Thermal decomposition effect of MgCo2O4 nanosheets on ammonium perchlorate-based energetic molecular perovskites
Er-hi An , Xio-xi Li , Hi-xi Zho , Ying-xin Tn , Xiong Co ,*, Peng Deng ,c,**
a School of Environment and Safety Engineering, North University of China, Taiyuan, 030051, Shanxi, People's Republic of China
b School of Engineering for Safety and Emergency Management, Taiyuan University of Science and Technology, Taiyuan, 030024, China
c State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing,100081, People's Republic of China
Keywords:AP-based energetic molecular perovskites MgCo2O4 nanosheets Thermal decomposition Catalytic performance
ABSTRACT Energetic molecular perovskites have attracted widespread attention in the fields of energy materials due to their high detonation performance.In this work,we reported the effect of MgCo2O4 nanosheets on the thermal decomposition of ammonium perchlorate (NH4ClO4, AP)-based energetic molecular perovskites (AP-based energetic molecular perovskites).The morphology and structure of the MgCo2O4 nanosheets were characterized.And their catalytic effect on the thermal decomposition of AP-based energetic molecular perovskites (H2pz)[NH4(ClO4)3](PAP-4), (H2dabco)[NH4(ClO4)3](DAP-4), (H2mpz)[NH4(ClO4)3](PAP-M4), and (H2hpz)[NH4(ClO4)3](PAP-H4) was analyzed.The results showed that MgCo2O4 nanosheets had excellent intrinsically catalytic performance towards enhancing the thermal decomposition of AP-based energetic molecular perovskites.After adding MgCo2O4 nanosheets, the thermal decomposition peak temperatures of PAP-4, DAP-4, PAP-M4, and PAP-H4 had been reduced by 35.7 °C, 48.4 °C, 37.9 °C, and 43.6 °C, respectively.And the activation energy (Ea) of the thermal decomposition of AP-based energetic molecular perovskites had been reduced, the Ea of PAP-H4 decreased by 46.4 kJ/mol at most among them.The catalytic mechanism of MgCo2O4 nanosheets for AP-based energetic molecular perovskites is analyzed.This work provides a reference for the future application of AP-based energetic molecular perovskites.
1.Introduction
Energetic molecular perovskites not only have simple preparation process and low cost, but strong detonation performance and thermal stability, which were pioneered by Chen's group.They have attracted more and more attention from the world [1-6].High-energy inorganic oxidizer and organic fuel were combined into high-symmetry ternary ABX3unit cells using a molecular assembly approach.Among them, the detonation performance of typical ammonium perchlorate-based molecular perovskites highenergy-density materials are superior[7-14].However,because of its high thermal decomposition threshold,it is difficult to minimize the ignition delay time, which limits its application possibilities[15-20].As we all know, adding a small amount of catalyst is a viable method of reducing thermal decomposition, reducing the activation energy, and promoting the specific heat release of energetic materials.
It is important to introduce suitable and useful catalysts to reduce the thermal decomposition of energetic molecular perovskites.Recently,it has been found that composite metal oxides are easier to accept electrons than single metal oxides due to its synergistic effect of different metal atoms, which improves the catalytic activity [21-25].Spinel oxides (AB2O4) show excellent electrochemical applications, thermal stability, and catalytic performance due to polymetallic atoms[26-31].At first,spinel oxides attracted much attention due to their high capacitance and good electrochemistry.In recent years,it was found that they have great catalytic properties, such as MgCo2O4, which has a low cost and good heat resistance.It exhibits a significant catalytic effect on the electro-oxidation of methanol[32]and the catalytic decomposition of nitrogen oxides [33-38].Meanwhile, there is also a small amount of research in the catalysis of energetic materials,and it is found that it is very superior to the thermal decomposition catalysis of AP, which reduces the thermal decomposition temperature and reduces the apparent activation energy [39-42].These reports revealed that MgCo2O4has excellent catalytic advantages.As a result, MgCo2O4was considered and designed here as a novel catalyst for the thermal decomposition of the typical AP-based energetic molecular perovskites.
In this work, the thermal decomposition effect of MgCo2O4nanosheets on AP-based energetic molecular perovskites was studied.The AP-based energetic molecular perovskites PAP-4,DAP-4, PAP-M4, and PAP-H4 were synthesized via molecular assemble strategy, and nanocrystalline spinel MgCo2O4nanosheets were prepared by the template-free hydrothermal method.The microscopic morphology and structure of nano crystalline spinel MgCo2O4nanosheets were characterized.The thermal decomposition processes of AP-based energetic molecular perovskites that with the nanocrystalline spinel MgCo2O4nanosheets added were studied.What's more, coupled TG-QMS was used to analyze the thermal decomposition catalytic mechanism.This work would provide a reference for the catalysis application of molecular perovskites high-energy-density materials in the future.
2.Experiment
2.1.Preparation of PAP-4, DAP-4, PAP-M4, PAP-H4
PAP-4,DAP-4,PAP-M4,and PAP-H4 were synthesized according to literature methods [16,40].The organic fuel molecules piperazine (pz), triethylenediamine (dacbo), 1-methylpiperazine (mpz),and homopiperazine (hpz) are dissolved in water at fixed molar ratios with ammonium perchlorate, and perchloric acid, and then slowly crystallized to obtain PAP-4, DAP-4, PAP-M4, PAP-H4.
2.2.Preparation of MgCo2O4 nanosheets
MgCo2O4nanosheets are prepared by a template-free hydrothermal method.Firstly, 0.3 mmol Mg(NO3)2·6H2O, 0.6 mmol Co(NO3)2·6H2O, and 3 mmol CO(NH2)2were dissolved in 10 mL deionized water and agitated for 0.5 h until the solution became clear.Secondly, the solution was transferred to a high-pressure reactor and kept at 120°C for 6 h.Then, the precursor was collected through filtration, washed multiple times with ethanol,and dried at room temperature.Finally, the dried precursor was calcined at 350°C for 4 h to obtain the black MgCo2O4nanosheets sample.
2.3.Materials characterization
The DX-2700 diffractometer was used to determine the crystal structure data of the material in the scan angle range of 5-80°.The microstructures and morphological characteristics of the materials were studied by scanning electron microscope(SEM,BCPCAS4800)and transmission electron microscope (TEM).
2.4.Catalytic activity measurement
PAP-4, DAP-4, PAP-H4, PAP-M4 were mechanically mixed with MgCo2O4nanosheets at a ratio of 95:5, respectively.DSC(SETARAM,Caluire,France)was used to test and record the thermal decomposition curve of mixtures and raw PAP-4, DAP-4, PAP-H4,PAP-M4 at 30-500°C under high-purity nitrogen (the heating rates were 5 K/min, 10 K/min, 15 K/min, 20 K/min), respectively.Under argon atmosphere, TG-QMS was used to test the mass spectrums of samples under 10 K/min and analyze its catalytic mechanism.
3.Result and discussion
3.1.Structure and morphology
The SEM results of MgCo2O4nanosheets made by the hydrothermal method (Fig.1(a) and Fig.1(b)) show that they have nano sheet structures with width size of 10 μm and thickness size of~20 nm.High specific surface areas from nano sheet structures are advantageous for exposing more redox-active sites.The structure of nanosheets is further explored by TEM result.Fig.1(d) indicates a single MgCo2O4nanosheet, which is consistent with the SEM results.The XRD pattern of MgCo2O4nanosheets was showed in Fig.1(a), and the sample's diffraction peak corresponded to the standard card(PDF:81-0667).Weak diffraction peaks indicated the nano-sized structure of sample, which was corresponding to the SEM and TEM results.There is no other diffraction peak from impurity, discovered on the peaks of MgCo2O4.All of above characterization results demonstrate the catalyst sample MgCo2O4nanosheet had been successful synthesized.
AP-based energetic molecular perovskites were characterized further.XRD patterns of AP-based energetic molecular perovskites(PAP-4, DAP-4, PAP-M4, and PAP-H4) prepared by the molecular assemble method were showed in Fig.2.The main diffraction peaks of the XRD patterns are correlated well with standard diffraction data of AP-based energetic molecular perovskites(CCDC:1956805,1528108,1956806,1956807).As shown in Fig.3,PAP-4,DAP-4,PAPM4, and PAP-H4 exhibited cube shapes owe to their cubic crystal structures.The surfaces of cubic particles are smooth.Especially,grains’morphology of DAP-4 and PAP-H4 in Fig.3(b)and Fig.3(d)is extremely regular.It was determined that the typical AP-based energetic molecular perovskites were synthesized.
3.2.Catalytic performance
With different heating rates (5 K/min,10 K/min,15 K/min, and 20 K/min),DSC curves of the raw materials and AP-based energetic molecular perovskites/MgCo2O4are shown in Fig.4.As shown in Fig.4(a), Fig.4(c), Fig.4(e) and Fig.4(g), raw AP-based energetic molecular perovskites have two decomposition peaks,including an endothermic peak(the change of organic fuel guest molecules from low-temperature phase to high-temperature phase[38-40])and a violent decomposition exothermic peak.The endothermic peaks of PAP-4,DAP-4,PAP-M4,and PAP-H4 are 232.2°C,275.5°C,222.0°C and 120.7°C, respectively.It was confirmed that the thermal decomposition temperature of these types of energetic materials is generally higher,of which the decomposition peak of DAP-4 is the highest at 394.4°C, and the other three are above 360°C.The unique molecular perovskite structure enhances the force in the molecule, so endow the thermal stability of AP-based energetic molecular perovskites.Furthermore, the thermal decomposition peak of AP-based energetic molecular perovskites is high and abrupt, implying that the thermal decomposition process of APbased energetic molecular perovskites is quick and intense.And in the presence of MgCo2O4nanosheets, the temperatures of endothermic peak are basically unchanged, but the thermal decomposition peaks of AP-based energetic molecular perovskites were significantly reduced, the PAP-4 decomposition temperature was lessened by 35.7°C,the decomposition temperature of DAP-4 was decreased to 346.0°C,the decomposition temperature of PAPM4 was lowered from 366.0 to 328.1°C, and the PAP-H4 decomposition temperature was reduced by 43.6°C.It indicated MgCo2O4nanosheets had a significant catalytic effect on the thermal decomposition of AP-based energetic molecular perovskites.And from the TG curve (Fig.5), it can be seen that the thermal weight loss after catalysis becomes smaller, which is due to the stable existence of MgCo2O4.
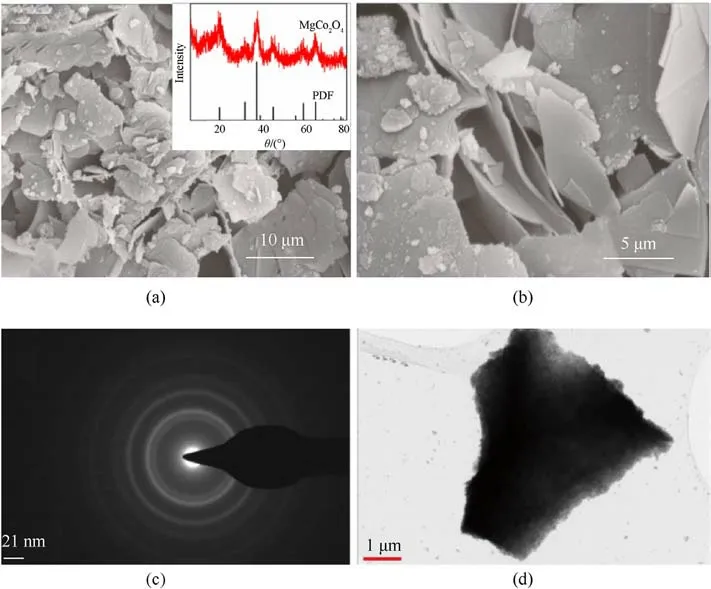
Fig.1. (a), (b) SEM patterns, insert pattern of XRD diffraction in (a) and (c), (d) TEM patterns of MgCo2O4 nanosheets prepared by hydrothermal method.
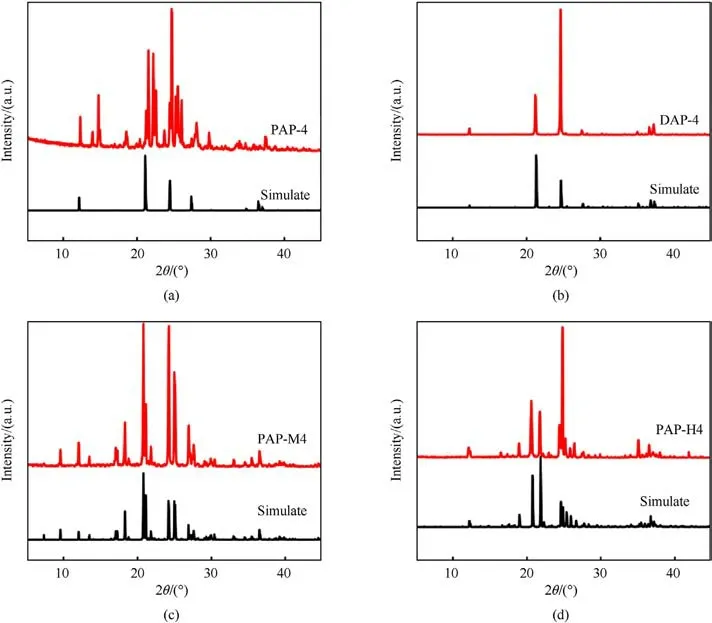
Fig.2. XRD patterns of PAP-4, DAP-4, PAP-M4, PAP-H4.
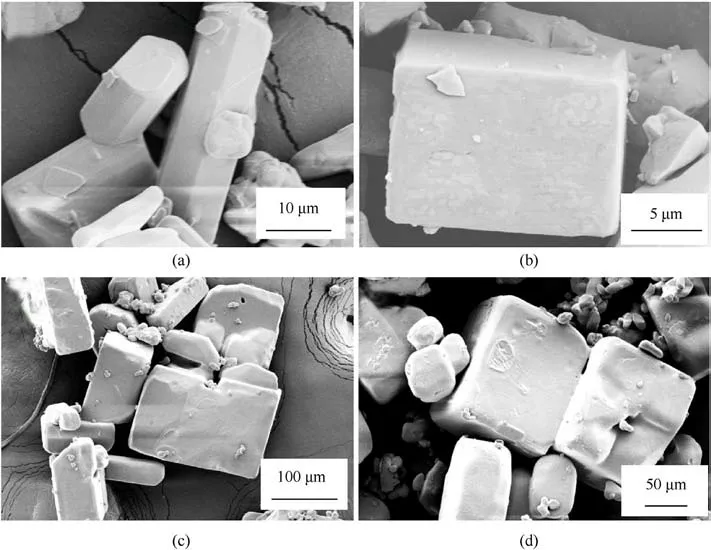
Fig.3. SEM images of (a) PAP-4, (b) DAP-4, (c) PAP-M4, and (d) PAP-H4.
Furthermore, the heat release of AP-based energetic molecular perovskites and mixtures was analyzed in Fig.6.It was found that the heat released by raw PAP-4, DAP-4, PAP-M4, and PAP-H4 is 2188 J/g,4142 J/g,2408 J/g,and 2328 J/g,respectively.After mixing with MgCo2O4nanosheets, the heat released is 2327 J/g, 4363 J/g,2603 J/g, and 2480 J/g.Compared with AP-based energetic molecular perovskites, the heat release from AP-based energetic molecular perovskites mixing with MgCo2O4nanosheets was enhanced significantly.Among the tested samples,the heat release of DAP-4 mixed with MgCo2O4nanosheets was increased the most,reaching 221 J/g, and the heat release of PAP-4 mixed with MgCo2O4nanosheets was increased the least, by 138 J/g.
In addition, the activation energy can be calculated by the Kissinger model (Eq.(1)).
In the formula,βiis the heating rate,Tpiis the peak temperature under βi,Ris the ideal gas constant,Ais the pre-factor,andEis the apparent activation energy.EandAcan be obtained from the slope and intercept of ln
For the raw AP-based energetic molecular perovskites and the mixtures, their kinetic parameters based on the DSC curses are shown in Table 1.The activation energies of raw PAP-4,DAP-4,PAPM4,and PAP-H4 were 178.1 kJ/mol,205.4 kJ/mol,168.3 kJ/mol,and 218.5 kJ/mol.After MgCo2O4nanosheets added,theirEadecreased to 151.5 kJ/mol, 179.8 kJ/mol, 152.9 kJ/mol, and 172.9 kJ/mol respectively.The catalyst MgCo2O4nanosheets has a nano sheet structure and a large specific surface area,which allows it to gather more heat per unit time and hence accelerate the thermal decomposition of AP-based energetic molecular perovskites.It revealed that MgCo2O4nanosheets with unique structure and properties have excellent thermal decomposition catalysis properties towards AP-based energetic molecular perovskites,which are beneficial for reducing the thermal decomposition temperature and activation energy of the decomposition process, and also intensifying the heat release.
3.3.Catalytic mechanism
To investigate the catalytic mechanism of MgCo2O4nanosheets on the decomposition of AP-based energetic molecular perovskites,coupled thermal analytic techniques (TG-QMS) were employed to perform a real-time and continuous investigation of the entire decomposition process.Previous works had analyzed the decomposition mechanism of AP-based energetic molecular perovskites[38-41].The thermal decomposition of AP-based energetic molecular perovskites is primarily influenced by the stability of the cage-like framework structure, which is made of cationand anion.The protonated organic fuel component is encased in the stable framework structure.The high temperature destroys the cage structure of cationsanions, releasing organic fuel molecules in the cage.It was found in Fig.7 that the main products of AP-based energetic molecular perovskites thermal decomposition are H2O, NO, CO2, N2, etc.After mixing with MgCo2O4nanosheets,the mass spectrum curves showed the trend of the peak temperature shifting to the low-temperature direction and coincided well with the DSC curve(Fig.4).
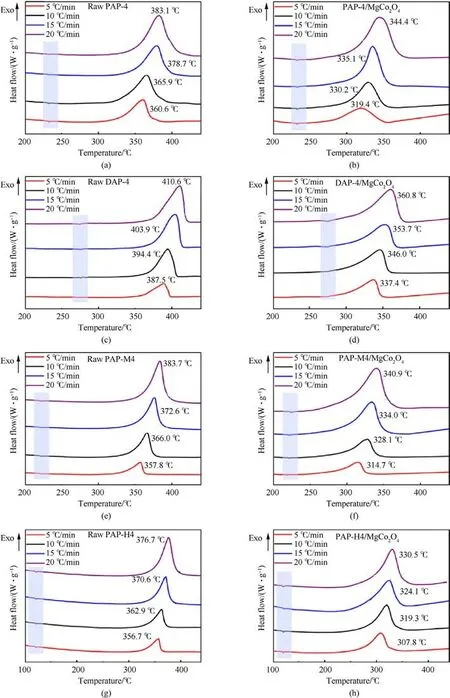
Fig.4. DSC curves of(a)Raw PAP-4,(b)PAP-4/MgCo2O4,(c)Raw DAP-4,(d)DAP-4/MgCo2O4,(e)Raw PAP-M4,(f)PAP-M4/MgCo2O4,(g)Raw PAP-H4,(f)PAP-H4/MgCo2O4 at different heating rates.
Based on the findings of the characterization and thermal analysis, a possible synergistic catalytic thermal decomposition mechanism of MgCo2O4nanosheets for AP-based energetic molecular perovskites was further proposed.In Fig.8, when MgCo2O4nanosheets are mixed, the multi-component effect and nano-layered structure of MgCo2O4,the Co3+or Mg2+in MgCo2O4nanosheets can provide electrons to promote the transfer of H+from the protonated organic fuel cations (H2dacbo2+, H2pz2+,H2mpz2+, H2hpz2+) andto, and generate NH3, organic fuel molecules (dacbo, pz, mpz, hpz), and HClO4macromolecules[22,24].At the same time,the superoxide ion()was produced by HClO4due to the electron transfer ability of MgCo2O4,then it reacts with organic fuel molecules and NH3to generate H2O,NO,NO2,N2,and CO2[42].In this process,more heat was greatly released.In the redox reaction cycle, electron transfer is further accelerated by catalysts, leading in that the AP-based energetic molecular perovskites are decomposed at a lower temperature and theEais further reduced.
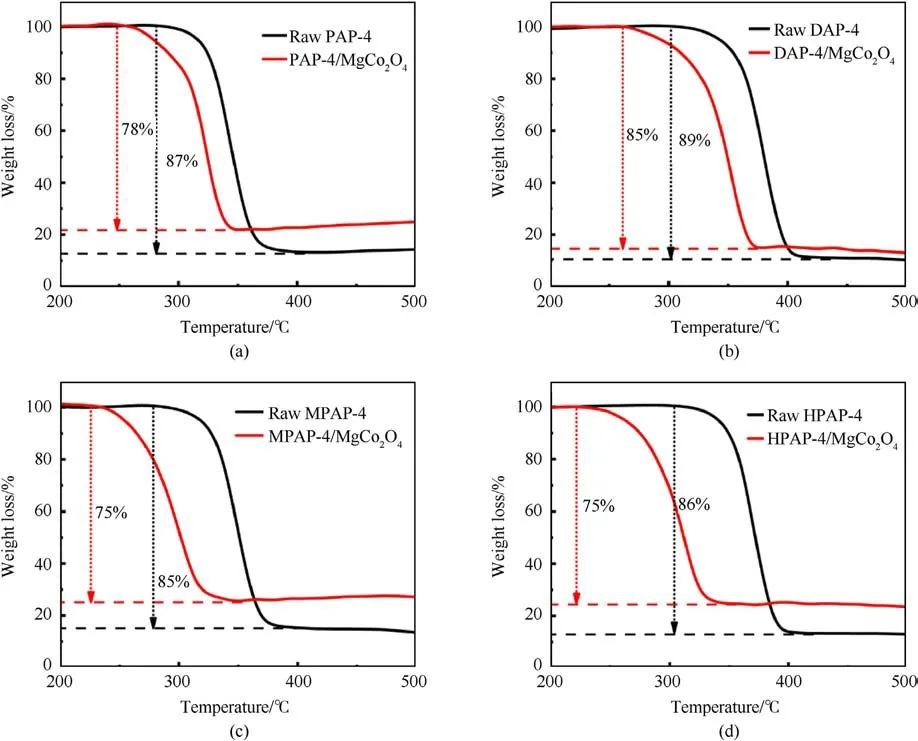
Fig.5. TG curves of (a) raw PAP-4, PAP-4/MgCo2O4, (b) raw DAP-4, DAP-4/MgCo2O4, (c) raw PAP-M4, PAP-M4/MgCo2O4, (d) raw PAP-H4, PAP-H4/MgCo2O4 at 10 °C/min.
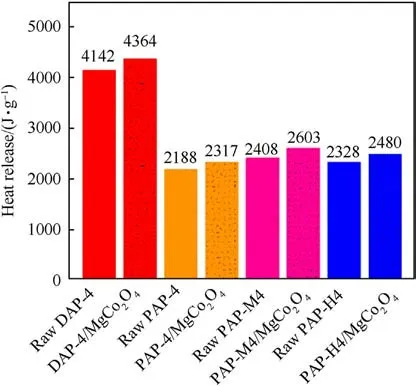
Fig.6. The heat release of raw AP-based energetic molecular perovskites and AP-based energetic molecular perovskites with 5 wt% MgCo2O4 nanosheets.
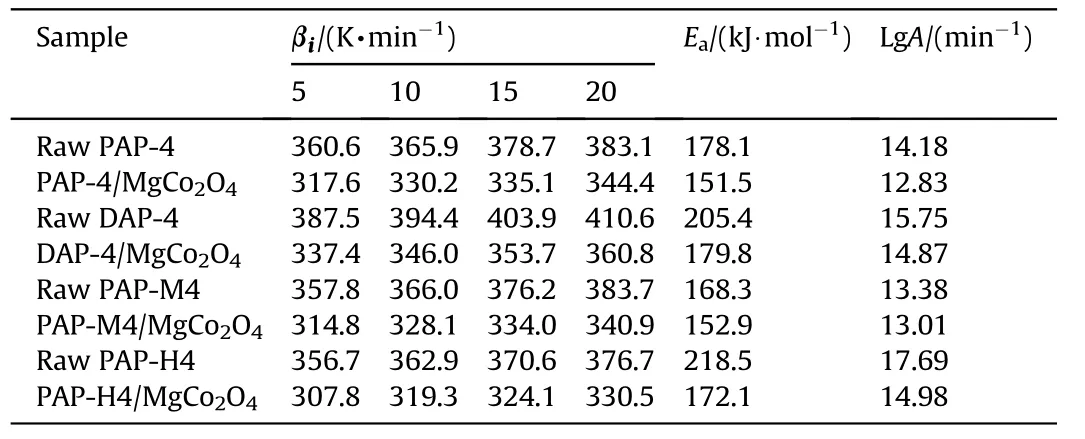
Table 1The kinetic parameters for AP-based energetic molecular perovskites’ decomposition with and without MgCo2O4.
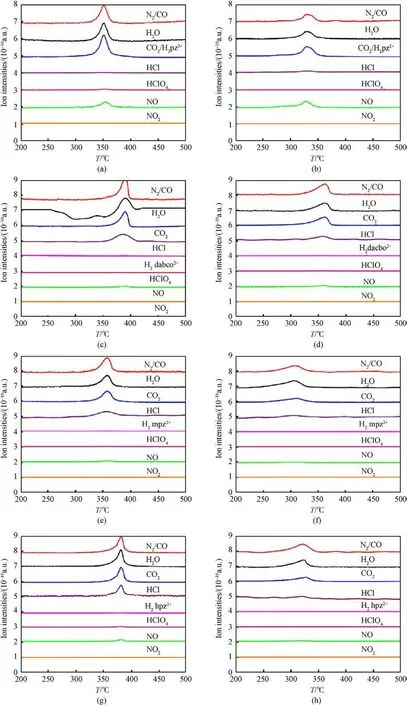
Fig.7. The mass spectra for(a)raw PAP-4,(b)PAP-4/MgCo2O4,(c)raw DAP-4,(d)DAP-4/MgCo2O4,(e)raw PAP-M4,(f)PAP-M4/MgCo2O4,(g)raw PAP-H4,(f)PAP-H4/MgCo2O4 at a heating rate of 10 °C/min.
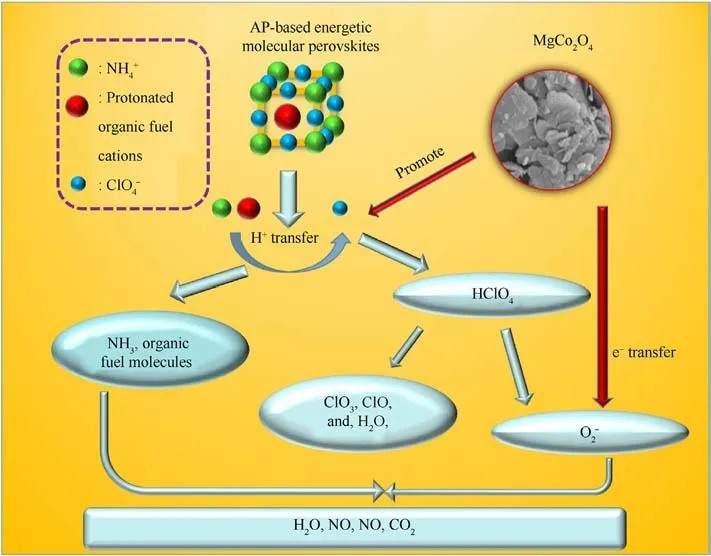
Fig.8. Schematic diagram of the decomposition mechanism of AP-based energetic molecular perovskites catalyzed by MgCo2O4 nanosheets.
4.Conclusions
In summary,a new type of multi-metal oxide MgCo2O4catalyst was used to study the thermal decomposition performance of APbased energetic molecular perovskites.We found that MgCo2O4nanosheets exhibit excellent catalytic activity against AP-based energetic molecular perovskites.When 5 wt% MgCo2O4nanosheets were added,the thermal decomposition peak temperatures of PAP-4, PAP-M4, DAP-4, and PAP-H4 were reduced by 35.7°C,48.4°C, 37.9°C, and 43.6°C, respectively.The heat release of APbased energetic molecular perovskites mixing with 5 wt%MgCo2O4nanosheets has increased significantly.The activation energy of the thermal decomposition of AP-based energetic molecular perovskites is reduced.TheEaof PAP-H4 was decreased by 46.4 kJ/mol at most among them.The possible catalytic mechanism of MgCo2O4nanosheets to AP-based energetic molecular perovskites was analyzed.The prepared MgCo2O4nanosheets have unique layered structures and multi-component effect, that effectively accelerate the collapse of the AP-based energetic molecular perovskites frame structure.The results also provide references for catalyzing the thermal decomposition of AP-based energetic molecular perovskites.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the National Natural Science Foundation of China(Grant No.21975227)and the Found of National defence Sci& Tech Laboratory(Grant No.6142602210306).
- Defence Technology的其它文章
- A comparative single-pulse shock tube experiment and kinetic modeling study on pyrolysis of cyclohexane, methylcyclohexane and ethylcyclohexane
- Investigation on thermal characteristics and desensitization mechanism of improved step ladder-structured nitrocellulose
- 2D metal-organic frameworks endow ammonium perchlorate with enhanced thermal effect
- Mechanical behavior of entangled metallic wire materialspolyurethane interpenetrating composites
- Modification of SDOF model for reinforced concrete beams under close-in explosion
- Air combat target maneuver trajectory prediction based on robust regularized Volterra series and adaptive ensemble online transfer learning

