity for business development in some recent areas (linked with climate change).
The cost of PHA was evaluated between 8 and 12 USD·kg-1depending on the grade and application(packaging vs.biomedical)[326].Chavez et al.[326]compared different techniques (different technical processes and sources)for the synthesis of PHA and PHB;and suggested using alternative carbon sources (including CO2)and found cost analyses ranging between 5 and 11 USD·kg-1.The cost evolution of natural biopolymers between 2018 and 2023(food grade; EUR·kg-1, source: Algaia SA, Saint-Lô, France) is reported in Table 12.
Table 12 Cost evolution of natural biopolymers between 2018 and 2023 (food grade; EUR·kg-1, source: Algaia SA, Saint-Lô, France)
8.Research Aspects and Prospects
The increasing waste generation of PBPs and poor waste management has prompted the scientific community to develop an alternative option in the form of biopolymer from agricultural,industrial,and residential waste.Although garbage is an appealing substitute for nonrenewable sources,it requires preliminary treatment and correct component characterization before it can be used.Thermal, chemical, and biological processes are all involved in the synthesis of a biopolymer,and they are all affected by a variety of operational conditions.All thermochemical treatments have the disadvantage of being chemical and energy-intensive procedures that produce unpleasant chemicals, whereas a biological approach can give a techno-economical alternative.The efficiency of biological processes for biopolymer synthesis is determined by the substrate composition and the microbial strains used.However,the method is coupled with a slow rate of biopolymer production and includes many factors.The control of many factors as well as complex biopolymer manufacturing routes is time-consuming,rendering the process ineffective.Furthermore, all the treatment techniques are inapplicable on a broad scale.It is necessary to overcome these constraints using mathematical models such as density functional theory,factorial design methodologies,principal component analysis, and response surface methodology.This will assess and optimize the influence of various parameters on biopolymer yield.Finally, it will allow a biopolymer to be employed as a CBE product for wastewater reclamation.
9.Conclusions
In recent years, the development and discharge of billions of tons of trash directly into water bodies have resulted in advancements in wastewater management to meet global statutory demands for clean water.The removal of emerging contaminants from wastewater is extremely important for the ecosystem.The current review article primarily summarizes the most recent significant information regarding biopolymers, including the most often used base materials (i.e., alginate, cellulose, chitosan, and others), and the recent synthesis, modification, and characterization strategies.The ingredients of biopolymers have the potential to substitute the traditional materials normally used in different wastewater treatment strategies such as activated carbon, alumina, silica, and so on.Among other materials, biopolymers may exhibit competitive behavior in terms of admirable capacity,cost-effectiveness,and biocompatibility.Several intriguing investigations related to employing different biopolymers and their composites through wastewater engineering reclamation technologies of coagulation/flocculation,adsorption,membrane technology,and photocatalysis have been collectively highlighted.Because of the biopolymer system’s biodegradability, biocompatibility, and renewability,biosorption technology has attracted a lot of interest in wastewater treatment.Although several fabrication techniques,such as electrospinning and 3D printing technology, are used to synthesize these components in biopolymers for wastewater treatment, the scaling-up of biopolymers for industrial applications ensures economic feasibility, and multi-toxin removal is a worry.The industrial application of these biopolymers on a large scale is still in its early stages.Furthermore, the regeneration and destiny of biopolymers following biosorption, as well as pollutant recovery, are critical in competing with existing materials for industrial wastewater treatment.As a result, additional quantitative and qualitative investigations and research are needed to comprehend and investigate the potential of biopolymers in drinking and wastewater treatment in order to preserve low-risk exposure to water contamination.
Future research studies for progressing the broad applications of biopolymers in wastewater treatment are highly recommended in terms of four main aspects; (i) increasing biopolymers sources,improving extraction technologies,and the discovery of additional resources, (ii) process optimization via identification of the modifying agents and modulating the operational parameters, and (iii)upgrading the depth of application research of elucidation of practical application conditions for cost-benefit analysis and process standardization.Believing in the displayed data, the development of low-cost, safe, multifunctional biopolymers will provide longterm solutions by replacing synthetic polymers with sustainable circular bioeconomy.
Authorship Contribution Statement
Ahmed M.Elgarahy:Conceptualization, Investigation, Data curation.M.G.Eloffy:Conceptualization, Methodology, Writing -original draft.Eric Guibal:Validation, Data curation.Huda M.Alghamdi:Validation, Data curation.Khalid Z.Elwakeel:Validation, Data curation.
Data Availability
No data was used for the research described in the article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors received no financial support for the research,authorship, and/or publication of this article.
 Chinese Journal of Chemical Engineering2023年12期
Chinese Journal of Chemical Engineering2023年12期
- Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
