lnflammatory myofibroblastic tumor diagnosed due to high intraocular pressure
Juan Zhu, Hong-Lei Liu, Hui Liu, Gang Li, Jian-Rong Liu, Wei-Wei Wang
1Shaanxi Eye Hospital, Xi’an People’s Hospital (Xi’an Fourth Hospital), Affiliated People’s Hospital Northwest University,Xi’an 710004, Shaanxi Province, China
2Department of Pathology, Xi’an People’s Hospital (Xi’an Fourth Hospital), Affiliated People’s Hospital Northwest University, Xi’an 710004, Shaanxi Province, China
Dear Editor,
I am writing this letter to present an unusual case of orbital inflammatory myofibroblastic tumor (IMT) diagnosed due to high intraocular pressure (IOP).
IMT is a rare mesenchymal, low‐grade malignant or borderline tumor, composed of differentiated myofibroblastic spindle cells, often accompanied by plasma cells and lymphocyte infiltration[1]. The onset in most patients is insidious; it frequently occurs in children and young adults, most commonly in the lungs, abdomen, and pelvic cavity, and rarely in the orbit[1‐3]. Recent reports showed that orbital IMTs are monocular[3‐8]. Their usual manifestations are proptosis and ptosis[3], although chemosis, diplopia, and periocular swelling can also be observed[4‐8]. Extraocular movement disorders can occur in cases of involvement of the extraocular muscles[8].Nevertheless, the present paper describes the first case of IMT diagnosed due to high IOP.
This study was approved by the Review Board of Xi’an People’s Hospital (Xi’an Fourth Hospital). Written informed consent to participate and publication was obtained from the patient.
CASE PRESENTATION
A 38‐year‐old male presented with ocular hypertension for two weeks detected during physical examination. The medical history of the patient was normal, with no family history or heredity. The best corrected visual acuity was 20/32 right and 20/25 left; IOP was 33.8 and 16.8 mm Hg,respectively. Motility testing showed right hypertropia and completely limited right supraduction and infraduction deficit.Examination revealed right conjunctival congestion and chemosis located at the inferior‐temporal quadrant with pain on ocular palpation (Figure 1). The results of slit‐lamp and fundus examinations were normal. B‐scan ultrasound showed oval low‐echo area below the right eye bulb (Figure 2). Orbital CT established ellipsoid soft tissue density focused at the inferior orbit of the right eye, with unclear boundary with the inferior rectus muscle (Figure 3). The orbital MRI findings were as follows: 13.5×32×18.5 mm3spindle shape at the inferior orbit with isointense on T1‐weighted images, slightly hyperintense on T2 (Figure 4A, 4B, 4D, 4F). Enhanced scanning showed inhomogeneous enhancement of the lesion, with unclear boundary with the inferior rectus muscle (Figure 4C, 4E, 4G).No abnormality was found by gonioscopy, visual field, OCT,fundus photo with an ultrawide view and ultrasonic biological microscopy. The IOP fluctuated from 28.2 to 33.4 mm Hg after topical anti‐glaucoma medications.
The patient underwent right orbitotomy and tumor removal in the Orbital Disease Center of Shaanxi Eye Hospital,Xi’an People’s Hospital (Xi’an Fourth Hospital). During the operation, the gray tumor tissue near the inferior rectus muscle was observed, which adhered to the inferior rectus muscle and had a capsule. The pathological results showed that the gray matter of the tumor section was composed of fragments of spindle fibroblasts and myofibroblasts, with no obvious atypia,no pathological mitosis, and visible infiltration of plasma cells and lymphocytes (Figure 5A). Immunohistochemistry was positive for anaplastic lymphoma kinase (ALK) (Figure 5B),smooth muscle actin (SMA) (Figure 5C), desmin, lymphocyte common antigen (LCA), CD34 and CD68. The tumor was negative for CD117. The Ki‐67 proliferation index was 5%. Fluorescencein-situhybridization (FISH) analysis was positive for an ALK gene rearrangement (Figure 5D). These features supported a diagnosis of orbital IMT. Postoperatively,the patient’s right eye was not treated with IOP lowering drugs, and the IOP fluctuated within 13‐15 mm Hg. Motility testing showed right hypertropia and right mild supraduction and moderate infraduction deficit. Prism was recommended to the patient to eliminate vertical diplopia. The patient was followed up for seven months after the operation, and no tumor recurrence or metastasis was detected. His IOP was normal.
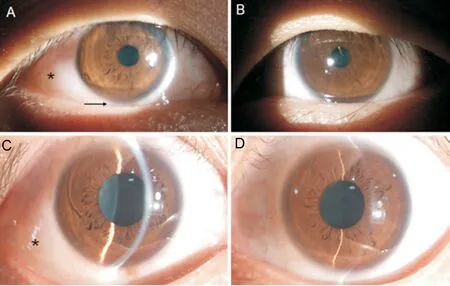
Figure 1 Biomicroscopic images A, C: The right eye showing circumscribed conjunctival edema (asterisk) and upward displacement of eyeball (arrow); B, D: Normal left eye.
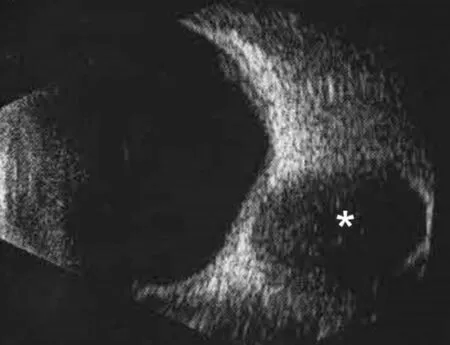
Figure 2 B-scan ultrasound image Asterisk indicates the tumor.
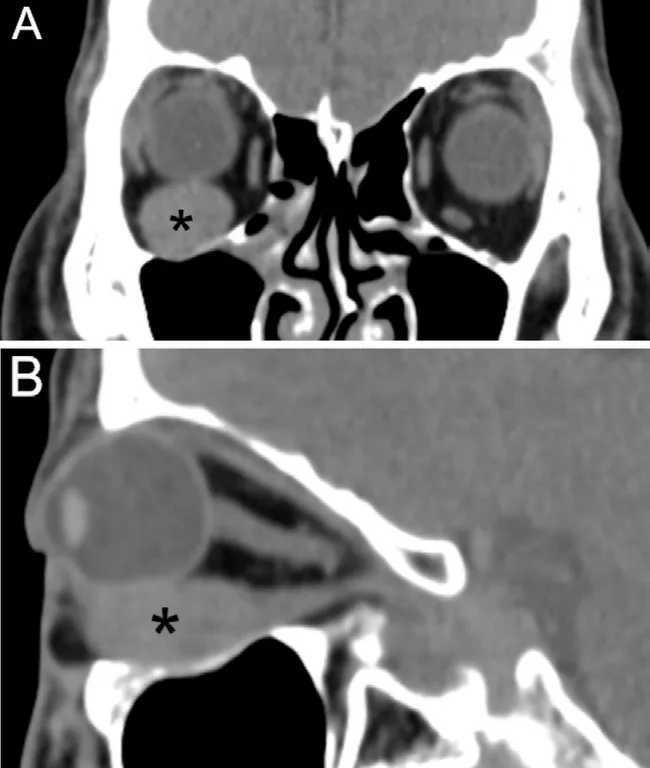
Figure 3 Computerized tomography images A: Coronal section; B:Sagittal section. Asterisk indicates the tumor.
DISCUSSION
IMT increases IOP, which is considered to be caused by the rise in the volume of the orbital contents by tumors and the subsequent obstruction of the blood reflux in the orbital venous system. These changes result in an elevate episcleral venous pressure and aqueous outflow obstruction, hindering the effectiveness of the control of refractory high IOP by drugs.To the best of our knowledge, this is the first reported case of a patient with elevated IOP caused by IMT. After complete tumor resection, the obstruction of the orbital venous was relieved, and IOP returned to normal ranges.
The pathogenesis of IMT is still unclear, and no characteristic imaging findings have been reported[3‐9]. The ocular ultrasound examination in this study showed hypoechoic lesions.Additionally, CT revealed the presence of localized lesions of the extraocular muscle that were equal in density. MRI findings detected soft tissue mass, isointense on T1‐weighted images but hyperintense on T2‐weighted images, which were enhanced by injection of contrast agent[3]. No difference was observed between the imaging examination results of benign and malignant tumor tissues, and thus our diagnosis had to be based on pathological examination results.
Immunohistochemistry can confirm the immunophenotype of myofibroblasts to identify other types of tumors[10]. Myogenic SMA and desmin detection have high specificity, and their positive reactions are important markers of IMT, with positive rates of 80%‐90% and 60%‐70%, respectively[3,11].Furthermore, ALK‐positive staining is of critical significance for the diagnosis of IMT, with a detected positive rate within 45%‐88%, which is closely related to the location of the tumor[3,10‐12].ALKgene fusion is critically involved in IMT development. TheALKgene, located at 2p23, encodes a 200‐kD tyrosine kinase receptor that is mainly expressed in the developing nervous system.ALKgene amplification can be observed in a few mesenchymal tumors, whereasALKgene rearrangement is rare, occurring mainly in IMT, with a positive rate ofALKgene rearrangement of 50%‐75%[10‐13]. In the case described here, ALK, SMA, desmin, and LCA staining results were positive. Moreover,ALKgene rearrangement was also positive by FISH, and thus the diagnosis was clear.
Surgical resection is the main treatment for orbital IMT.In patients with unresectable or recurrent orbital IMT,glucocorticoid or non‐corticosteroid anti‐inflammatory therapy, chemotherapy or low‐dose radiotherapy can also be considered[1‐4,7‐8]. In recent years, targeted therapy for ALK has achieved good results. The tumor in patients with ALK‐positive test results can shrink or even disappear after oral crizotinib administration[14]. Targeted drugs are also of substantial significance for refractory, relapsing, and metastatic IMT patients[13,15]. The recurrence rate of IMT is 24%‐37%, and the metastasis rate is 8%‐11%. Long‐term follow‐up durations of IMT patients are critical as the liver and the brain are the most common sites of metastasis[3,7,11]. Good prognosis in most patients with tumor recurrence and metastasis is achieved through radiotherapy and chemotherapy, combined with targeted drugs and other comprehensive treatment methods.In this case, the positive expression of ALK was found by immunohistochemical staining, and ALK gene rearrangement was further detected by FISH. The tumor was completely surgically resected, with no recurrence or metastasis detected during the seven‐month follow‐up period.
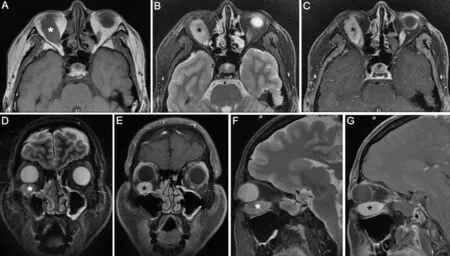
Figure 4 Magnetic resonance imaging photos showing isointense in T1, slight hyperintense in T2, and inhomogeneous enhancement after gadolinium administration A: Transverse section of T1; B: Transverse section of T2; C: Transverse section after gadolinium administration; D:Coronal section of T2; E: Coronal section after gadolinium administration; F: Axial section of T2; G: Axial section after gadolinium administration.Asterisk indicates the tumor.
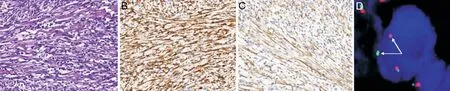
Figure 5 Histopathologic images A: The spindle cells in fascicular arrangement with inflammatory cells infiltration (hematoxylin-eosin, ×200);B: Immunohistochemistry using anaplastic lymphoma kinase (ALK) antibody highlights the spindle cells (×200); C: Immunohistochemistry using smooth muscle actin antibody highlights the spindle cells (×200); D: Fluorescence in-situ hybridization (FISH) shows rearrangement of the ALK gene with split of the red and green signals (arrows) (×1000).
The limitation of our study was the relatively short follow‐up time, and long‐term follow‐up duration is required.
To conclude, in cases of localized chemosis in young people with refractory high IOP, timely ocular palpation and extraocular movement examination should be done, which is conducive to the detection of orbital mass lesions. Elevated episcleral venous pressure and blocked aqueous outflow is observed in subjects with orbital venous obstruction due to orbital tumor. In such cases, IOP increases, hindering the effective control by IOP‐lowering drugs. Nevertheless,orbitotomy and timely tumor removal normalize the orbital venous drainage and can reverse IOP to normal ranges.
ACKNOWLEDGEMENTS
Authors’ contributions:Zhu J drafted the manuscript. Liu HL interpreted the patient’s data. Liu H acquired and interpreted the pathological data of the patient. Li G and Liu JR critically revised the manuscript. Wang WW contributed to conception,design and critically revised the manuscript. All authors have read and approved the final manuscript.
Foundations:Supported by the Xi’an Science and Technology Plan Project (No.21YXYJ0044); the Research Incubaiton of Xi’an People’s Hospital (Xi’an Fourth Hospital; No.BS‐2).
Conflicts of Interest: Zhu J, None;Liu HL, None;Liu H,None;Li G,None;Liu JR, None;Wang WW,None.
 International Journal of Ophthalmology2023年1期
International Journal of Ophthalmology2023年1期
- International Journal of Ophthalmology的其它文章
- Visual perception alterations in COVID-19: a preliminary study
- COVID-19 pandemic impact on ocular trauma in a tertiary hospital
- Apolipoprotein A1 suppresses the hypoxia-induced angiogenesis of human retinal endothelial cells by targeting PlGF
- Comparison of vegetable oils on the uptake of lutein and zeaxanthin by ARPE-19 cells
- Identifying a novel frameshift pathogenic variant in a Chinese family with neurofibromatosis type 1 and review of literature
- Recurrence risk factors of intravitreal ranibizumab monotherapy in retinopathy of prematurity: a retrospective study at one center
