Insight into the green route to dimethyl succinate by direct esterification of bio-based disodium succinate using CO2 and CH3OH
Yu-Gao Wang, Xiao-Chen Han, Bao Gu, Hua-Shuai Wu, Jun Shen
School of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, China
Keywords:Succinate Carbon dioxide Direct esterification Reaction mechanism Reaction kinetics Kinetic modeling
ABSTRACT The fermentation for succinic acid production outperforms other methods by low energy consumption and environmental benignity, with the resulting products mainly as disodium succinate (DSA).By directly esterifying DSA using CO2 and CH3OH, it is expected to avoid the use of inorganic acids.By high-resolution mass spectrometry analysis and theoretical calculation, this study establishes that the reaction consists of three steps, i.e., first forming 3-carboxypropanoate, then monomethyl succinate(MMS),and finally dimethyl succinate(DMS).A detailed kinetic analysis is further performed,the results demonstrate that the transformation of DSA to MMS is regarded to be a second-order reaction for reactant DSA, while the transformation of MMS to DMS is a first-order reaction for reactant MMS.The activation energy for the generation of MMS from DSA is 37.15 kJ·mol-1,and that for the generation of DMS from MMS is 85.80 kJ·mol-1, indicating the latter one is the rate-determining step.
1.Introduction
Succinic acid (SA) is an important synthetic intermediate for various chemicals and polymers [1,2].The current industrial production of SA comes from the hydrogenation of maleic anhydride generated from the hydrogenation of petrochemical [3].However,the large-scale production of SA from petrochemicals is inevitably limited by the fluctuating price and reserve of petroleum.Other than aligning with the current low-carbon economy[4],the biological synthesis of SA by the fermentation of lignocellulose and starch has the obvious advantages of low cost for the raw material and environmental benignity [5].
With intensifying public environmental awareness and enacting ‘‘plastic ban”, the demand for biodegradable plastics such as poly(butylene succinate) is dramatically increasing [6].As the starting material for synthesizing poly(butylene succinate), the production of SA and its homologous esters has become increasingly pressing [7].Therefore, it is necessary to achieve stable and sustainable production of SA and dimethyl succinate (DMS) [8].The polymerization of DMS by the current transesterification polymerization method [9] is a facile approach as compared with the direct melting polymerization [10] required for SA.Considering the polymerization method, DMS is an ideal polymer precursor[11].The manufacture of desired chemicals from biomass is a very challenging task [12].Under hypoxia conditions, corticium glutamate strains are required for producing SA [13].During biological fermentation, a continuous addition of an alkaline solution is required to maintain the fermentation broth close to neutral[14], which is necessary for the efficient production of carboxylic acids[15]by microorganisms.Thus,the generated carboxylic acids in the fermentation tank are in the form of carboxylates [16], and the classical process of converting succinate to DMS usually involves the acidification of succinate to SA using inorganic acids and subsequent esterification with alcohols to generate DMS[17].However,it is not environmentally benign during the acidification of succinate because a large amount of inorganic acid is consumed, thus generating a large amount of waste water.
The research group of Straathof used a strong anionic resin containing quaternary ammonium salt functional groups to adsorb succinate in the fermenter, and then directly produce DMS with chloroethane as a methylation reagent,greatly reducing the energy consumption and waste production [18].However, chloride compounds as reagents do not comply with the policy of environmental protection.So direct esterification has been upgraded by replacing chloroethane with benign dimethyl carbonate (DMC)[19,20].Nevertheless, the current production cost of DMC is relatively high [21].It is urgent to reduce CO2emissions, because excessive CO2emissions disrupt the natural carbon cycle balance,also aggravate global warming and natural disasters [22-24].Meanwhile,CO2is a valuable C1 resource,the capture[25],storage[26], and utilization [27] of CO2have been attracted increasing attention.CO2and methanol could be used for synthesizing DMC[28-30].Straathof et al.[31] adopted CO2and methanol as esterification reagents, achieved the direct esterification of succinate,and realized the fixation of CO2to a certain extent.Moreover, the formed carbonate as a byproduct could be recycled into the fermenter to maintain the fermentation broth close to neutral.The recent work of Straathof’s research group [31] greatly facilitated the direct esterification of succinate.However,due to the inherent instability of ion exchange resin,the esterification of succinate was only conducted at a low reaction temperature (<100 °C), resulting in only an 18% mole yield of DMS after 20 h.
In the absence of resin, Kulkarni et al.[32] have achieved the direct esterification of some monocarboxylates at high temperature(170-175°C)with CO2and methanol as esterification reagents for the first time.The yields of methyl acetate and methyl lactate reached up to 81% and 81.2% only after 5 h, respectively.Furthermore, it is worth investigating the application of this method for the direct esterification of succinate.
Based on the current research progress, this study would focus on the direct esterification of bio-based dicarboxylates using CO2and methanol as esterification reagents.With disodium succinate(DSA) as a model compound, the reaction conditions, reaction mechanism and kinetics in the direct esterification process were investigated by high resolution mass spectrometry (HRMS), theoretical calculations and simulation.
2.Materials and Methods
2.1.Materials
The majority of reagents were used as analytically pure,such as SA (>98.0%), DSA (>99.0%), CH3OH (99.5%), monomethyl succinate(MMS) (>98.0%), and DMS (99.0%).Acetonitrile was chromatographically pure.Phosphoric acid was used at 85% (mass),hydrochloric acid at 36% (mass) and the purity of CO2was greater than 99.9%.Deionized water was used for all reactions.
2.2.Experimental section
As displayed in Fig.1, DSA was dissolved in 50 ml CH3OH, and then transferred into a 250 ml stainless-steel,magnetically stirred autoclave.The solution inside the autoclave was purged with CO2three times, and the autoclave was pressurized with CO2to a desired pressure at room temperature,heated to an indicated temperature within 30 min, and maintained for different time.Then the autoclave was immediately cooled to room temperature in an ice water bath.The resulted mixture in the autoclave was subjected to HRMS and high performance liquid chromatography(HPLC) analysis.
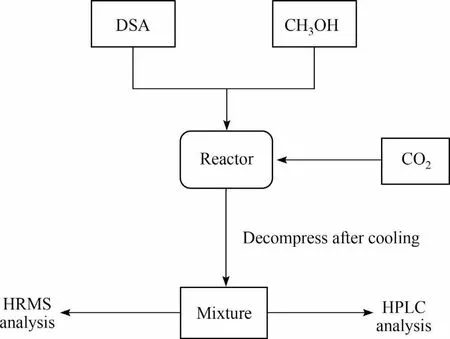
Fig.1.Experimental procedure of esterification reaction of DSA, CH3OH and CO2.
2.3.Analytical methods
The qualitative analysis of the product was conducted by HRMS of Bruker solariX 70 (Germany), equipped with an electrospray ionization source in positive and negative modes.The moisture content in the reaction sample before and after the reaction was analyzed by the Karl Fisher titration method using automatic Karl Fischer instrument (Metrohm AG, Switzerland).
For the chromatographic analysis, the mixture of the reaction products was acidified,and the amount of SA was used to indicate the amount of DSA in the reaction.The quantitative analysis was conducted with Agilent 1260 high performance liquid chromatography (HPLC) with ZORBAX SB-C18 chromatographic column(5 μm × 4.6 mm × 250 mm).The mobile phase was acetonitrile and 0.10% phosphoric acid aqueous solution.The flow rate was set at 1.0 ml·min-1.The UV detection wavelength was 210 nm.The injection volume was 20 μl, and the column temperature was 35 °C.The eluent containing SA, MMS and DMS was detected by the gradient elution program, and the standard liquid chromatography and the corresponding linear relationship were shown in Fig.2 and Table 1.The products were quantitatively analyzed via HPLC by using the external standard method.The conversion rate of DSA, the yield and the selectivity of MMS and DMS were calculated as follows (unit of amount: mol):
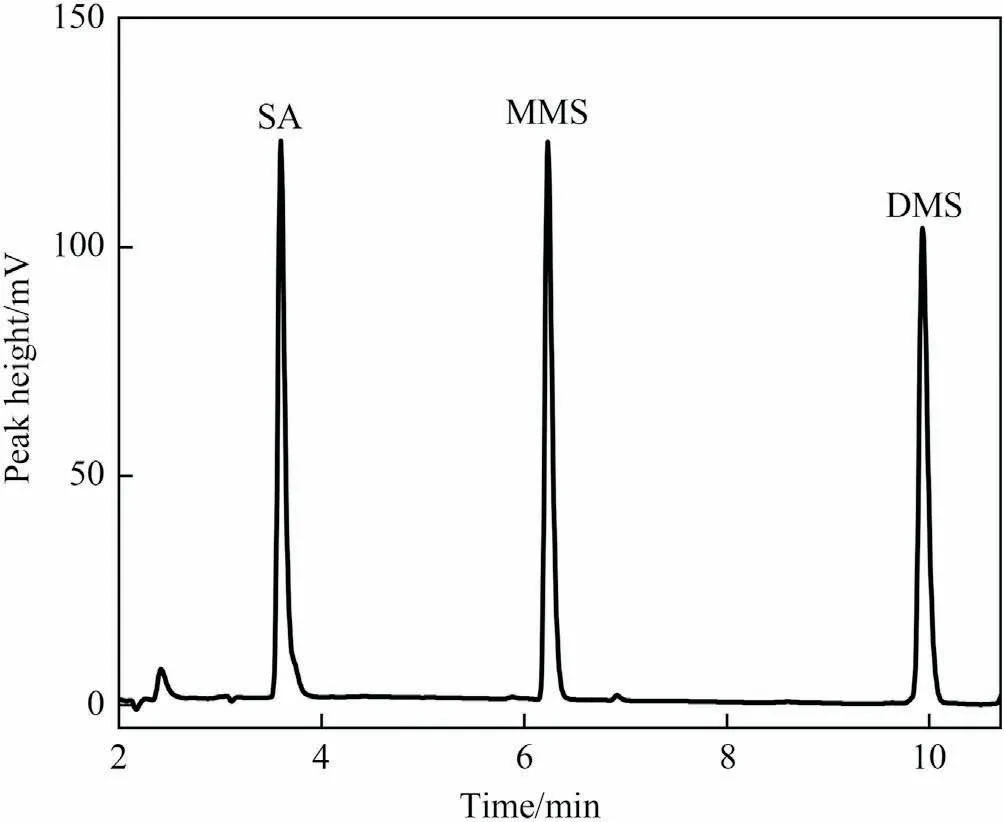
Fig.2.HPLC spectrum of pure SA, MMS and DMS.
3.Results and Discussion
3.1.Influence of reaction conditions on the direct esterification of DSA
The reaction temperature is a dictating factor for the esterification reaction.As shown in Fig.3(a), the conversion rate of DSA increased first and then approached steady with increasing reaction temperature.Before the reaction temperature reached 150°C,the yield of MMS in the system increased rapidly and then decreased gradually with the increased temperature.The yield of DMS increased with raising reaction temperature, but the increasing rate was faster at the temperature higher than 160°C.The data indicated that the reaction temperature within a certain range would facilitate the conversion rate of DSA, and the increase of temperature was conducive to the conversion of MMS to DMS,which could be attributed to that more MMS were activated to cross the reaction barrier to form the product DMS at the high temperature.The yield of DMS gradually increased, while the yield of MMS initially increased to the maximum and then decreased with the reaction time evolution in Fig.3(b).As shown in Fig.3(c), the conversion rate of DSA and the yield of MMS gradually increased with the elevating initial pressure of CO2, while the yield of DMS remained basically unchanged,which suggested that the increased initial pressure of CO2could only increase the conversion of DSA to MMS.This might be due to that the equilibrium shifted to the precursor of MMS from DSA at the high initial pressure of CO2, which resulted in the increased yield for MMS.As displayed in Fig.3(d),the conversion rate of DSA and the yield of MMS and DMS gradually decreased, but the selectivity of DMS almost remained constant with the increased addition of DSA.It was suggested that the initial concentration of DSA maybe not be involved in the rate equation,or this reaction was not the rate-determining step for the final product of DMS.
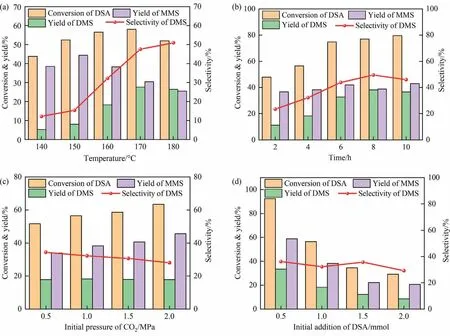
Fig.3.Reaction conditions for the conversion of DSA,yields of MMS and DMS and selectivity of DMS in DSA esterification reaction(reaction conditions:(a)reaction time 4 h,initial pressure of CO2 1.0 MPa, initial DSA dosage 1.0 mmol; (b) reaction temperature 160 °C, initial CO2 pressure 1.0 MPa, initial DSA addition 1.0 mmol; (c) reaction temperature 160 °C, reaction time 4 h, DSA initial dosage 1.0 mmol; (d) reaction temperature 160 °C, reaction time 4 h, initial pressure of CO2 1.0 MPa).
3.2.Reaction pathways for the direct esterification of DSA
For the determination of reaction pathways, it is important to first determine the specific distribution of the products.The crude reaction mixture from the reaction of DSA with CH3OH and CO2consisted of carboxylic acid ester,carboxylic acid,and carboxylate,and it was difficult to be directly analyzed by HPLC or gas chromatography/mass spectrometry.HRMS could achieve the product self-separation and then analyze the components with high resolution.Also, the molecular composition of each component in the product mixture could be inversely derived from the accurate molecular weight.In this study, the resulting mixture from the direct esterification of DSA with CH3OH and CO2was directly analyzed by HRMS.As shown in Fig.4 and Table 2, some components were determined, which were DMS (Compound 1), sodium 4-methoxy-4-oxobutanoate (Compound 2), DSA (Compound 3),methyl carbonate (Compound 4), MMS (Compound 5), 3-carboxypropionate (Compound 6), and sodium 3-carboxypropanoate (Compound 7).
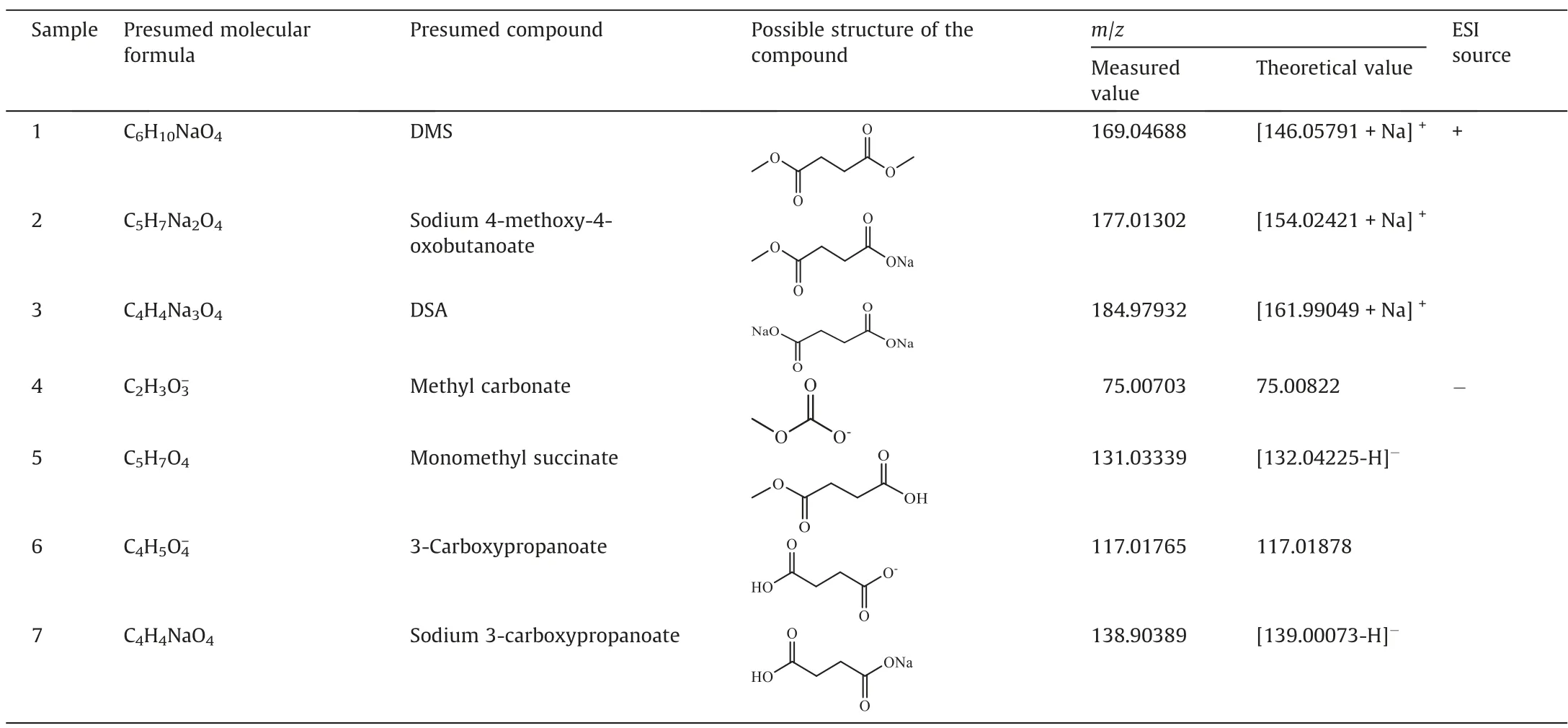
Table 2 Possible molecular formulas obtained by HRMS detection of the resulting mixture after reaction
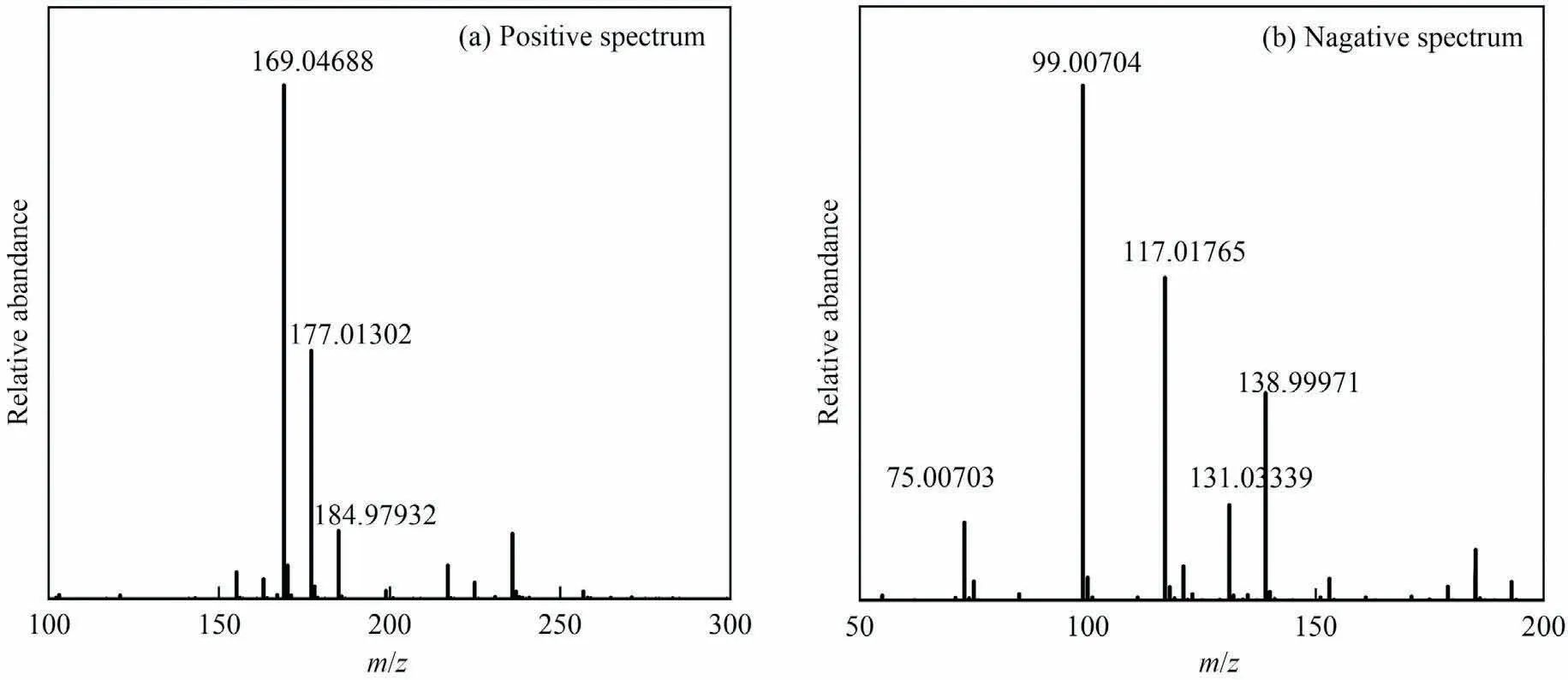
Fig.4.HRMS spectra of the crude reaction mixture after reaction (reaction conditions: reaction temperature 160 °C, reaction time 2 h, initial CO2 pressure 1.0 MPa, DSA addition 1.0 mmol).
On the basis of HRMS analysis,it was proposed that the process of DSA direct esterification reaction.Diphenyldiazomethane has been reported as an active probe to intercept acidic substances generated in alcohol solution under CO2atmosphere, for verifying the generation of alkylcarbonic acid [33].Then, the formation and dissociation of alkyl carbonates in alcohol and CO2systems were determined by UV spectroscopy [34].The results indicated that CO2reacted with methanol and formed alkylcarbonic acid, which then dissociated to free H+.In this study, a large amount of CO2and CH3OH were in the reaction system, thus, CO2and CH3OH could react to form methyl carbonic acid,then dissociate to release H+as shown in Eqs.(1) and (2), which were further confirmed by the presence of methyl carbonate (Compound 4) in the product mixture detected by HRMS.The conductivity of CH3OH was 2.43 μS·cm-1, but the conductivity of the reaction mixture system reached up to about 800 μS·cm-1after adding 0.50-2.0 mmol DSA,which evidenced the dissociation of DSA in CH3OH as displayed in Eq.(3).Meanwhile, a trace amount of water in CH3OH could be reduced by reacting with CO2to generate carbonic acid and release H+, which could be confirmed by decreasing water content from 0.15% to 0.04% by Karl Fischer method.Monosodium monosuccinate dissociated from DSA possibly associated with H+released from methyl carbonic acid to produce sodium 3-carboxypropanoate (Compound 7), which was detected by HRMS as shown in Eq.(4).
There would be two reaction paths for the subsequent reaction of 3-carboxypropanoate (Compound 7).3-Carboxypropanoate might react with CH3OH to generate sodium 4-methoxy-4-oxobutanoate (Compound 2), and then Compound 2 could further react with H+to form MMS.Alternatively, 3-carboxypropanoate might react with CH3OH to directly form MMS.To certify the reaction path of 3-carboxypropanoate,the theoretical calculation with Gaussian 16 was conducted to optimize the structure of compounds by the B3LYP method of density functional theory for the most stable molecular structures and the thermodynamic parameters.It was found that at the reaction conditions of 140 °C and 3.1 MPa, ΔG was 143.09 kJ·mol-1for the direct generation of MMS from 3-carboxypropanoate and CH3OH, suggesting that this process cannot occur under these reaction conditions.But it was thermodynamically favorable (ΔG = - 10.89 kJ·mol-1) to form sodium 4-methoxy-4-oxobutanoate (Compound 2) from 3-carboxypropanoate and CH3OH under the same conditions.Therefore,the theoretical calculation along with HRMS results suggested that 3-carboxypropanoate first reacted with CH3OH to generate 4-methoxy-4-oxobutanoate (Compound 2), and then Compound 2 reacted with H+to generate MMS, as summarized in Eqs.(5) and(6).The high pressure of CO2could expedite the formation of 3-carboxypropanoate(Compound 7)from DSA by shifting the chemical equilibrium, and 3-carboxypropanoate (Compound 7) was the precursor for MMS generation.Therefore, MMS generation was increased at the high pressure of CO2.
In Fig.5,the mole fraction of SA decreased,and the mole fraction of MMS increased first and then decreased,but the mole fraction of DMS increased gradually with the reaction time evolution.Meanwhile, the selectivity of MMS decreased and the selectivity of DMS increased gradually, which was consistent with the feature of continuous reaction [35].In other words, the formed MMS further reacted with CH3OH to form DMS.However,both MMS(Compound 5) and 4-methoxy-4-oxobutanoate (Compound 2) were observed in the reaction mixture from HRMS analysis.Therefore,there might be two possible reaction paths for the formation of DMS, similar to MMS generation.The theoretical calculation by Gaussian 16 software was also conducted for thermodynamics.At the reaction conditions of 140°C and 3.1 MPa,the direct formation of DMS from the reaction of 4-methoxy-4-oxobutanoate (Compound 2)with CH3OH was less possible as ΔG was 140.87 kJ·mol-1.On the other hand, the reaction of MMS with CH3OH was highly possible to generate DMS under the same reaction conditions given that the calculated ΔG was-13.12 kJ·mol-1in Eq.(7).
The common esterification between a carboxylic acid and alcohol is an equilibrium-limited reaction.To obtain a high yield of esters,it is necessary to remove the generated water from the reaction system,which could be achieved by chromatographic reactors[36] and reactive distillation [37].Interestingly, the formed water could be immediately consumed by forming carbonic acid and then releasing H+during the direct esterification of disodium succinate, which would spontaneously expedite esterification and lead to negligible water content in the reaction mixture.
3.3.Kinetic modeling of DSA direct esterification
According to the results of HRMS analysis and the theoretical calculations, the reaction of DSA direct esterification possibly involved the first generation of sodium 3-carboxypropanoate,then forming MMS, and finally producing DMS, as summarized in Eqs.
(1)-(7).For convenient kinetic modeling investigation, the major reaction route was therefore briefly described as exhibited in Eqs.(8) and (9).
To further verify the above proposed reaction pathways of DSA direct esterification, a detailed kinetic study was performed in the present work.
3.3.1.Kinetic model description
In a batch reactor,the changes in molar concentrations of components were expressed as follows:
where the subscript A, B, M, E and W represent DSA, CH3OH,MMS,DMS and H2O,respectively;R1and R2are the rate equations of Eqs.(8) and (9), respectively.
The rates of reactions(8)and(9)were assumed as to follow the power-law type:
where k1and k2the reaction rate constants of Eqs.(8) and (9),respectively, and α and β as the adjustment coefficients, with a range of 0 to 1.
3.3.2.Determination of rate constants
The parameters of the proposed kinetic models consist of rate constants and adjustment coefficients.The kinetic models were fitted to the experimental data by using MATLAB software and the regression results were shown in Fig.6.When α = 1 and β = 0,the average absolute deviation (MARR) between the experimental data and the calculated values from the kinetic models was 16.84%,which was the lowest among the kinetic models,as shown in Fig.6.The obtained rate constants of the best fitted kinetic model were listed in Table 3.With the increased reaction temperature from 140 to 170 °C, the reaction rate of Eq.(8) increased rapidly from 482.87 to 937.19 L2·mol-2·h-1and then slowly to 942.22 L2·mol-2·h-1, while that of Eq.(9) increased steadily from 2.14 to 11.64 L·mol-1·h-1.
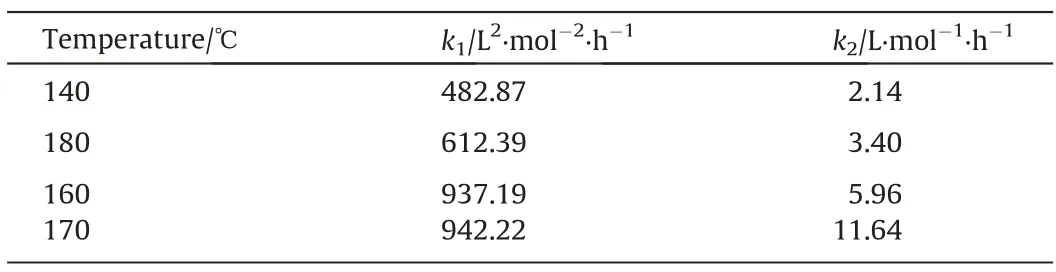
Table 3 Fitting results of reaction rate constants k1and k2 (α=1, β=0)
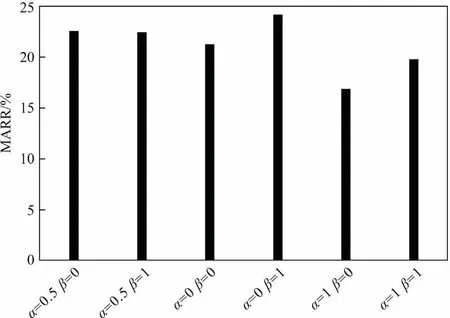
Fig.6.The MARR values between the experimental data and the calculated values from the proposed kinetic models.
The temperature dependence of the rate constants kiis evaluated according to the Arrhenius-type equation:
where Aiis the pre-exponential factor, Eiis the activation energy(kJ·mol-1), and R is the universal gas constant (J·mol-1·K-1).Thus,the activation energy and pre-exponential factor of each reaction could be obtained by the linear fitting of lnk and 1/T.Fig.7 showed the plot of lnk1and lnk2versus 1/T.The pre-exponential factors and the activation energies were obtained by linear fitting and presented in Table 4.
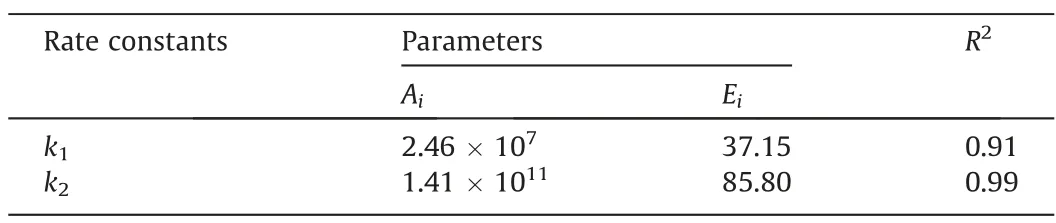
Table 4 Fitting results of pre-exponential factors and activation energies
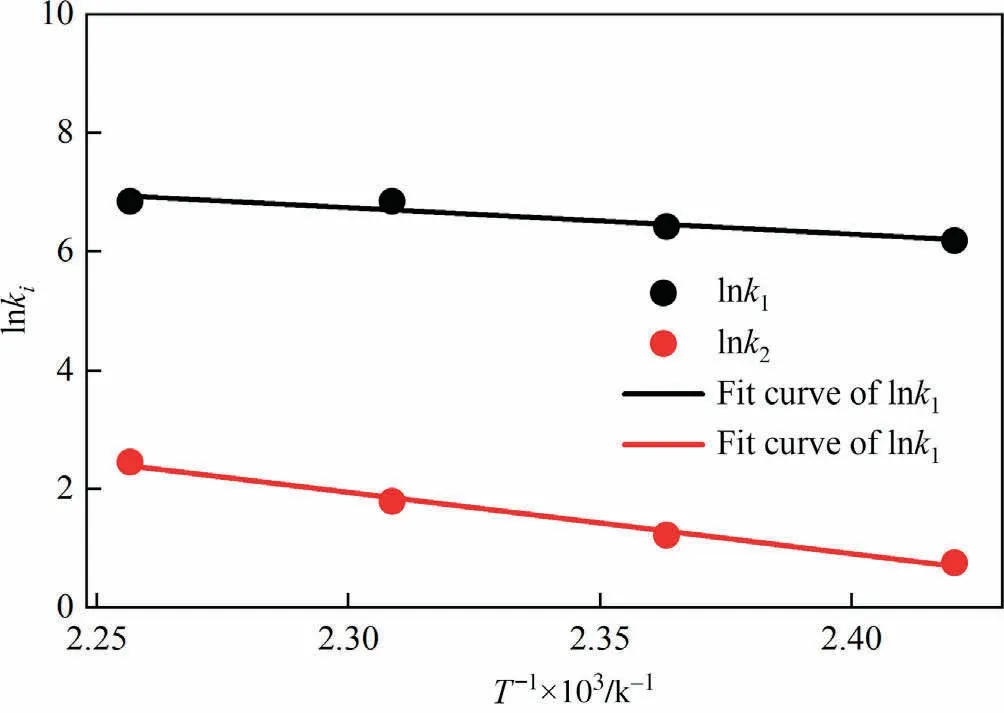
Fig.7.The fitting curve of lnk vs.1/T for reaction.
As shown in Table 4, the activation energies for MMS to react with methanol to form DMS was 85.80 kJ·mol-1, which was obviously larger than that for DSA to form MMS.This meant that compared to the formation of MMS from DSA, the formation of DMS from MMS need to conquer a larger barrier.Considering α = 1 and β = 0, the consuming rate of DSA and the formation rate of DMS could thus be expressed as:
According to Eqs.(19) and (20), for the conversion of DSA to MMS, it could be considered as a second-order reaction for DSA,and a first-order reaction for methanol.As for the conversion of MMS to DMS, it was regarded as a first-order reaction for both MMS and methanol.
3.3.3.Model comparison with experiments
With the rate equations obtained by data regression, the best fitted model predicts the variation of composition of DSA, MMS and DMS with time during the reaction process of DSA direct esterification under different reaction temperatures, as shown in Fig.8.
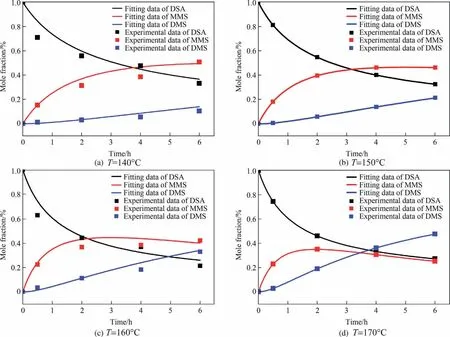
Fig.8.Variation of the mole fraction of DSA,MMS and DMS with reaction time at different reaction temperatures(initial pressure of CO2 was 1.0 MPa,initial dosage of DSA was 1.0 mmol).
It could be seen clearly from Fig.8 that at all the investigated temperatures, great agreement was observed between the experimental data and calculated values from the best fitted model.As shown in Fig.8, the proposed model predicted that the mole fraction of DSA decreased rapidly at first and then decreased slowly at all the investigated temperatures along with the reaction, and the consumption rate of DSA was only accelerated a bit with the increasing reaction temperature.This was consistent with the fact that the barrier for DSA to react with methanol was not very high.The cases for the production of MMS and DMS got complicated under different reaction temperatures.As shown in Fig.8(a) and(b), more MMS were formed than DMS at a temperature of 140 °C and 150 °C, and the mole fraction of MMS increased to a maximum value and that of DMS increased gradually with the increasing of reaction time.However, as reaction temperature increased to 160°C and 170°C,the mole fraction of MMS increased to a maximum value and then tended to decrease and that of DMS continuously increased.Especially,when the temperature reached 170°C,more DMS were generated and the time for MMS reaching a maximum value was shortened.The fact could be attributed to that the energy barrier for the transformation of MMS to form DMS was more than twice larger than that for the transformation of DSA to form MMS.When the temperature increased to 160 °C or higher,the reaction for MMS to further form DMS with a higher barrier was accelerated more compared to that for DSA to form MMS.This means more MMS were consumed to further form DMS, which led to a lower net production of MMS and a higher production of DMS.According to the kinetic analysis, it was more difficult for MMS to further form DMS during the reaction of DSA direct esterification in comparison to the transformation of DSA to MMS.Therefore, in order to improve the reaction results, it encourages us to design a proper catalyst to lower the barrier of the transformation of DSA to MMS.
4.Conclusions
The reaction pathway of DSA direct esterification with CO2and CH3OH was determined by HRMS analysis of the reaction mixture and theoretical calculation.The results verified that CO2and CH3-OH reacted to produce methyl carbonate for facilitating the esterification of DSA under the investigated reaction conditions.A detailed kinetic analysis was performed to further confirm the proposed reaction pathways.The activation energy for the reaction from DSA to MMS was 37.15 kJ·mol-1, and 85.80 kJ·mol-1for the reaction from MMS to DMS.In the future study, the conversion of monoester to diester could be accelerated by suitable catalysts.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by Natural Science Foundation of Shanxi Province (202203021221069 and 202103021223063);National Natural Science Foundation of China (21706172).
 Chinese Journal of Chemical Engineering2023年12期
Chinese Journal of Chemical Engineering2023年12期
- Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
