Polybenzoxazine/organosilicon composites with low dielectric constant and dielectric loss
Manlin Yuan, Xin Lu, Xiaoyun Ma, Hao Lin, Angui Lu, Liyan Shao, Zhong Xin
State Key Laboratory of Chemical Engineering, School of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
Keywords:Polybenzoxazine Organosilicon Composite Dielectric constant
ABSTRACT The evolution of electronic communication technology raises higher requirements for low dielectric constant(low-k) materials.For this, a benzoxazine functional organosilicon (HP-aptes) with dense Si—O—Si crosslinking networks and large sterically hindered tert-butyl groups was prepared by the sol-gel method.Then, a series of polybenzoxazine composites (PPHP) were prepared from intrinsically low dielectric constant bis-functional benzoxazine monomer(P-aptmds)and HP-aptes.The double crosslinking networks of polybenzoxazine and organosilicon further increased the crosslinking density and decreased the dipole density of composites, which endowed the composites with enhanced low-k properties.When the content of HP-aptes is 30% (mass), the crosslinking density was 2.05 × 10-3 mol·cm-3,while that of PP-aptmds was 3.31×10-3 mol·cm-3.In addition,the dielectric constant and dielectric loss of PPHP composite at 1 MHz could reach 2.61 and 0.0056, respectively.
1.Introduction
Low dielectric constant(low-k)polymer materials are becoming increasingly important in the field of electronic communication and have received considerable attention in recent years [1-3].Polymers such as epoxy, polytetrafluoroethylene, and polyimide have been applied commercially [4-6].However, the evolution of electronic communication technology puts forward higher requirements for the dielectric properties of low-k polymer materials.
As a thermosetting resin,polybenzoxazine exhibits many excellent properties, such as good thermal stability, hydrophobicity,outstanding chemical resistance, and intrinsic low dielectric constant [7-10].Moreover, the molecular design of benzoxazine is extremely flexible.It is practicable to introduce low polarizability groups or bulky groups into the molecular structure of polybenzoxazines.Feng et al.[11] prepared a polybenzoxazine containing carboxyl groups using diphenolic acid and furfurylamine as raw materials, which have good thermal and dielectric properties.Zhang et al.[12] designed a low-k polybenzoxazine containing ortho-maleimide,which could be further polymerized to form benzoxazole.However, pure polybenzoxazine resins are still difficult to satisfy the requirement of low-k electronic application.
The organic-inorganic hybrid composite is an effective approach to enhance the dielectric properties.Materials containing Si—O bands such as polyhedral oligomeric silsesquioxane (POSS),polydimethylsiloxane (PDMS), and SiO2particles could endow composites with high thermal stability and good optical properties[13,14].Zhao et al.[15]prepared polybenzoxazine/POSS nanocomposites with a dielectric constant of 2.64 and a water contact angle of 127.7°.Asrafali et al.[16] prepared polybenzoxazines/SiO2nanocomposites whose dielectric constant and dielectric loss decreased with the addition of SiO2.Kumar et al.[17] prepared a series of silane functionalized bio-silica reinforced polybenzoxazine composites and found silica can significantly improve the dielectric and thermal properties.Casarino et al.[18] prepared a benzoxazine functional polysilsesquioxane using sol-gel method and copolymerized it with conventional benzoxazine to obtain a series of composites with enhanced thermal resistance and hydrophobic properties.
In this study, we designed a novel benzoxazine monomer (Paptes) with reactive ethoxy groups and large sterically hindered tert-butyl groups and prepared a benzoxazine functional organosilicon (HP-aptes) with dense Si—O—Si crosslinking network by a facile sol-gel method.Then, a series of organic-inorganic hybrid composites containing intrinsically low dielectric constant benzoxazine monomer(P-aptmds)and HP-aptes were prepared to further enhance the dielectric properties and improve thermal properties.The structure of benzoxazine monomers was characterized by Fourier transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance spectroscopy (NMR).The curing behavior and thermal properties of benzoxazine monomers and composites were further investigated.To ensure the application in the field of electronic communication materials, the dielectric properties were evaluated by a broadband dielectric impedance spectrometer.
2.Materials and Methods
2.1.Materials
Trichloromethane, ethanol, ammonia 25% (mass) in water, and hydrochloric acid 35% (mass) in water were purchased from Sinopharm Chemical Reagent Co., Ltd.Paraformaldehyde, sodium hydroxide and 4-tert-butylphenol were obtained from Shanghai Titan Scientific Co., Ltd.1,3-bis(aminopropyl)tetramethyldisilox ane (APTMDS) and 3-aminopropyltriethoxysilane (APTES) were purchased from Shanghai Macklin Biochemical Co.,Ltd.All chemicals were of analytical grade and used as received.
2.2.Synthesis of 6-(tert-butyl)-3,4-dihydro-3-(3-(triethoxysilyl)propyl)-2H-1,3-benzoxazine (P-aptes)
To obtain P-aptes,APTES,and paraformaldehyde were dissolved in trichloromethane and added to a flask.Then the mixture was heated to 40 °C under stirring conditions and kept for 1 h.Subsequently, 4-tert-butylphenol was added to the flask upon further heating up to 85 °C.After 4 h of reaction, the crude product was taken out and washed with NaHCO3aqueous and deionized aqueous in turn.After removing the solvent and water by rotary evaporation and freeze drying, the light-yellow viscous P-aptes was obtained.The synthesis route of P-aptes is shown in Fig.1.FT-IR(KBr, cm-1): 1321 (swing vibration of methylene in oxazine ring),1233(asymmetric Ar—O—C stretching),1123(C—N—C stretching),1104 and 1080 (Si—O—C stretching), 939 (stretching vibrations of oxazine ring) (Fig.S1 in Supplementary Material).1H NMR(600 MHz, CDCl3, δ): 6.56-7.03 (—ArH), 4.71 (O—CH2—N), 3.85(N—CH2—Ar).13C NMR (600 MHz, CDCl3, δ): 82.53 (O—CH2—N),58.40 (N—CH2—Ar).29Si NMR (600 MHz, CDCl3, δ): -45.17 (C—Si—O) (Fig.S2).
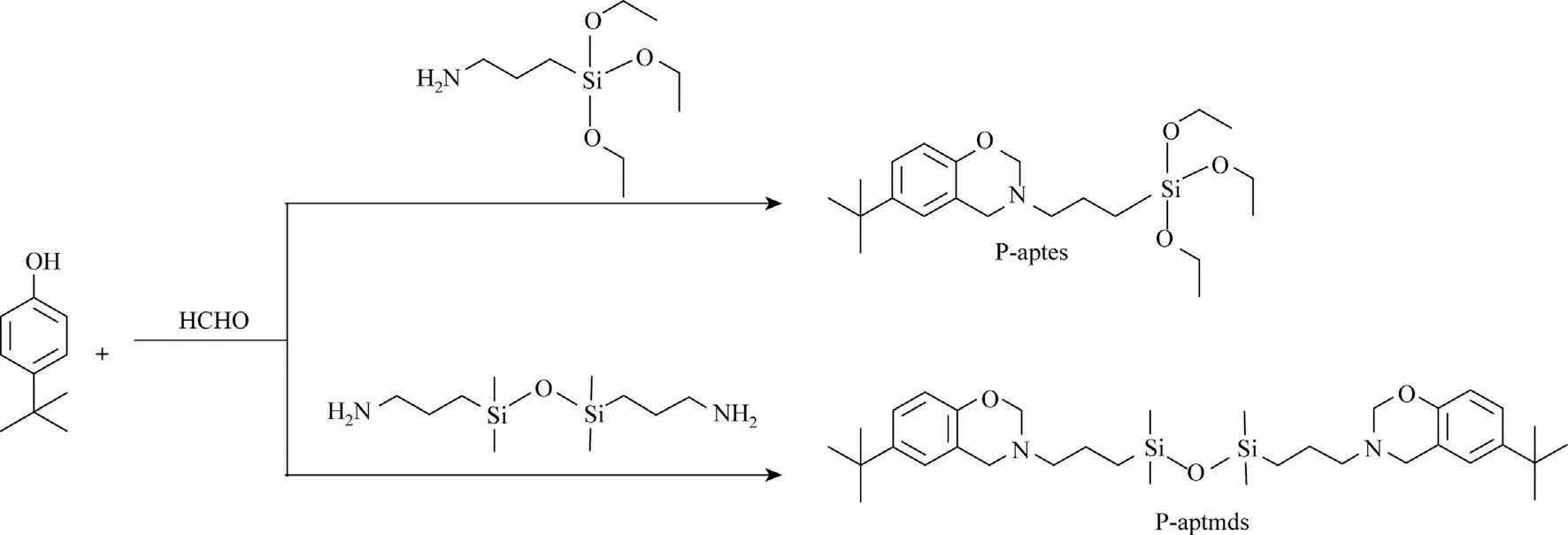
Fig.1.Synthesis routes of benzoxazine monomers (P-aptes and P-aptmds).
2.3.Synthesis of 1,3-bis(3-(6-(tert-butyl)-3,4-dihydro-2H-1,3-benzoxazine-3-yl)propyl)-1,1,3,3-tetramethyldisiloxane (P-aptmds)
P-aptmds was synthesized according to the Ref.[19].In detail,APTMDS and paraformaldehyde were dissolved in trichloromethane and added into a three-neck flask.Then the system was heated to 60 °C under stirring conditions.Subsequently,4-tert-butylphenol was added to the flask upon further heating up to 80 °C.After keeping for 6 h, the crude reaction product was extracted with NaOH aqueous, HCl aqueous, and deionized water in turn.Trichloromethane and water remaining in the organic phase were removed by rotary evaporation and freeze drying to afford yellow viscous liquid.The synthesis route of P-aptmds is shown in Fig.1.FT-IR (KBr, cm-1): 1321 (swing vibration of methylene in oxazine ring), 1253 (Si-CH3bending), 1232(asymmetric C—O—C stretching), 1124 (C—N—C stretching), 1055(Si—O—Si stretching), 938 (stretching vibrations of oxazine ring)(Fig.S3).1H NMR (600 MHz, CDCl3, δ): 6.60-7.10 (—ArH), 4.78(O—CH2—N), 3.93 (N—CH2—Ar).13C NMR (600 MHz, CDCl3, δ):82.00(O—CH2—N),54.31(N—CH2—Ar).29Si NMR(600 MHz,CDCl3,δ): 7.62 (C—Si—O) (Fig.S4).
2.4.Synthesis of organosilicon (HP-aptes)
As shown in Fig.2,a benzoxazine functional organosilicon(HPaptes)was prepared by a sol-gel method.P-aptes was dissolved in a solvent containing of ethanol and water (3:1, volume ratio), and then ammonia 25% (mass) in water was added.The mixture was stirred at room temperature for 18 h and then centrifuged.The sediment was washed with ethanol and ultrapure water in sequence.The white powder obtained after vacuum drying was named as HP-aptes.
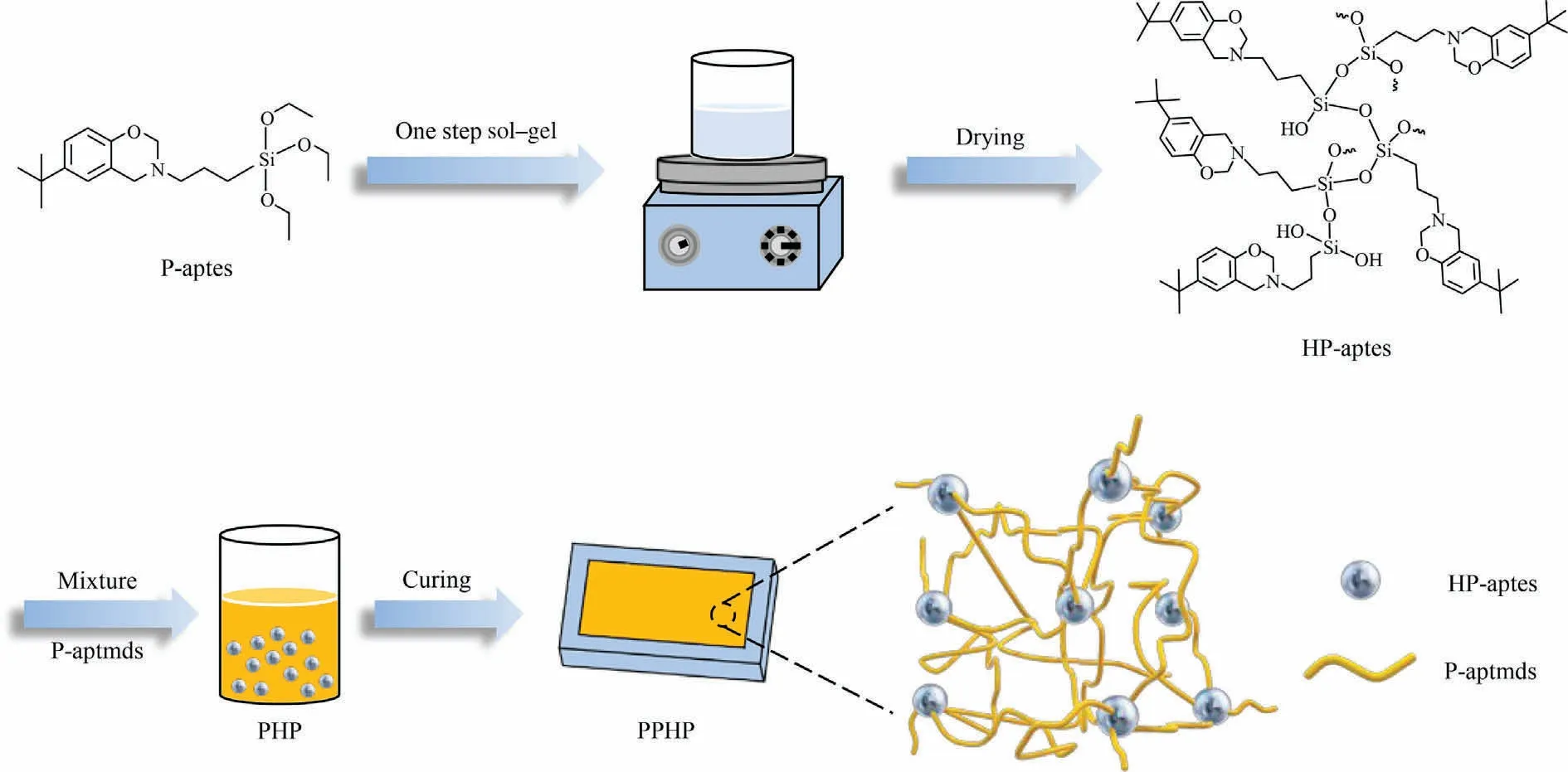
Fig.2.Preparation route of PPHP composites.
2.5.Preparation of polybenzoxazine composites (PPHP)
As shown in Fig.2,five samples containing different proportions of P-aptmds and HP-aptes were prepared and noted as P-aptmds,PHP10, PHP20, PHP30, and HP-aptes, respectively.For instance,PHP10 meant the mass ratio of P-aptmds and HP-aptes was 9:1.These five samples were first placed into the mold,and then placed into a vacuum drying oven at 70 °C for 1 h.PP-aptmds, PPHP10,PPHP20, PPHP30, and PHP-aptes were obtained after the subsequent curing process: 120 °C (1 h), 150 °C (1 h), 180 °C (1 h),200 °C (1 h), 210 °C (1 h) and 220 °C (6 h).
2.6.Characterizations
Fourier transform infrared spectroscopy.FT-IR was measured with a Thermo Fisher Scientific Nicolet IS10 analyzer (America)using the KBr pellet method.All spectra were scanned 32 times with a resolution of 4 cm-1in the range of 4000 cm-1-400 cm-1.
Nuclear magnetic resonance spectroscopy.One dimensional1H NMR,13C NMR,and29Si NMR spectra were recorded on a Bruker ASCEND NMR spectrometer (Germany) in deuterated chloroform.Solid-state29Si NMR spectrum was measured on a Bruker Advance III 500 MHz spectrometer (Germany).
Surface properties measurement.Roughness testing was performed with Bruker Dimension ICON atomic force microscopy(Germany).Micro morphology was collected using an FEI Nova NanoSEM 450 scanning electron microscopy (America).Element distribution(EDS)was characterized by an EDAX TEAMEDS energy dispersive spectroscopy (America).The optical test was implemented using a WGT-S transmittance/haze meter (China).
Hydrophobic properties measurement.The water absorption test was conducted by IPC-TM650-2.6.2.1.The mass change of samples before and after immersion in water at room temperature for 24 h was recorded.The static water contact angle was tested with a Dataphysics OCA20 contact angle measuring instrument(Germany).
Thermal properties analysis.The curing behavior of samples was monitored by a TA Q2000 differential scanning calorimetry(America)from 50°C to 300°C at a rate of 10°C·min-1under nitrogen atmosphere.The mass loss of samples was characterized using a TA SDT Q600 Thermogravimetric analyzer (America) from 40 °C to 800 °C at a rate of 10 °C·min-1under nitrogen atmosphere.A Mettler-Toledo DMA1 dynamic mechanical analyzer (America)was employed to determine the storage modulus E′and loss factor tan(δ) using double cantilever mode.
Dielectric property measurement.The dielectric properties of polybenzoxazines were tested using a Novocontrol Concept 40 broadband dielectric spectrometer (Germany).The test frequency range is from 100 Hz to 1 MHz.
3.Results and Discussion
3.1.Structure of HP-aptes organosilicon
The structure of organosilicon,namely HP-aptes,was characterized by FT-IR, and the result was shown in Fig.3(a).Similar to the FT-IR spectrum of P-aptes, the absorption peaks at 939, 1233 and 1321 cm-1indicate the typical C-H out-of-plane bending vibration, C—O—C stretching vibration and CH2wagging in the oxazine ring respectively.The absorption peaks at 1503,900 and 819 cm-1represent the 1,2,4-trisubstituted benzene ring.Signals at 1023 and 1124 cm-1are absorption peaks of Si—O—Si stretching vibration.The absorption peak at 3434 cm-1is Si—OH stretching vibration peak.
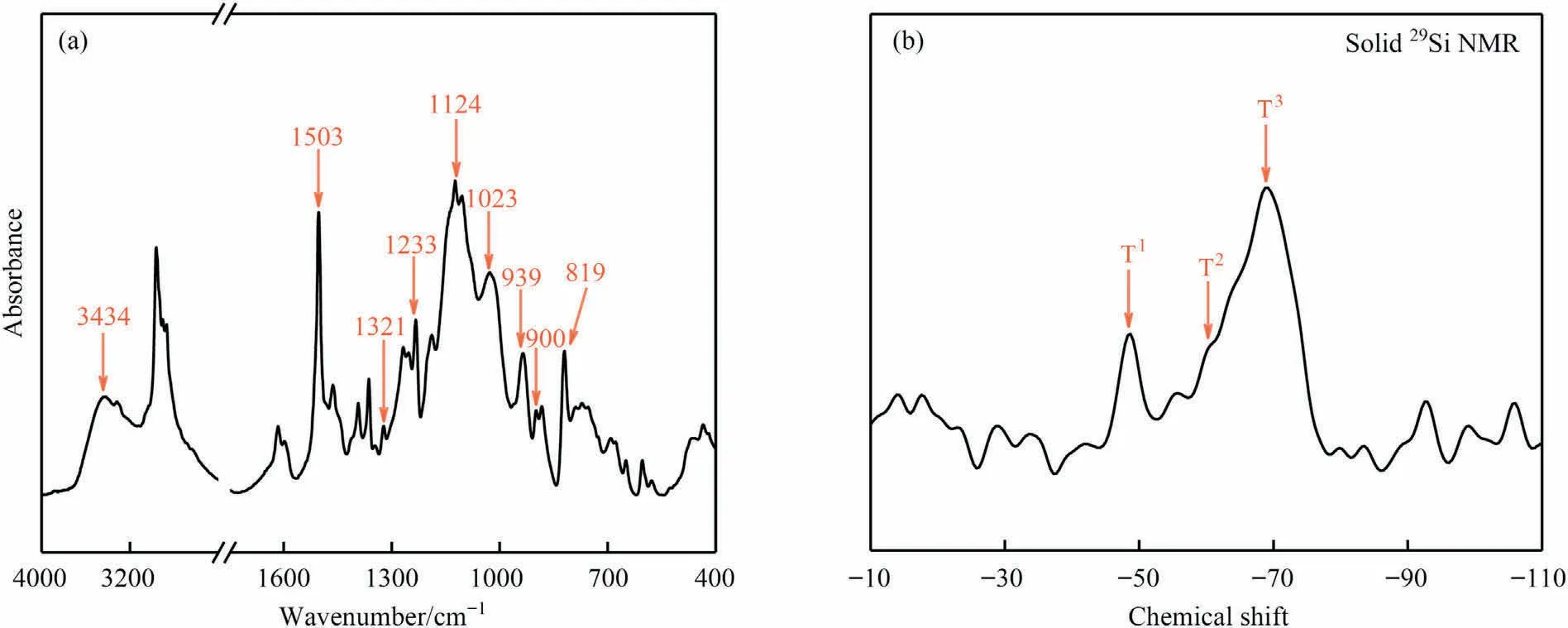
Fig.3.(a) FT-IR spectrum and (b) solid-state 29Si NMR spectrum of HP-aptes.
The crosslinking degree of the siloxane structure in HP-aptes was characterized by solid-state29Si NMR.Chemical shifts of silicon atoms are referred to using the traditional terminology Tn[RSi(OSi)n(OH)3-n].Fig.3(b) shows a major resonance peak at-68.34 which corresponds to the completely condensed T3.This indicates that most of the silicon atoms are cross-linked at all three reactive sites.The peaks near-64.47 and-48.28 correspond to the linear T2and the terminal T1, respectively.The positions of these response peaks are in good agreement with those reported in the literature [20,21].
3.2.Curing behavior of PHP mixtures
Fig.4 shows the DSC curves of PHP mixtures and PPHP composites,and the specific data are displayed in Table 1.P-aptmds shows an exothermic peak with an onset temperature Tonof 225°C and a peak temperature Tpeakof 261 °C, while HP-aptes displays a broader exothermic peak with a Tonof 154 °C and a Tpeakof 203 °C.HP-aptes exhibits a relatively low enthalpy ΔH due to the volatilization of the water produced by polycondensation of the silicon hydroxyl groups during heating.As the content of HPaptes increases, the exothermic peak of PHP mixtures gradually shifts to the lower temperature and the enthalpy value ΔH gradually decreases.When the proportion of HP-aptes is 30%(mass),the Tpeakand ΔH of PHP30 are 233 °C and 72.73 J·g-1, respectively.As shown in Fig.4(b),no thermal events were detected in the thermograms of polymers.The results indicate that the ring-opening polymerization reaction was completed after the curing process described in Section 2.1.

Table 1 DSC data of P-aptmds/HP-aptes with different compositions
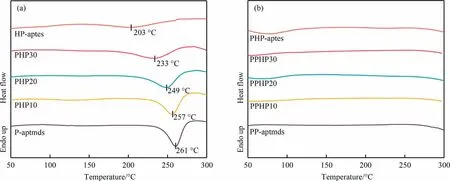
Fig.4.DSC curves of (a) PHP mixtures and (b) PPHP composites.
FT-IR analysis was employed to confirm the molecular structure of composites,and the results are exhibited in Fig.5.For qualitative analysis,the symmetric deformation of C—H in the methyl group at 1364 cm-1was designated as the benchmark and all curves were normalized.The absorption peaks at 839 and 795 cm-1are stretching vibration peaks of C—Si.The broad peaks between 1150 and 1000 cm-1are Si—O—Si stretching vibration peaks.The absorption peak at 1254 cm-1is the bending vibration peak of Si—CH3.The absorption peaks at 1321, 1232 and 939 cm-1related to the oxazine ring in Fig.S1 and Fig.S3 completely disappear.In addition,absorption peaks of 1,2,3,5-tetrasubstituted benzene ring at 1484 cm-1and phenolic hydroxyl at 3432 cm-1are observed.The FT-IR results prove that the oxazine ring has been fully opened and the polybenzoxazine crosslinking network has formed.
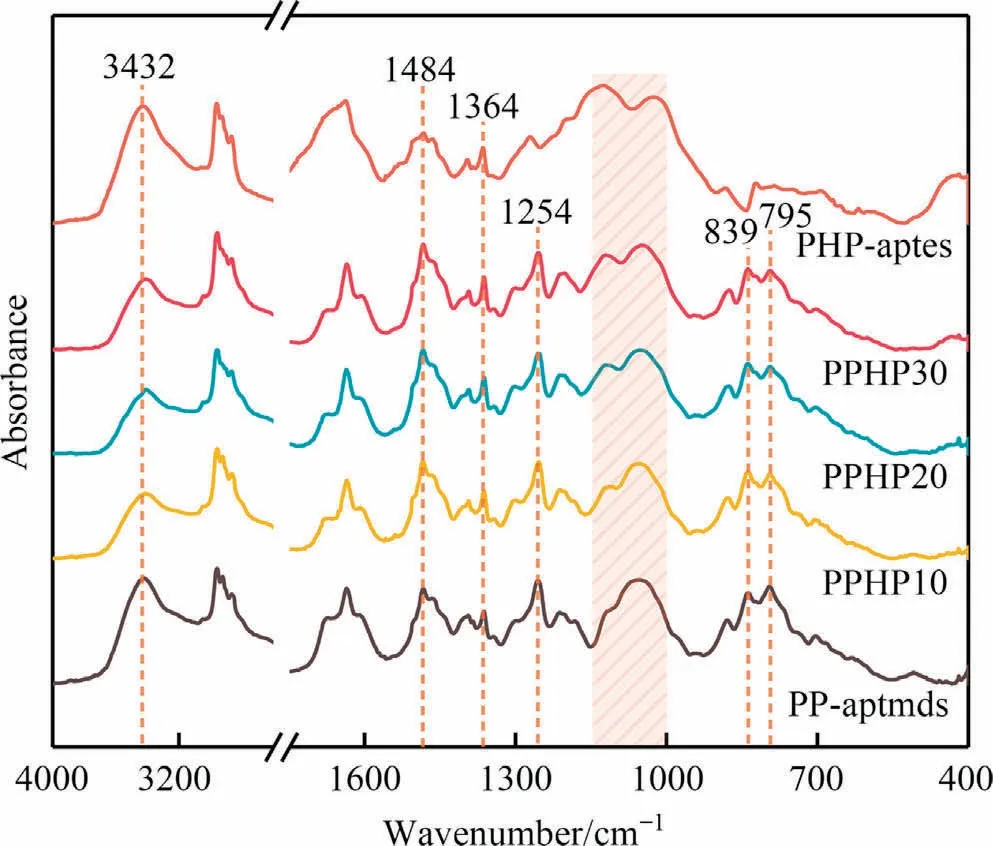
Fig.5.FT-IR curves of polybenzoxazines.
3.3.Surface and optical properties of composites
A well-defined microstructure is quite significant to endow unique properties of polymer materials.The microscopic and macroscopic morphologies of polybenzoxazines are displayed in Fig.6.All samples are reddish brown and transparent.Moreover,polybenzoxazines obtained become more and more transparent and light-colored as the content of HP-aptes increases.When the magnification was ×20000 as shown in Fig.6 (a)-(d), the surface of all samples is smooth and flat, and there are no cracks or other defects.While pure HP-aptes cannot form a film by itself due to its rigidity.This indicates that P-aptmds has superior film-forming properties as a matrix material.In addition, HP-aptes has excellent compatibility with P-aptmds because of the structural similarity.EDS characterization was conducted and the surface scanning results of element Si are shown in Fig.6 (e)-(h).The element Si is evenly distributed on polybenzoxazines, which demonstrates that the organosilicon HP-aptes has good dispersibility in the pre-pared polybenzoxazines.
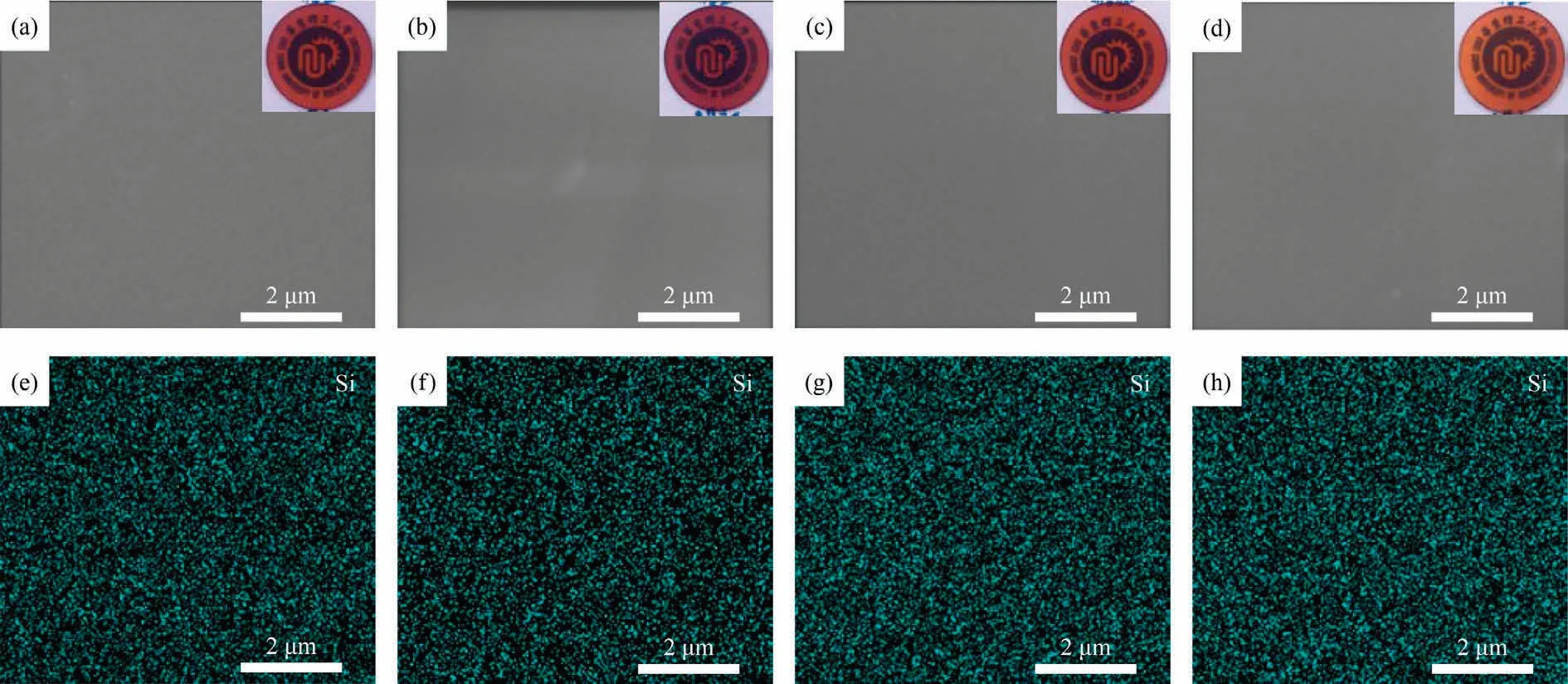
Fig.6.SEM and EDS images of (a), (e) PP-aptmds, (b), (f) PPHP10, (c), (g) PPHP20 and (d), (h) PPHP30 (the inset is the corresponding macro morphology).
Atomic force microscope was employed to further characterize the surface roughness of polybenzoxazines.The sampling range is 2 μm × 2 μm.Fig.7 shows height images of polybenzoxazines prepared.Table 2 displays the average of roughness (Ra) and the root mean square of roughness (Rq).PP-aptmds has a smooth surface whose Raand Rqare 0.667 nm and 0.832 nm respectively.Organosilicon HP-aptes did not lead to a significant increase in roughness and all composites have a Rqbelow 1 nm, which could prove the excellent compatibility between P-aptmds and HP-aptes.
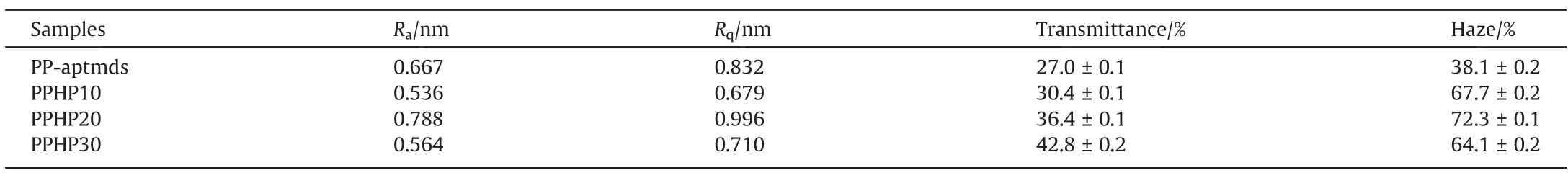
Table 2 Optical and surface properties of polybenzoxazines
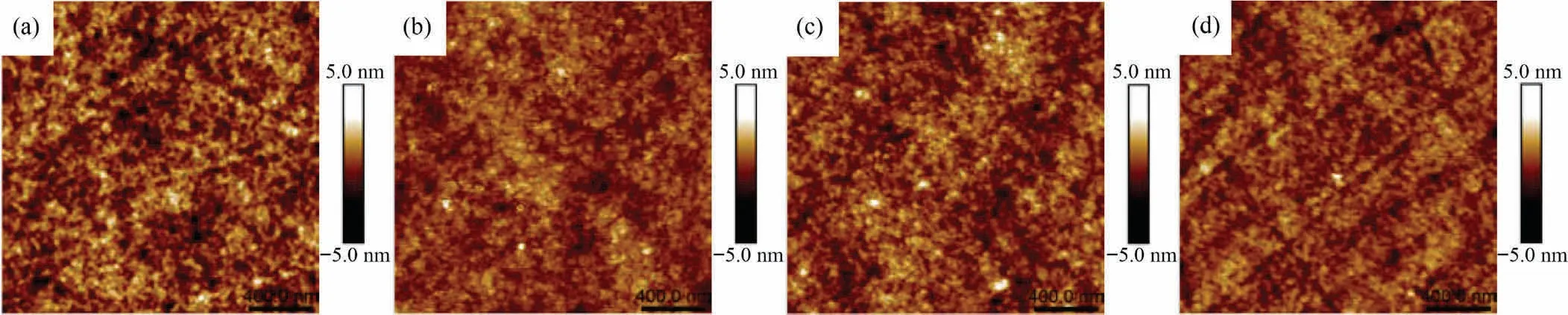
Fig.7.Height images of (a) PP-aptmds, (b) PPHP10, (c) PPHP20 and (d) PPHP30.
In order to further characterize the light transmittance of polybenzoxazines,optical tests were employed to obtain the transmittance and haze value, and the results are shown in Table 2.The transmittance and haze values of polybenzoxazines gradually increase after adding HP-aptes.After adding 30% of HP-aptes, the transmittance of polybenzoxazines resin increased from 27.0% to 42.8%; the haze value of polybenzoxazine increased from 38.1%to 64.1%.
3.4.Hydrophobic properties of composites
Hydrophobicity is quite important for low-k materials because the dielectric constant of water at room temperature exhibits a remarkably high value [22].Fig.8 displays the water absorption and static water contact angle of all prepared polybenzoxazines.After being soaked in water for 24 h at room temperature, the water absorption of all prepared polybenzoxazines is relatively low.The water absorption of PPHP20 is 0.23%±0.02%.Polybenzoxazines generally have low surface energy and good hydrophobicity[23].The water contact angles of PP-aptmds,PPHP10,PPHP20 and PPHP30 are 99.9°, 101.6°, 105.2° and 106.8°.The abundant Si—O—Si segments and alkyl chains in the molecular structure contribute greatly to the hydrophobicity of prepared composites.The results show that all prepared polybenzoxazines have good hydrophobicity,which is conducive to the improvement of dielectric properties.
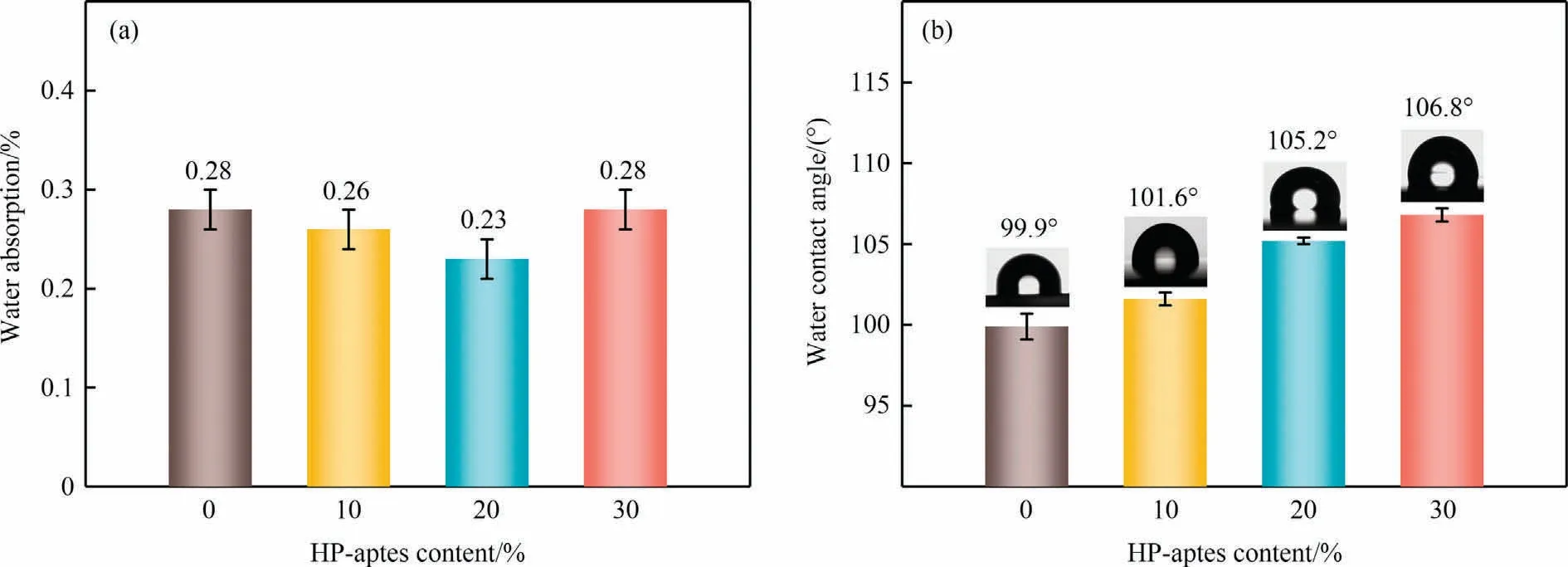
Fig.8.(a) Water absorption and (b) water contact angle of polybenzoxazines.
3.5.Thermal properties of composites
The effect of HP-aptes on the dynamic thermomechanical properties of composites was investigated by dynamic mechanical analyzer (DMA).As shown in Fig.9 and Table 3, composites with the rigid Si—O—Si crosslinking network have a E’ of 2834-3518 MPa at 30 °C, which is higher than 2560 MPa of PP-aptmds.The peak temperature of tan(δ) is recorded as the glass transition temperature (Tg).As shown in Fig.9(b), the three composites all have higher Tgthan PP-aptmds, which proves that the mobility of molecular chains gradually decreases with the increase of HPaptes.
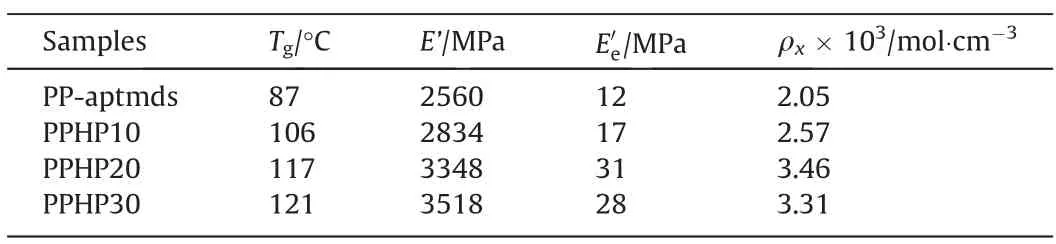
Table 3 DMA data of PPHP composites

Fig.9.DMA thermograms of polybenzoxazines: (a) storage modulus E′ and (b) tan(δ).
Nielsen proposed that the crosslinking density of polymers can be quantitatively calculated from the equilibrium storage modulus in the rubbery region using Eq.(1) [24].
The TGA and DTG thermograms of composites are displayed in Fig.10, and the specific data are shown in Table 4.With the increase of temperature, the mass loss of PP-aptmds is the most severe, and the char yield (CY) is only 11.1% when temperature is 800 °C.While PHP-aptes exhibits the best thermal stability, and the CY is as high as 54.0%.The temperature when PPHP10,PPHP20 and PPHP30 lose 5% of mass (T5%) are 294 °C, 296 °C and 297 °C,respectively.Since HP-aptes is rich in Si—O bonds which have high bond energy (460 kJ·mol-1), HP-aptes could effectively improve the thermal stability of the composites.In addition, HP-aptes can increase the crosslinking density of the composites, which is also beneficial to the thermal stability of composites.
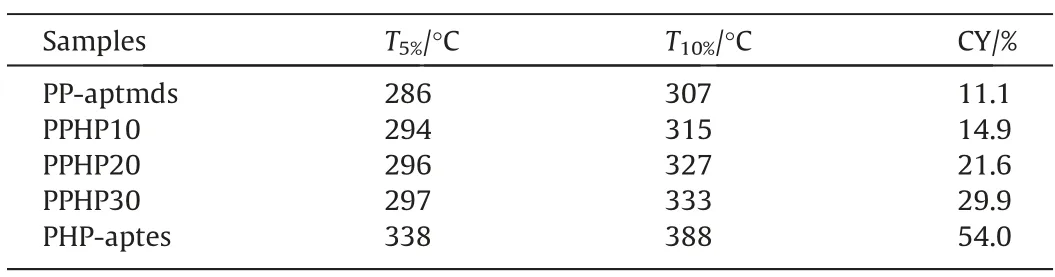
Table 4 TGA results of polybenzoxazines

Fig.10.(a) TGA and (b) DTG curves of polybenzoxazines.
As shown in Fig.10(b), all obtained polybenzoxazines exhibit multiple mass loss events.The thermal mass loss of polybenzoxazines could be divided into two stages: the evaporation of amines and the degradation of phenol structures [25].The products after C—N bond cleavage in HP-aptes still have high thermal stability due to the dense Si—O—Si crosslinking network connected to the aminopropyl group.In addition, as the content of HP-aptes increases, the content of oxazine ring in the composites would decrease, and the content of C—N bonds would also decrease.Therefore, when the temperature is lower than 500 °C, the mass loss rate of polybenzoxazines decreases with the increase of HPaptes content.
3.6.Dielectric properties of composites
The dielectric constant and dielectric loss Tan (δ) could affect the propagation speed and loss of the signal in the dielectric mate-rials[26].Therefore,it is of great importance to reduce the dielectric constant and loss of polymer materials.Dielectric properties of prepared polybenzoxazines are displayed in Fig.11 and Table 5.Due to the large sterically hindered tert-butyl and non-polar siloxane segment,PP-aptmds exhibit a relatively low dielectric constant of 2.79 at 1 MHz.The addition of organosilicon HP-aptes which possesses a large number of Si—O—Si cross-linked networks can reduce the dipole density and the polarizability of the composites.As the content of HP-aptes increases, the dielectric constant of polybenzoxazines gradually decreases.The dielectric constant of PPHP30 at 1 MHz dropped to 2.61.In addition,current works about composites containing polybenzoxazine and silicon were listed in Table 5.Compared to them, PPHP prepared in this work has a relatively low dielectric constant and tan(δ), which proves the organic-inorganic hybrid method used is effective for reducing the dielectric constant.
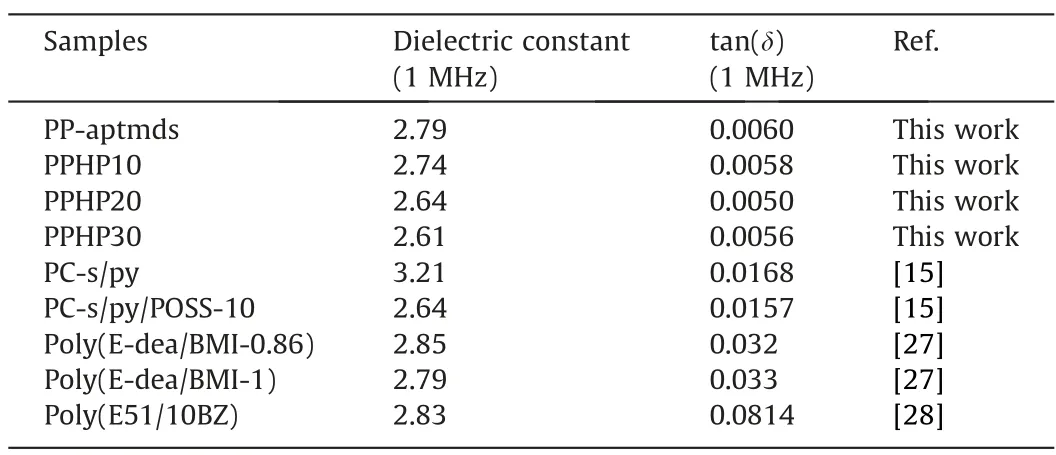
Table 5 Dielectric properties data of polybenzoxazines
As shown in Fig.11(b), the tan (δ) of PP-aptmds at 1 MHz is 0.0060.The dense polybenzoxazine/Si—O—Si double crosslinking network of composites can make molecular chains difficult to move,which is beneficial to the reduction of dielectric loss.Therefore,the composites all exhibit lower dielectric loss than that of PPaptmds because of the greater crosslink density.In addition, the intrinsic nature of the Si—O—Si crosslinking network can also reduce the dielectric loss of polymers [29].The dielectric loss of PPHP10 and PPHP20 are reduced to 0.0058 and 0.0050 respectively.The dielectric loss of PPHP30 has increased slightly, but it is still lower than pure PP-aptmds.It might be caused by the interfacial effect caused by a large number of HP-aptes.
4.Conclusions
In summary, a series of organic-inorganic hybrid composites PPHP were prepared.Benefiting from the excellent compatibility between P-aptmds and HP-aptes,composite films are flat and uniform.When the content of HP-aptes is 30% (mass), the water absorption and water contact angle of PPHP30 is 0.28% and 106.8° respectively.The rigid Si—O—Si crosslinking network increases the storage modulus of composites from 2560 MPa to 3518 MPa,while increasing T5%from 286°C to 297°C.In addition,abundant oxazine rings in HP-aptes endow composites with extra crosslinking sites and high crosslinking density.Meanwhile, HPaptes reduces the dipole density of composites, which reduces the dielectric constant of PPHP30 to 2.61 at 1 MHz.These obtained composites exhibit great potential in the field of electronic communication.
CRediT Authorship Contribution Statement
Manlin Yuan:Conceptualization,Methodology,Validation,Formal analysis, Investigation, Writing - original draft.Xin Lu:Resources, Methodology, Writing - review & editing, Supervision,Project administration.Xiaoyun Ma:Writing - review & editing,Visualization.Hao Lin:Writing - review & editing, Visualization.
Angui Lu:Writing-review&editing,Formal analysis.Liyan Shao:Writing-review&editing,Formal analysis.Zhong Xin:Resources,Supervision, Funding acquisition.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Innovation Program of the Shanghai Municipal Education Commission (2019-01-07-00-02-E00061)and the Shanghai Municipal Science and Technology Commission (21520761100).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.06.024.
 Chinese Journal of Chemical Engineering2023年12期
Chinese Journal of Chemical Engineering2023年12期
- Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
