Influence of support properties on selective oxidation of 2-methylnaphthalene on vanadia-molybdena based catalyst
Yi Yu, Fanfan Li, Xiaocong Li, Guoji Liu, Li Xu,, Xingchuan Yang,
1 School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, China
2 School of Chemical and Printing-Dyeing Engineering, Henan University of Engineering, Zhengzhou 450007, China 3 Kaifeng Wujing Industrial Co.Ltd, Kaifeng 475000, China
Keywords:2-Methylnaphthalene Catalysis Partial oxidation Support Interactions
ABSTRACT In this work, the catalytic performance of vanadia-molybdena loaded on TiO2, MgO, ZSM-5, NaY and Mordenite was investigated in the selective oxidation of 2-methylnaphthalene (2-MN) to 2-naphthaldehyde(2-NA).Results show that strong interactions between supports(TiO2,ZSM-5)and active components can promote the dispersion of active component.Monolayer VOx and MoOx are the main form on the catalyst surface,which is beneficial to the 2-NA selectivity.However,the corresponding weak interactions between Mordenite and active components may lead to the production of oxide crystals and the separation of active components,thus reducing the 2-NA selectivity.Due to the high Na content,new crystal NaVMoO6 forms on the NaY surface, which is inactive in the reaction.For V-Mo/TiO2, V and Mo can be inserted into the TiO2 lattice,changing the electronic structures of active components and support and improving the activity of surface oxygen species.This investigation highlights an important consideration on supports properties when designing supported catalysts.
1.Introduction
Selective oxidation of hydrocarbons to their corresponding aldehydes,acids and anhydride is a primary reaction in fine chemical production.These oxygen functionalized intermediates are main raw materials to produce resins, rubber, fiber and polymer materials [1-3].Unfortunately, industrial scale selective oxidation processes for the manufacture of most fine chemicals are still accomplished in toxic or caustic solvents by using the high-value metal salts such as potassium dichromate and potassium permanganate till now [4,5].The resulting environmental problems have seriously affected the development of human society.Intense studies tried to develop new and environmentally friendly catalytic oxidation methods in recent years,which can effectively substitute conventional stoichiometric processes by using metal catalysts combined with green oxidants such as air, O2and H2O2[6,7].And lots of achievements have been made in developing new catalytic methods and catalysts, for example in the field of oxidation of ethane, propane and toluene.However, there still exists challenging in terms of achieving high conversion without over oxidation [8].The selective oxidation of 2-methylnaphthalene (2-MN) to 2-naphthaldehyde (2-NA) is a typical example.
2-NA is an important intermediate to synthesize medicine,spices and pesticides [9,10].It is manufactured industrially through the oxidation of 2-naphthalene nitrile or 2-naphthoyl chloride in liquid phase, which contains the characterizations of high production cost,equipment corrosion and harsh reaction conditions [11].Our research group put forward and developed a greener gas-solid reaction method by oxidizing the cheap 2-MN,and vanadia-molybdena based catalysts exhibit excellent catalytic performance in this reaction [12,13].
For the supported vanadia-molybdena based catalysts, it is widely believed that active species morphology and supports properties are the two main factors that affect the catalytic performance [14,15].As for the active component, the polymerized or isolated species usually exhibit different conversion and selectivity, and the reasons for these effects are still a controversial topic[16,17].When comes to the selective oxidative dehydrogenation(ODH)of methanol and ethanol with two e-reaction,the bridging V-O-support bond are considered as the active site, and the isolated and oligomeric active species show the highest active and selectivity.While for reactions involve the participation of O atom,the catalytic activity is proved to increase with the surface coverage of active components [18,19].This conclusion is consistent with our previous research result that the most active and selective species are vanadia nearly monolayer covered on the support surface[12,13].In this case,the active component is most affected by the support, avoiding various catalytic behaviors caused by exposure of different crystal planes on its surface.Excessive load of VOxand MoOxover one-monolayer capacity will result in the creation of V2O5crystals, which is inactive in the oxidation reaction[20].
The status and activity of VOxand MoOxare also closely related to the nature of the support material.Kaichev et al.[21] reported the ODH of ethanol over vanadia supported on TiO2, ZrO2, Al2O3and SiO2support, oxide monolayer are more likely to form on TiO2surface.While for ZrO2, Al2O3and SiO2, the V2O5crystallites exist regardless of the VOxcontent because of the weak interactions.Wachs et al.[22]had put forward that the activity of surface VOxcan be associated with the Sanderson cation electronegativity of support.Schomäcker et al.[23] correlated the apparent activation energies of ODH of propane and methanol and suggested that the oxygen defect formation enthalpies or the reducibility of the active component can be used as a descriptor of its catalytic activity.However, studies on the influence of the support on the active component in the selective oxidation of aromatics have been rarely reported.In addition,the mechanism of the effect of different support surface properties on the dual active components(VOx/MoOx)remains unclear.
Herein,in this work,we dispersed VOxand MoOxon five different materials with different surface properties and pore structure.These catalysts were applied in the gas-solid oxidation of 2-MN to 2-NA in order to evaluate how support materials and properties affect catalysts behavior in this reaction.
2.Experimental
2.1.Catalysts preparation
Anatase TiO2(99.8%) and MgO (99.0%) were supplied by Macklin reagent company.ZSM-5 (Si/Al = 25), NaY (Si/Al = 5) and Mordenite(Si/Al= 25)were bought from Nankai Catalyst Company.In a typical synthesis process, a certain amount of NH4VO3(99%,Macklin) and (NH4)6Mo7O24·4H2O (99%, Macklin) were dissolved in aqueous oxalate solution.Then, certain amounts of promoters K2SO4and K2S2O7were dissolved.After different supports impregnation,the catalysts were dried at 110°C overnight.Subsequently,they were calcined at 500 °C for 2 h in air.The load of V is 5.8%(mass), which has been proved the optimal value in this reaction.The molar ratio of V to Mo is 1:1.
2.2.Catalyst characterization
The X-ray powder diffraction (XRD) was measured on a Bruker D8 Advance diffractometer with Cu Kα radiation.Texture properties of different catalysts were measured by Quantachrome iQ2.0.2 g samples were pretreated at 300°C under vacuum before testing.Fourier transform infrared (FTIR) spectra were determined by Perkin-Elmer Spectrum GX in the range of 4000-400 cm-1.Raman spectroscopy measurements were recorded by LabRAM HR Evo spectrometer with a 514 nm maser.TEM and HRTEM were performed by TalosF200 FEI with an accelerating voltage of 200 kV.
Chemisorption of NH3or O2was measured by using a TP-5080D instrument (Xianquan, Tianjin).For the NH3-TPD measurement,0.1 g catalysts were pretreated at 300°C in a He atmosphere.Then,the catalyst was subsequently cooled to 80 °C and saturated with the NH3/He blend (20% (vol)).After the desorption of physically adsorbed NH3at 110 °C, TCD signals were collected from 80 to 550 °C at 10 °C·min-1.For O2-TPD, 0.1 g catalysts were pretreated at 300 °C, and then placed in an atmosphere of oxygen.When the desorption of physisorbed oxygen is complete, the experiment was performed from 80 to 1000 °C.H2-TPR was performed by the same equipment, 0.1 g catalysts were heated in N2atmosphere at 300 °C.After the catalysts were cooled to room temperature, the TPR was proceed in H2/N2(5% (vol)) atmosphere from 30 to 900 °C at 10 °C·min-1.XPS was measured on Thermo Fisher Scientific ESCALAB 250Xi instrument with Al Kα irradiation.The binding energies were calibrated by using carbon 1 s peak at 284.8 eV.
2.3.Activity testing
Partial oxidation of 2-MN was carried out in a continuous-flow fixed-bed reactor with a stainless steel reaction tube (10 mm I.D.).Before testing, all catalysts were pressed into wafers, crushed and sieved to a pellet size between 0.18 and 0.23 nm.Typically, 0.5 g catalysts mixed with 2.5 g silica sand with the same particle size were loaded in the isothermal zone of reaction tube.A type K thermocouple was placed at the vertical center of the catalyst bed to monitor the actual temperature.The catalysts were pretreated under air atmosphere at 400 °C for 2 h before exposing them to the reactants.After the pretreatment, 2-MN was evaporated into the air stream by bubbling at 120 °C.All gas transfer lines were wrapped in heating strips and kept at 150 °C to prevent the liquidation and condensation of reactant gas (molar ratio of 2-MN to O2was 1:160).The flow of reactant mixture was adjusted by mass flow controller and the gas hourly space velocity (GHSV) was kept at 10000 h-1.Chemical species in the feed and reactor effluent stream were absorbed by 1,4-dioxane and quantitatively measured by gas chromatograph (Agilent 7890B) equipped HP-5 column.Black experiment results showed that the steel reaction tube and silica sand had no catalytic ability for 2-MN.Each set of experiment at a certain temperature was repeated three times for the average.The 2-MN conversion (%), 2-NA selectivity (%) and 2-NA yield (%)were calculated based on the inlet and out let concentrations:
where 2-MNin, 2-MNout, 2-NAoutand 2-MNconvertedrepresent the inlet 2-MN moles, the outlet 2-MN moles, the outlet 2-NA moles and the converted 2-MN moles.
2.4.Theoretical calculations
Mulliken charges and projected density of state (PDOS) of VMo/TiO2catalyst was calculated by using Materialsstudio 2017.Catalysts structural optimizations were performed using the GGA-PBE exchange correlation functional form and DNP basis set.Monolayer VOxand MoOxwere placed on a two layer (4 × 2)TiO2(0 0 1) surface to simulate catalyst system.The vacuum space of all models was set 2 nm.K-points mesh used to optimize the super cell is 3 × 2 × 1.
3.Results and Discussion
3.1.Catalytic activity
In this reaction, apart from the target 2-NA, the main byproducts were ring-opening phthalic anhydride, 4-methylphthalic anhydride and 2-methyl-1,4-naphthoquinone.Fig.1 shows the catalytic performances of V-Mo/ZSM-5, V-Mo/NaY, V-Mo/Mordenite, V-Mo/MgO and V-Mo/TiO2.Within the range of reaction temperature 370-410 °C, the 2-MN conversion over V-Mo/MgO is always lower than 20%, and no target 2-NA is produced.It can be considered that MgO support used for selective oxidation of alkanes is not active in this reaction.At temperatures range from 340 to 410 °C, V-Mo/TiO2shows the highest activity,followed by V-Mo/Mordenite >V-Mo/ZSM-5 >V-Mo/NaY.From Fig.1(b), 2-NA selectivity over V-Mo/TiO2is lower than that over V-Mo/zeolite catalysts at 340-360 °C, indicating that V-Mo/TiO2can achieve better performance only at a certain temperature.At temperatures 360-390 °C, the 2-NA selectivity over V-Mo/TiO2remains at a high level, while it decreases markedly for V-Mo/zeolite catalysts.V-Mo/TiO2presents the highest yield 35.31% of 2-NA at temperature 390 °C.
Since the catalysts are being developed for use in fine chemical production, the catalytic stability of the optimal V-Mo/TiO2catalyst was measured and the results are shown in Fig.2.At the optimum reaction temperature of 390°C,the 2-NA yield remains about 35%for a long time.After 40 h of reaction,the reaction temperature is reduced to 360 °C and the 2-NA yield maintains at around 18%.When the reaction is carried out for 60 h,the catalyst performance does not change significantly after the reaction temperature is increased to 390 °C.These indicate that V-Mo/TiO2catalyst has strong catalytic stability.
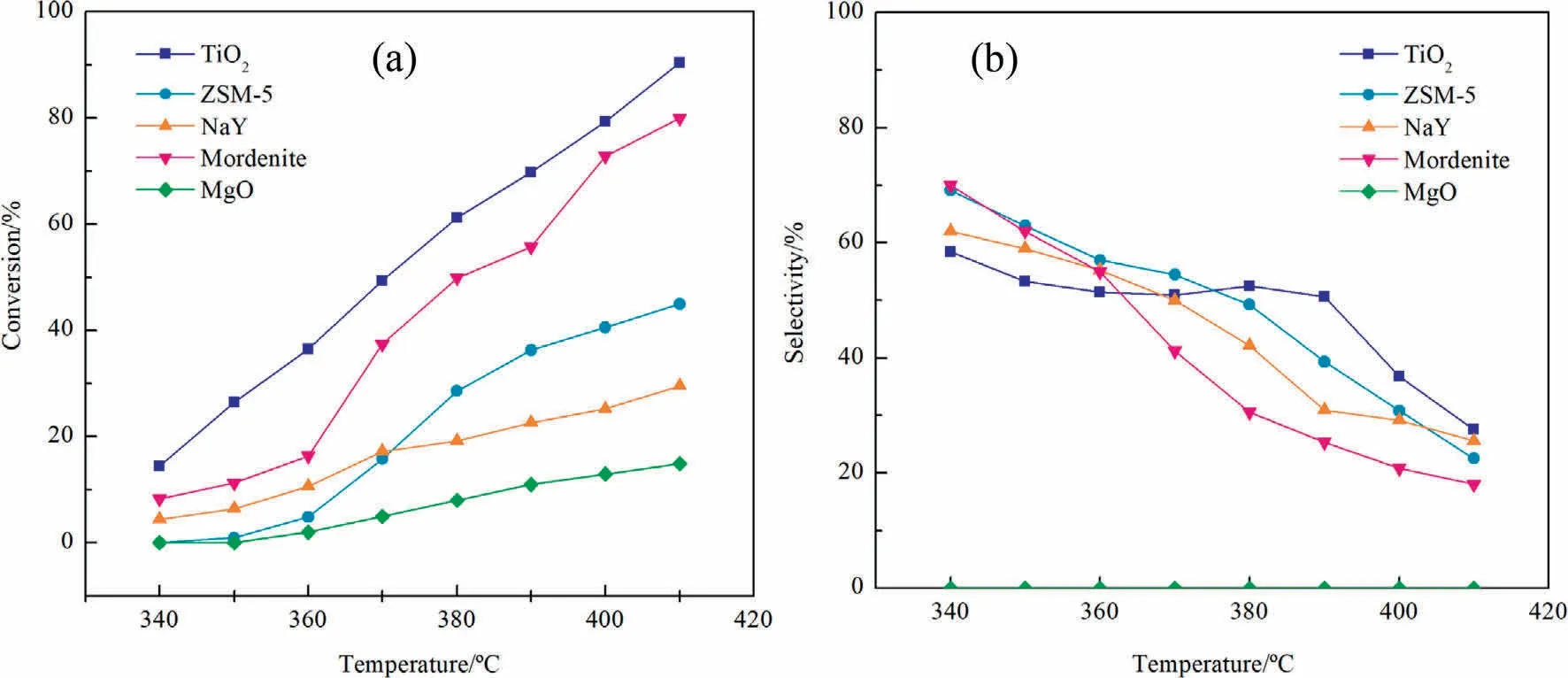
Fig.1.2-MN conversion (a) and 2-NA selectivity (b) on different catalysts.
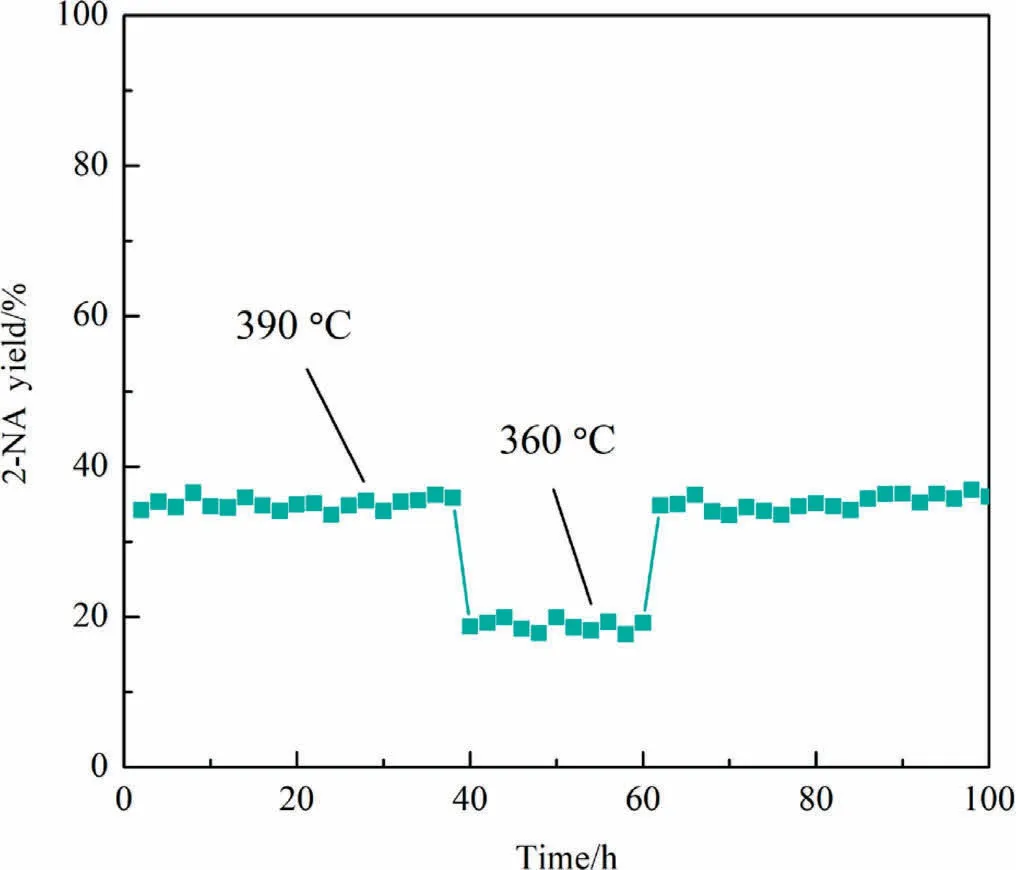
Fig.2.Stability test of V-Mo/TiO2 in the oxidation of 2-MN.
3.2.Structural properties
In order to explore the existence forms of active components VOxand MoOx, the catalysts were characterized by XRD.From Fig.3, peaks representing support materials can be observed for all catalysts.There are no obvious V2O5and MoO3diffraction peaks for V-Mo/TiO2, indicating the surface VOxand MoOxare well dispersed or in the amorphous structure [24,25].A few characteristic peaks of VO2(JCPDS PDF#76-0673)can be detected for V-Mo/ZSM-5 and peaks of V2MoO8(JCPDS PDF#47-1081)can be identified for V-Mo/MgO, suggesting that the dispersion of active components decreases on these two supports.While for V-Mo/NaY and V-Mo/Mordenite, the peaks of V2O5(JCPDS PDF#45-1074) and MoO3(JCPDS PDF#47-1081)are even observed,showing that the dispersion of VOxand MoOxon these two supports is poor.For V-Mo/NaY,diffraction peaks of a new ternary phase NaVMoO6instead of support are observed, this indicates that high content of Na can react with VOxand MoOxduring the catalysts preparation process.In addition, the peaks of AlVMoO7are also detected.MgMoO4, the product of the reaction between MgO and Mo, is also found for VMo/MgO [26].Studies have shown that the active component dispersion on the support surface is related to the interaction strength between them[27].Hence,VOxand MoOxmonolayer tend to form on the TiO2surface due to the strong interaction, so as to ZSM-5.While the V2O5and MoO3crystals are easily formed on the NaY and Mordenite surface even when the V and Mo loading is lower than that of monolayer, which is due to the weak interaction[28].In addition, the appearance of crystallization on zeolites may due to the presence of more microporous structures in the material, which hinders the diffusion of active ions during the impregnation process.The strong alkali properties of MgO promote the dispersion of acidic active components on its surface to some extent.As a result,only a small number of crystals exist from Fig.3.
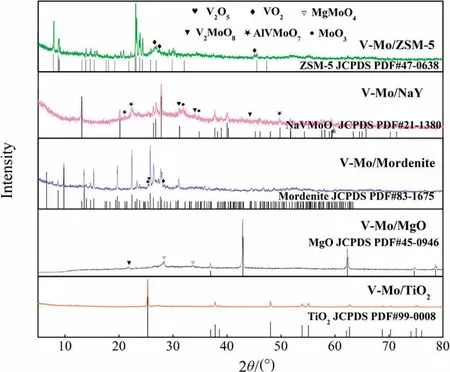
Fig.3.XRD patterns of catalysts with different supports.
Studies on vanadia based catalysts have shown that crystalline VOxspecies,especially V2O5crystals,are not favorable for selective oxidation, because V2O5formed on the support will expose different crystal faces and thus has different selectivity for various products[29,30].MoO3also has this feature.Moreover,V2O5and MoO3crystals show more inactive when compared with monolayer dispersed active species in the reports of toluene and p-xylene selective oxidation [31,32].Catalytic testing results in this study also confirm this conclusion, that V-Mo/TiO2has the optimal catalytic performance.And 2-NA selectivity over V-Mo/ZSM-5 with less oxide crystals was higher than that over V-Mo/NaY and V-Mo/Mordenite.However, the 2-MN conversion of V-Mo/ZSM-5 is relatively low, indicating that the dispersion of active components is only one factor affecting the activity.
In order to more intuitively distinguish the presence form of active components on the surface of TiO2and zeolite supports.The morphology of V-Mo/TiO2and V-Mo/NaY were characterized through TEM and HRTEM.From Fig.4(a1)-(a5), no obvious aggregation and corresponding oxide crystals are found.In addition, only the anatase TiO2phase can be detected as they are identified by the spacing distance of 0.35 from (1 0 1) planes in Fig.4(a6).These demonstrate that VOxand MoOxexist in a highly dispersed form on TiO2surface.While for V-Mo/NaY, there are a lot of fine particles around the support crystals from Fig.4(b1).These particles are confirmed NaVMoO6crystals due to the pacing distance of 0.32 nm from (-1 1 1) planes.In addition, EDSmapping in Fig.4(b4)-(b6)shows that the dispersion of crystal particles on NaY surface is uniform.
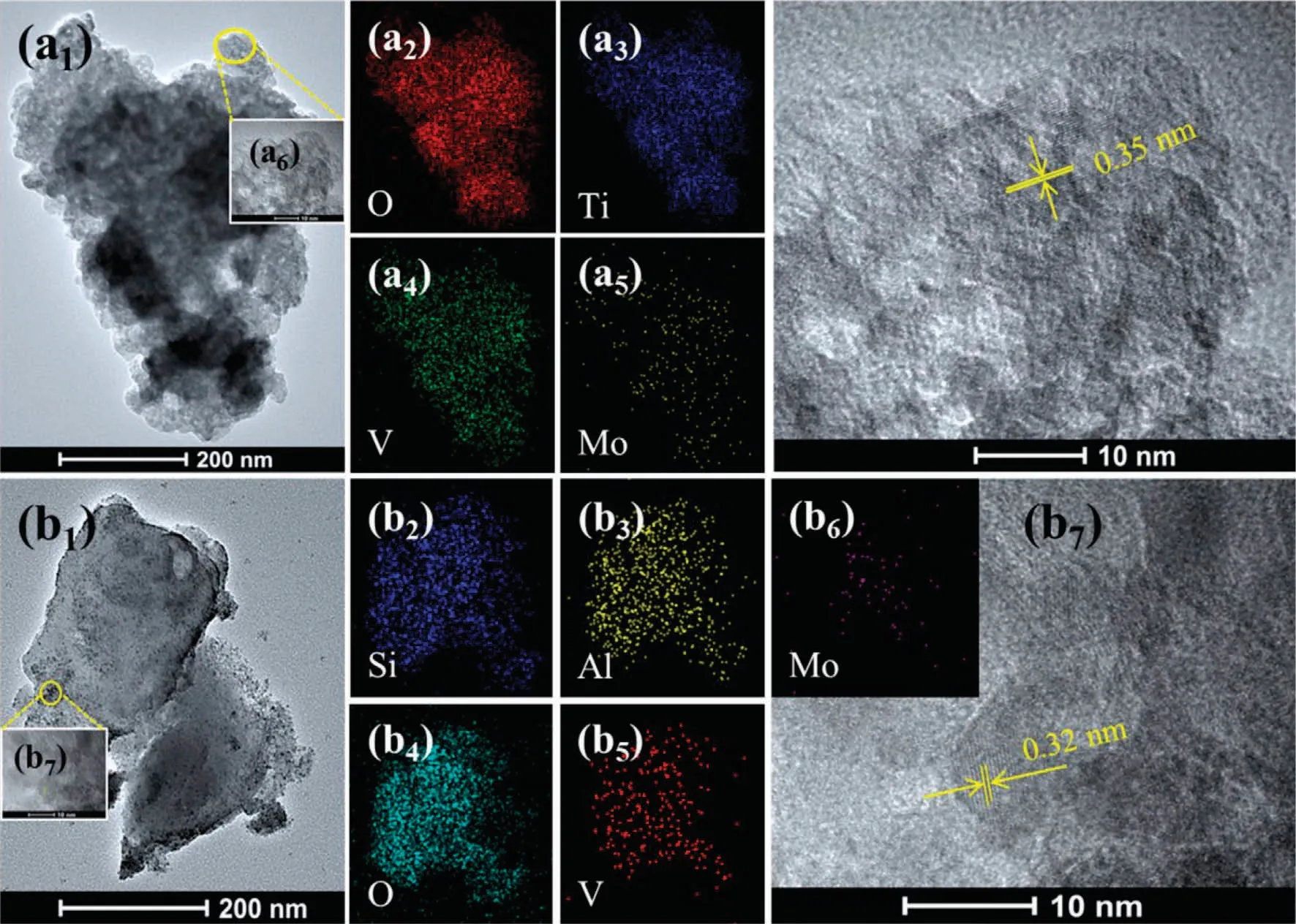
Fig.4.TEM, HRTEM and EDS-mapping images of (a) V-Mo/TiO2 and (b) V-Mo/NaY.
Table 1 shows the texture values of V-Mo/support.Catalysts exhibit the higher BET surface area when zeolites are used as support, V-Mo/NaY shows the largest surface area 456 m2·g-1, while V-Mo/TiO2has the smallest value (4.33 m2·g-1) among catalysts.Active components dispersion is usually related to the surface area of catalyst, and larger value will be more conductive to the metal oxides dispersion.However, in this study, the dispersion of VOxand MoOxon the zeolites with large specific surface area is not as good as that of TiO2, indicating that the polymerization degree of VOxand MoOxis strongly affected by VOx/MoOx-support interactions for these catalysts [26].The pore properties of catalysts also affect the diffusion of reactants and products molecules.Catalysts with zeolites as support show typical microporous and mesoporous characteristics.For V-Mo/ZSM-5 and V-Mo/NaY,the micropores surface area and volume account for more than 50% of the total value, respectively.The molecular diameters of 2-MN and 2-NA are both larger than 0.7 nm.While the average pore sizes of V-Mo/ZSM-5 and V-Mo/NaY are 1.69 and 2.54 nm,which would inhibit reactants and product molecules diffusion in the pore during the reaction.Because the α position on the naphthalene ring of 2-MN is also active, the narrow pore makes the naphthalene ring more likely to collide with the active site, which leads to the ring opening reaction to produce anhydride rather than 2-NA.In addition, with the increase of reaction temperature, the thermal motion intensifies,making the aromatic ring easier to contact with the active site.V-Mo/TiO2and V-Mo/MgO contain only mesoporous surface area and pore volume without micropores based on t-plot method, which is benefit for the improvement of 2-NA selectivity.However, V-Mo/MgO has almost no catalytic activity and 2-NA selectivity, suggesting that other surface properties of this catalyst are not conducive to the gas phase oxidation of 2-MN.
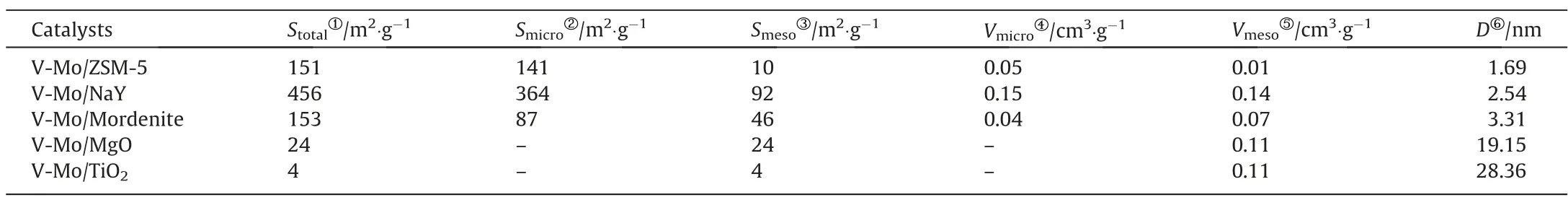
Table 1 The texture values of catalysts with different supports
3.3.Vibrational character
Fig.5 displays FTIR spectra of V-Mo/Support catalysts.Absorption peaks at 3500 and 1630 cm-1represent the O-H and H-O-H of physical adsorbed water.The wide peak between 500 and 900 cm-1of V-Mo/TiO2represents the bending vibration of Ti-O-Ti in anatase TiO2[33].Peaks at about 1070 and 458 cm-1of V-Mo/Mordenite, V-Mo/ZSM-5 and V-Mo/NaY belong to the stretching and bending vibration of Si-O-Si in silica [34].All catalysts contain characteristic peak of 1410 cm-1that represents the asymmetric stretching vibration of covalent sulfate species formed by sulfur oxide and support, indicating that the added promoter K2SO4in the preparation process can interact with different supports [33].For V-Mo/TiO2, peaks at about 1200 cm-1representing the interaction between VOx,support and sulfuric acid species can be observed.But in zeolites support catalysts, this peak has some degrees of deviation, indicating differences in interaction between active components and support.Small peak at 1030 cm-1of V-Mo/TiO2belongs to the terminal V=O in crustal V2O5,indicating that there are a small amount of V2O5microcrystals on the surface of V-Mo/TiO2, which cannot be detected by XRD[14].The infrared spectra of the three zeolites support catalysts do not contain this absorption peak, which may due to the covering by peak of support.For V-Mo/TiO2, peak at 960 cm-1indicates that VOxmainly exists in the form of monolayer [35].However, it disappears in the infrared spectra of the zeolites support catalysts.Peak at 786 cm-1representing V-O-Mo bond cannot be found for VMo/TiO2, which may due to the strong characteristic peak of support [36].
The weak absorption peak at 910 cm-1represents terminal Mo=O in surface MoOx[37].The larger intensity of this peak for V-Mo/zeolites,especially for V-Mo/NaY,demonstrates the low dispersion of MoOx.The absorption peak at 725 cm-1in V-Mo/NaY represents the characteristic vibration of V-O-Al in AlVMoO7solid solution [27].But it disappears in V-Mo/ZSM-5 and V-Mo/Mordenite, which may due to the low Al content in these zeolites.
Raman spectra of V-Mo/TiO2,V-Mo/ZSM-5 and V-Mo/NaY were characterized to acquire more detailed structural information about surface active components.From Fig.6, characteristic peaks corresponding to the surface active components of the catalysts are mainly in the range 700-1000 cm-1.Peak at 771 cm-1represents the vibration of V-O-Mo bond [38].When compared with V-Mo/NaY, the peak intensity of V-Mo/TiO2and V-Mo/ZSM-5 is larger,indicating that the interaction between the active components VOxand MOxover TiO2and ZSM-5 is stronger than that over NaY.The peak near 990 cm-1represents the terminal Mo=O or V=O bond.And peaks near 842 and 950 cm-1reflect the V-O-V and Mo-O-Mo bonds in two-dimensional form on the catalyst surface [39,40].The intensity of these two characteristic peaks for NaY is weak, which may result from the surface crystals.
3.4.Acidic properties
Acidic properties of V-Mo/TiO2were studied by NH3-TPD.From Fig.7(a), V-Mo/Mordenite and V-Mo/ZSM-5 catalysts contain two ammonia desorption peaks at 220 and 350 °C, corresponding to weak and dium-strong acid sites [41].V-Mo/Mordenite contains larger number of the two acid sites compared with V-Mo/ZSM-5.V-Mo/NaY contains a large amount of weak and few dium-strong acid sites, this may due to weak acidity of Al2O3in the zeolite framework [42].When compared to V-Mo/Mordenite, V-Mo/TiO2shows weaker acid strength and smaller number of acid sites.VMo/MgO almost contains no NH3desorption peaks, which is related to the basic properties of MgO, resulting in the poor catalytic performance in 2-MN oxidation.For zeolites supported catalysts, 2-MN conversion is positively related to the acid sites numbers.However, catalyst V-Mo/TiO2with relatively small amount of acid sites displays much higher activity.To explain this phenomenon, three pure zeolites were characterized by NH3-TPD.
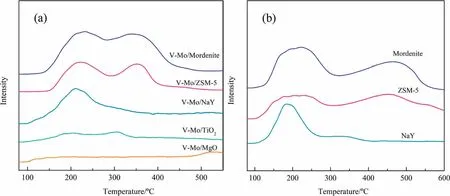
Fig.7.NH3-TPD spectra of (a) V-Mo catalysts with different supports and (b) various pure supports.
From Fig.7(b), Mordenite and ZSM-5 contain two peaks at about 200 and 480 °C, demonstrating weak and strong acid sites.While NaY contains peaks at 280 and 330 °C, ascribable to weak and dium-strong acid sites.To contrast TPD patterns in Fig.7(a)and(b),it can be concluded that active components VOxand MoOxoccupy part of the acid sites of supports.And the surface acid sites of V-Mo/TiO2are derived from the dispersed active component.The acid sites of V-Mo/Mordenite, V-Mo/ZSM-5 and V-Mo/NaY are derived not only from active component but also from the supports [43].According to the active testing results, large number of strong acid sites may cause the low 2-NA selectivity over V-Mo/Mordenite.And the low activity of V-Mo/NaY may result from the insufficient dium-strong acid sites [14].These phenomena are consistent with our previous result that weak and diumstrong acid sites need to maintain a certain ratio [13].In addition,the strong acidic skeleton aluminum in zeolite has a strong adsorption effect on aromatic aldehydes, leading to the difficulty of desorption of them from the catalyst surface [44].Therefore, the selectivity of 2-NA decreases sharply at high temperatures on the corresponding catalysts.
3.5.Reducibility
In the process of temperature-programmed reduction, excessive temperature will cause transformation in the catalyst crystal structure.For example,peaks near 800°C may result from the speciation of V2O5crystals from polymerized vanadia at high temperature [45].Therefore, we only analyze the reduction peak before 800 °C in H2-TPR spectra (Fig.8).Except for V-Mo/Mordenite, the H2-TPR curves of the other three catalysts only contain a reduction peak in a large temperature range, and there is no relatively isolated reduction peak of vanadia and molybdena species,indicating that the interaction between VOxand MoOxof these three catalysts is stronger than that of V-Mo/Mordenite.The one main peak can be attributed the overlap of reduction peaks of VOxand MoOx,including V5+to V4+and V3+, and Mo6+to Mo5+[42,43].Reduction peaks for V-Mo/ZSM-5 and V-Mo/TiO2at around 584 and 612 °C can be assigned to the monolayer VOxand MoOx[45].V-Mo/TiO2shows higher reduction temperature than V-Mo/ZSM-5,which may result from the strong interactions between VOx/MoOxand TiO2[42].Previous studies suggested the interaction between MoOxand support could promote the electron transfer process between support and VOx, such interaction would lead to the reduced reducibility of MoOx[46].V-Mo/NaY shows higher reduction temperature by compared with V-Mo/ZSM-5 and V-Mo/TiO2, which is related to the oxide crystal NaVMoO6on the surface of NaY [47].For V-Mo/Mordenite, the reduction peaks near 577 and 675 °C represent the reduction of molybdena and vanadia,respectively[44].Results show that the synergy between the two active components on Mordenite is weaker,and there exists a certain amount of V2O5and MoO3crystals on catalyst surface.
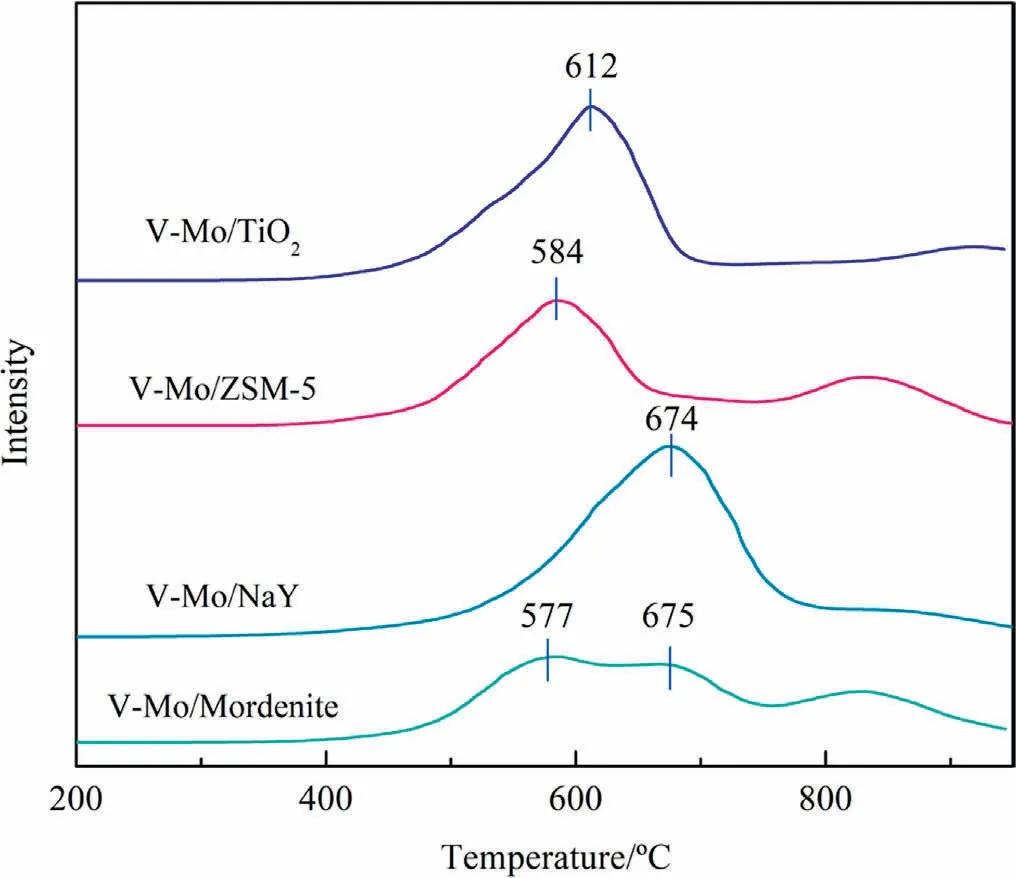
Fig.8.H2-TPR spectra of different catalysts.
3.6.Electronic properties
The electronic structure and surface oxygen species of V-Mo/TiO2, V-Mo/NaY and V-Mo/ZSM-5 were emphatically investigated by XPS in this section due to same Si/Al ratio in ZSM-5 and Mordenite.From Fig.9(a), all the three catalysts contain two typical bands that represent V5+and V4+according to deconvolution.The significant binding energies diversities of the V5+and V4+indicate that the electronic environment around vanadium is different in the catalysts with different supports [48].The 2p3/2orbital of V5+in V-Mo/TiO2is located at 516.6 eV, which is lower than the 517 eV in pure V2O5crystal [49].Studies have shown that when V5+enters the lattice of TiO2to replace a Ti4+,V5+will attract electrons around Ti4+, which will increase the outer electrons density of V5+and weaken the shielding effect on inner electrons.Consequently, the 2p3/2and 2p1/2orbitals of V move to the low binding energy [50].In addition, the inductive effect of V5+induces the decreased electron density of Ti4+and increases electron delocalization.As a result,the 2p3/2and 2p1/2orbitals of Ti move towards the high binding energy.Combined with the BE shifts of Ti,we can conclude some V atoms insert into the TiO2lattice and produce a strong interaction with supports, the electron transfer is then enhanced.However, the interaction between VOx/MoOxand unreducible zeolite supports is weak [51].
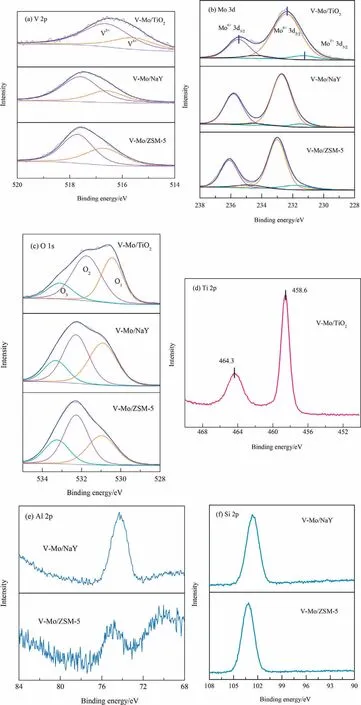
Fig.9.XPS patterns of different catalysts: (a) V 2p; (b) Mo 3d; (c) O 1s; (d) Ti 2p; (e) Al 2p; (f) Si 2p.
Fig.9(b) shows the XPS bands for Mo 3d of different samples.Molybdenum exists in the form of Mo6+and Mo5+,indicating there is an electron transfer between VOxand MoOx:V4++Mo6+↔V5++Mo5+[44,52].Compared with pure MoO3, the BE of Mo 3d5/2in VMo/TiO2shifts to a lower field, which may due to the increase of outer electron density caused by Mo insertion into the adjacent V2O5or TiO2lattice [53].In contrast, the Mo 3d orbital of V-Mo/NaY and V-Mo/ZSM-5 show less BE offsets.From Table 2 and Fig.9(a), (b), V-Mo/NaY has the highest content of V5+and Mo6+among three catalysts.And V 2p shows a larger offset than Mo 3d, suggesting that the interaction between support and VOxis stronger.This is related to the easy formation of AlVMoO7from Al2O3and V [54].

Table 2 XPS results of different components over catalysts with different supports
Peak fitting results and BEs of O 1s are shown in Fig.9(c) and Table 2.Three peaks can be attributed to the lattice oxygen (O1),oxygen vacancy (O2) and surface-adsorbed hydroxyl (O3), respectively[51,52,54].V-Mo/TiO2contains more oxygen vacancies than V-Mo/NaY and V-Mo/ZSM-5.Vacancies can active gas oxygen after the oxygen consumption.Therefore, more reactive oxygen species means more reactive sites, and hence the catalyst shows higher activity.From Fig.9(e), (f), 2p BE of Al and Si shifts to the higher fields, indicating that there is also an interaction between VOx,MoOxand zeolite supports [54].
3.7.O2-TPD results
O2-TPD was measured to study activity and mobility of surface oxygen.In general,for vanadia-molybdena based catalysts,oxygen desorption peaks at T <550 °C are ascribed to the physically adsorbed oxygen and weak chemically adsorbed oxygen.Peaks at 550 °C <T <800 °C can be attributed to chemically adsorbed oxygen on vacancies, peaks at T >800 °C represent the lattice oxygen[55,56].From Fig.10, it is observed that the peak at 550 °C <T <800 °C weakens in the order: V-Mo/TiO2>V-Mo/ZS M-5 >V-Mo/Mordenite ≈V-Mo/NaY,suggesting that different supports can affect the oxygen vacancies on vanadia-molybdena based catalysts.The O2-TPD results of oxygen adsorbed on oxygen vacancies are also consistent with the XPS results.Furthermore, V-Mo/TiO2shows the relative low desorption temperature than the other catalysts,indicating the higher oxygen mobility and the gas oxygen is easier to active over this catalyst[57].According to our previous studies in organic oxidation [14], more vacancies and higher oxygen mobility stand for the fast reduction of gas oxygen and their transformation into active nucleophilic oxygen, which means the higher selectivity of target product.V-Mo/Mordenite shows two desorption peaks at around 611 and 710 °C, this may because of the weak interactions between VOxand MoOx, which agree with the H2-TPR outcomes.
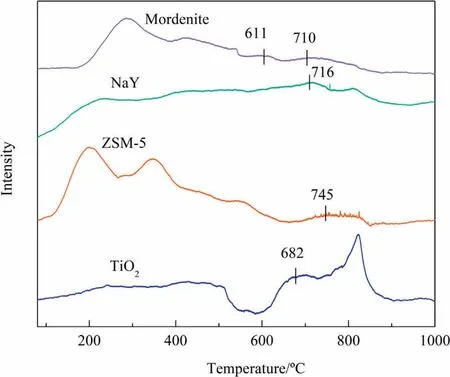
Fig.10.O2-TPD profiles of different VOx-MoOx/Support catalysts.
3.8.Theoretical calculations
From the characterization above, the advantage of TiO2as a support is that V and Mo can be inserted into the TiO2lattice to promote the catalytic performance.Therefore, to further understand the promotion effect of V-Mo co-doping in TiO2from the microscopic point of view, we calculated the Mulliken charges and density of states (DOS) of V-Mo/TiO2(clean) and V-Mo/TiO2(V-Mo co-doped) by the DFT method.The models of monolayer VOx(50%) - MoOx(50%) on the (0 0 1) surface of clean and V-Mo co-doped TiO2(Fig.11)were established based on the works of Vittadini et al.[58].There are three kinds of oxygens on the V-Mo/TiO2surface, terminal oxygen (O1), bridging oxygen (O2) and triply coordinated oxygen (O3).O3is always inert because of their hindrances in the reaction [13,14].Therefore, O1and O2coordinated with V and Mo are considered as the active sites.
The calculated Mulliken charges of O, V, Mo and Ti of different catalysts are shown in Table 3,and the serial number of the corresponding atom is indicated in Fig.11.From Table 3, the Mulliken charges of V, Mo and Ti decrease after V-Mo co-doping, while the absolute value of Mulliken charges of O increase.These indicate that the electronic structures of support and active components have changed due to the electron transfer and the catalytic performance has been affected eventually.The increased absolute value of Mulliken charges of oxygen species indicates that the charge density around them increases.Generally, surface reactive oxygen species can be regarded as nucleophilic active sites in the process of methyl oxidative dehydrogenation.Therefore, oxygen species in V-Mo/TiO2(V-Mo co-doped)with higher electronegativity show greater catalytic activity.In addition,the activity of bridged oxygen(O2) is higher than that of terminal oxygen (O1).
PDOS of V,Mo and O were calculated to explore how does V and Mo insertion affect the band structure of V-Mo/TiO2.From Fig.12,conduction band (CB) contains unoccupied V 3d and Mo 4d orbitals, while valence band (VB) is formed from O 2p, V 3d and Mo 4d orbitals.After V-Mo co-doping in TiO2, the band gap reduces from 0.156 eV to 0.053 eV,which is in favor of the electron transitions and the O removal during the reaction.In addition,for V-Mo/TiO2(V-Mo co-doped), the V 3d and Mo 4d orbitals of CB shift to the lower energy and their states increase, suggesting the V 3d and Mo 4d unoccupied orbitals are more likely to accept electrons and the transfer of electrons from support to the active component is facilitated[59],which is in consist with the XPS results.It is possible to see that in V-Mo/TiO2(Mo doped)the p orbitals of O1(terminal oxygen)and O2(bridging oxygen)are at higher states in the regions closer to the Fermi level, indicating that the V and Mo insertion into TiO2support can improve the reactivity of surface oxygen species [59,60].
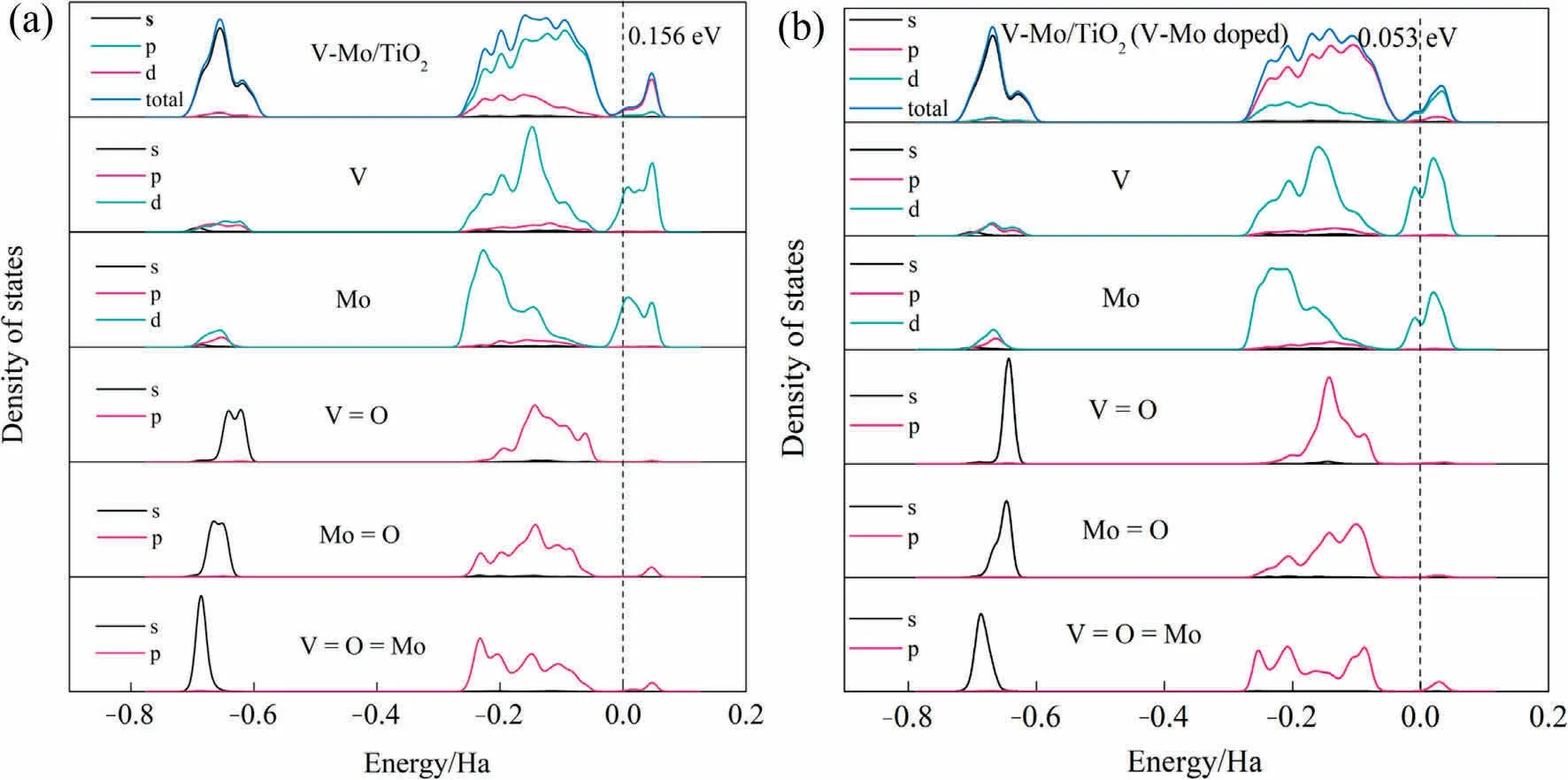
Fig.12.PDOS of V, Mo and O of monolayer VOx and MoOx on (a) clean TiO2 and (b) V-Mo co-doped TiO2 (1 Ha = 27.212 eV).
3.9.Discussion
From the characterization and catalytic testing, we can conclude that the factors that affect the catalytic effect are not unilateral.And the support not only supports the active species,but also participates in the whole catalytic process.The pore structure of catalysts shows certain effect on the reaction.More micropores in catalyst will seriously hinder the diffusion of 2-MN and 2-NA with large molecular diameter,resulting in the formation of phthalic anhydride.At the same time,the presence of microporous structure may hinder the diffusion of active cations and lead to the appearance of surface oxide crystals during the preparation of catalyst.The abundant acid sites and strong acidity on the surface of zeolite make it difficult for 2-NA to desorbed from the catalyst surface at high temperature, resulting in deep oxidation to phthalic anhydride.Poor activity of V-Mo/MgO is connected with the weak acidity and low acid concentration, indicating that the strongly basic support is not suitable for the 2-MN oxidation.
The active components-support interaction also determines the degree of VOxand MoOxpolymerization.For TiO2and ZSM-5, the active components exist in the form of monolayer polymerization or a small amount of microcrystals, which results in the high 2-NA selectivity on V-Mo/TiO2and V-Mo/ZSM-5.However, some V2O5and MoO3crystals exist on NaY and Mordenite surface, and lots of NaVMoO6crystals can also be detected on NaY surface due to the high Na content in NaY.Exposure of the different crystal faces of the crystal will lead to a decrease in selectivity.In addition,the acidity of crystalline oxides are stronger than that of highly dispersed ones, which will also lead to the deep oxidation of 2-NA.The interactions between supports and the active components also affect the reducibility and electron valence state of element.Weak interactions between active components and Mordenite can also lead to the independent existence of VOxand MoOx.Reports have shown that pure vanadia exhibits higher activity than pure molybdena and the mixed oxides.However,it is also prone to cause deep oxidation.Hence,V-Mo/Mordenite shows higher 2-MN conversion but lower 2-NA selectivity [61].Although the active component dispersed in a monolayer on the surface of ZSM-5 provides high aldehyde selectivity, its catalytic activity is relatively weak.This is related to the lack of oxygen vacancies on its surface, the weak mobility of surface oxygen and the poor electron transport capacity within the catalyst.The different pore structures (8 MR/10 MR/12 MR)may be responsible for the different interfacial interactions of V-Mo/ZSM-5 and V-Mo/Mordenite [62].For V-Mo/TiO2, the insertion of V and Mo in TiO2lattice contributes to the higher activity of terminal and bridging oxygens.
4.Conclusions
To study how the supports affect the physicochemical properties of surface VOxand MoOxin the selective oxidation of 2-MN,supported vanadia-molybdena based catalysts had been prepared by using TiO2, MgO, ZSM-5, NaY and Mordenite as supports.Results show that the factors that affect catalyst performance are manifold.The new crystalline phase NaVMoO6on NaY shows inactive performance.And the poor dispersion of VOxand MoOxon Mordenite due to the weak interactions leads to the low 2-NA selectivity.The stronger interactions between supports (TiO2and ZSM-5) and active components is in favor of the formation of monolayer VOxand MoOx, facilitating the production of 2-NA.In addition, V-Mo/TiO2shows more oxygen vacancies, higher mobility and activity of surface oxygen than the other catalysts due to the insertion of V and Mo in TiO2lattice, which can accelerate the activation of gas oxygen.
Data Availability
The data that has been used is confidential.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Henan Province Science and Technology Research Project (232102321041).
 Chinese Journal of Chemical Engineering2023年12期
Chinese Journal of Chemical Engineering2023年12期
- Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
