人工种植帚状香茶菜中木脂素类成分的研究*
陈素萍,胡 坤,孙汉董,普诺·白玛丹增△
(1.中国科学院昆明植物研究所 植物化学与西部植物资源持续利用国家重点实验室,云南 昆明 650201;2.中国科学院大学,北京 100049)
1 Introduction
Isodon scoparius is mainly distributed in northwestern Yunnan Province,China,and it is commonly used as an antipyretic agent by local inhabitants.Previous phytochemical investigations of I.scoparius collected in Shangri-La indicated that its major chemical constituents were ent-clerodane diterpenoids[1-2].Noteworthily,the discovery of cyclobutane-bearing meroditerpenoids and complicated dimeric ent-clerodanoids from I.scoparius,along with their fascinating immuno suppressive activities and the completion of their organic synthesis,marked one of the most fantastic breakthroughs in research on Isodon species in recent years[3-9].To achieve sustainable utilization of plant resources,I.scoparius was artificially cultivated in Kunming Botanical Garden with seeds collected from Shangri-La.Subsequently,phytochemical research on this plant material was undertaken by our research group.Previous study on the cultivated plants has led to the identification of two cyclobutane-bearing meroditerpenoids,including a novel one characterized by an oxygen insertion reaction in its proposed biosynthesis[10].In this work,we continued to investigate the chemical constituents of artificially cultivated I.sco-parius,which led to the isolation and identification of a series of lignans,including a new compound,scopariunan(1),along with five known ones,1-hydroxypinoresinol(2)[11],tanegool-7′-methylether(3)[12],7′-methoxylariciresinol(4)[12],erythro-2,3-bis-(4-hydroxy-3-methoxyphenyl)-3-methoxy-propanol(5)[13],threo-2,3-bis-(4-hydroxy-3-methoxyphenyl)-3-methoxy-propanol(6)[14].Herein,we report the details of the isolation and structure determination of these compounds.
2 Results

Scopariunan(1)was obtained as colorless oil and its molecular formula was determined as C22H26O7by a negative HRESIMS ion peak at m/z([M-H]-,401.1609,calcd for 401.1606),implying ten degrees of unsaturation.The1H NMR data together with HSQC spectrum showed four methoxy groups at δH3.88 (3H,overlapped,H3-1"),3.88(3H,overlapped,H3-2"),3.89(3H,s,H3-3")and 3.82 (3H,s,H3-4"),five aromatic hydrogens at δH6.98(1H,dd,J=8.2,2.0 Hz,H-6),6.95(1H,d,J=2.0 Hz,H-2),6.84(1H,d,J=8.2 Hz,H-5),6.80(1H,s,H-2′)and 6.70(1H,s,H-6′).The13C NMR and DEPT spectra exhibited 22 resonances,which can be categorized into four methyls,three methylenes,seven methines(including five aromatic carbons and one oxygenated carbon),and eight nonprotonated carbons(including one carbonyl and seven aromatic carbons).Through the analysis of 2D NMR spectra,compound 1 was inferred to be a dihydrobenzofuran lignan with ester group and four methoxy groups.The HMBC correlations from H-7 (δH6.05),H-8(δH4.32)and H3-4"(δH3.82)to C-9(δC171.5)indicated that C-9 was oxidated to a methyl ester.Besides,the HMBC correlations between H3-1"(δH3.88)to C-3(δC149.4),H3-2"(δH3.88)to C-4(δC149.4)and H3-3"(δH3.89)to C-5′(δC144.5)revealed the substitutional positions of the remaining methoxy groups(Figure 1).As for the relative configuration of compound 1,the coupling constants between H-7 and H-8(8.6 Hz)revealed that these two protons adopted cis arrangement.Then,quantum chemical calculations were run on(7S,8R)-1T(1Ta),with 1T being the truncated structure of 1(to be specific,the-CH2-CH2-CH2-OH chain in 1 was replaced by a methyl in 1T to avoid redundant conformers).The calculated3JH-7/H-8of 9.8 Hz at B972-SCRF/pcJ-1(chloroform)//M06-2X-D3/6-311G(d,p)level of theory verified the established relative configuration of compound 1.The calculated ECD spectrum of 1Ta at CAM-B3LYP-SCRF/6-31+G(2d,p)(methanol)//M06-2X-D3/6-311G(d,p)level of theory appeared as nearly image of the experimental ECD spectrum(Figure 2),indicating that the absolute configuration of 1 was 7R,8S.Thus,the structure of compound 1 was established.
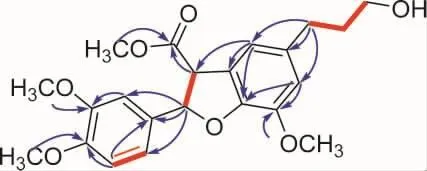
Fig.1 1H-1H COSY(red bold)and selected HMBC(blue arrow)correlations of 1.
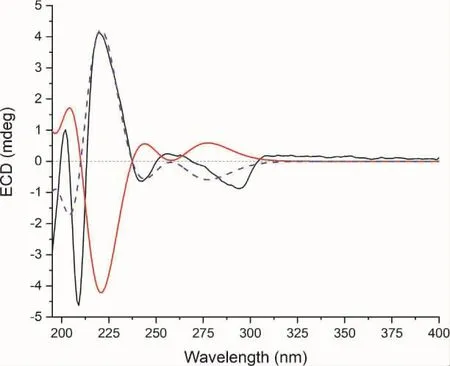
Fig.2 Experimental ECD spectrum of 1 (black).Calculated ECD spectra of(7S,8R)-1T(1Ta)(red solid line)and(7R,8S)-1T(1Tb)(blue dotted line)at CAM-B3LYP-SCRF/6-31+G(2d,p)(methanol)//M06-2X-D3/6-311G(d,p)level of theory(shift=+11 nm,full width at half maximum=0.6 eV).
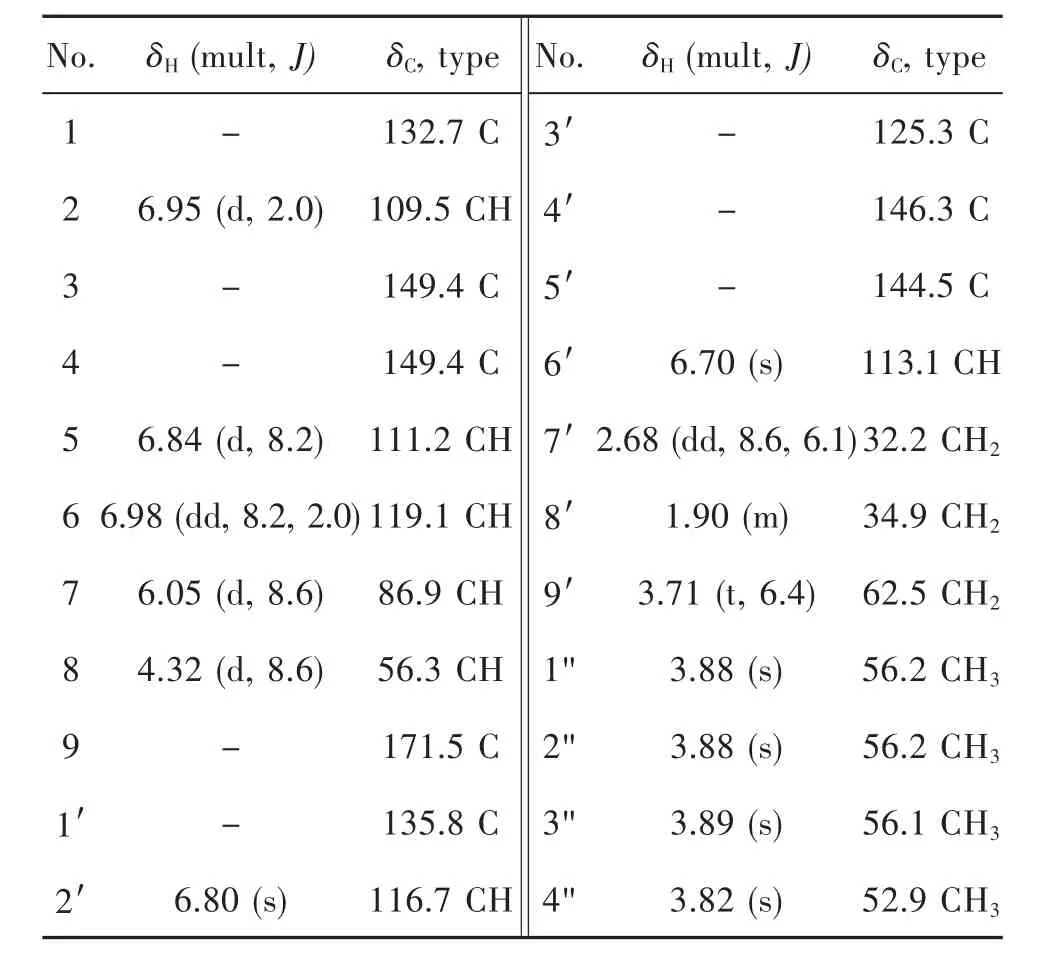
Tab.1 NMR data(δ in ppm,J in Hz)of 3′,4-O-dimethylcedrusin methyl ester(1)a
The five known lignans were identified by comparison of their spectroscopic data with those reported in literatures,and they were identified as 1-hydroxypinoresinol(2),tanegool-7′-methylether(3),7′-metho-xylariciresinol(4),erythro-2,3-bis-(4-hydroxy-3-methoxyphenyl)-3-methoxy-propanol(5),threo-2,3-bis-(4-hydroxy-3-methoxyphenyl)-3-methoxy-propanol(6).
3 Experimental details
3.1 General experimental procedures Optical rotation was measured with a JASCO P-1020 polarimeter.UV spectrum was obtained using a Shimadzu UV-2401 PC spectrophotometer.Experimental ECD spectrum was measured on a Chirascan instrument.A Tensor 27 spectrophotometer was used for scanning IR spectroscopy with KBr pellets.HRESIMS data was acquired on an Agilent 6540 QSTAR TOF time-of-flight mass spectrometer.1D and 2D NMR spectra were recorded on Bruker DRX-500,600 and 800 spectrometers with TMS as internal standard.Chemical shifts(δ)are expressed in parts per million(ppm)with reference to the solvent signals.Column chromatography was performed on silica gel(80-100 mesh and 100-200 mesh),Lichroprep RP-18 gel(40-63 μm,Merck,Darmstadt,Germany),MCI gel(75-150 μm,Mitsubishi Chemical Corporation,Tokyo,Japan),and Sephadex LH-20(Pharmacia,Uppsala,Sweden).Semi-preparative HPLC was performed on an Agilent 1260 liquid chromatograph with a Zorbax SB-C18(9.4 mm×250 mm)column.Fractions were monitored by TLC,and spots were visualized by heating silica gel plates sprayed with 10% H2SO4in EtOH.
3.2 Plant materials The Isodon scoparius was artificially cultivated in Kunming Institute of Botany and the seeds were collected from Shangri-La,Yunnan Province,China.Experimental materials were their aerial parts.
3.3 Extraction and isolation The air-dried and powdered aerial parts of Isodon scoparius(20 Kg)were extracted with 70% aqueous acetone.With filtration and concentration,the resultant extract was partitioned with EtOAc and n-BuOH respectively,and then evaporated in vacuo to afford a crude extract(0.75 Kg in EtOAc and 0.14 Kg in n-BuOH).The n-BuOH extraction was subjected to column chro-matography on silica gel eluted with a CHCl3-Me2CO gradient system (1∶0,9∶1,8∶2,7 ∶3,6 ∶4,0 ∶1,v/v)to yield six fractions,A-F.Fraction C(CHCl3-Me2CO 8∶2,82 g)was decolorized by MCI gel with methanol-H2O(90∶10,v/v)and methanol(100%,v)system to afford fractions C1 and C2.Fraction C1 was subjected to RP-18 silica gel with gradient system(methanol-H2O,from 30%∶70% to 100% ∶0%,v/v)to yield subfractions C1/1-8,subfraction C1-5 was purified by repeated silica gel(eluted with petroleum ether-Me2CO 10∶1 to 0∶1,v/v),Sephadex LH-20(MeOH system)and semipreparative HPLC(MeCN-H2O,35∶65,v/v)to yield 1(1.5 mg),2(1.1 mg),3(2.0 mg),4(1.2 mg),5(2.3 mg)and 6(1.0 mg).
Scopariunan (1):Colorless oil; [α]D19.4:-59.60(MeOH,c 0.095);ECD(MeOH)λmax(logε):202(0.25),209(-1.13),220(1.01),242(-0.16)and 294(-0.21)nm;UV(MeOH)λmax(logε):204(3.90),226(3.34)and 282(2.86)nm;IR(νmax):3436,2932,1737,1619,1518,1265,1142 and 1026 cm-1.HRESIMS at m/z 401.1609([M-H]-,calcd for 401.1606).1H and13C NMR data,see Table 1.
1-Hydroxypinoresinol(2):Yellow oil;C20H22O7;1H NMR(600 MHz,CDCl3):δH6.99(1H,d,10.9,H-5),6.96(1H,d,10.9,H-5′),6.95(1H,d,5.5,H-2),6.92(1H,d,5.5,H-2′),6.88 (1H,dd,10.9,5.5,H-6),6.81 (1H,dd,10.9,5.5,H-6′),4.85 (1H,d,5.0,H-7),4.80(1H,s,H-7′),4.54(1H,t,9.0,H-9′a),4.11(2H,m,H-9),3.87(6H,s,H-3,3′-OCH3),3.75(1H,dd,9.0,6.4,H-9′b),3.13(1H,m,H-8).δC(150 MHz,CDCl3):147.2(s,C-3′),146.9(s,C-3),146.2(s,C-4),145.7(s,C-4′),132.6(s,C-1′),127.2(s,C-1),119.9(d,C-6),119.8(d,C-6′),114.9(d,C-5′),114.5(d,C-5),109.6(d,C-2),109.2(d,C-2′),91.9(s,C-8′),88.0(d,C-7′),86.0(d,C-7),74.9(t,C-9),71.9(t,C-9′),60.3(d,C-8),56.2(q,C-3-OCH3),56.1(q,C-3′-OCH3).
Tanegool-7′-methylether(3):Yellow oil;C21H26O7;1H NMR(800 MHz,DMSO-d6):δH6.83(1H,brs,H-2′),6.81(1H,brs,H-2),6.75(1H,d,8.0,H-5′),6.70(1H,overlapped,H-5),6.70 (1H,overlapped,H-6),6.70(1H,overlapped,H-6′),4.51(1H,d,7.4,H-7),3.95 (1H,d,8.9,H-7′),3.76 (3H,s,H-3′-OCH3),3.73(3H,s,H-3-OCH3),3.54(1H,overlapped,H-9′a),3.44(1H,overlapped,H-9′b),3.49(2H,overlapped,H-9),3.02(3H,s,H-7′-OCH3),2.50(1H,m,H-8′),2.07(1H,m,H-8).δC(200 MHz,DMSO-d6):147.7(s,C-3′),147.5(s,C-3),146.3(s,C-4′),145.7(s,C-4),133.6(s,C-1),131.3(s,C-1′),120.4(d,C-6′),118.8(d,C-6),115.2 (d,C-5′),115.1 (d,C-5),111.0(d,C-2′),110.4(d,C-2),85.7(d,C-7′),83.3(d,C-7),69.1 (t,C-9′),61.5 (t,C-9),55.8 (q,C-7′-OCH3),55.7(q,C-3′-OCH3),55.6(q,C-3-OCH3),53.8(d,C-8),48.3(d,C-8′).
7′-Methoxylariciresinol(4):Yellow oil;C21H26O7;1H NMR(800 MHz,DMSO-d6):δH6.85(1H,br s,H-2),6.75(1H,brs,H-2′),6.72(1H,overlapped,H-6),6.71(1H,overlapped,H-5′),6.71(1H,overlapped,H-5),6.62(1H,br d,8.0,H-6′),4.50(1H,d,7.4,H-7),4.03 (1H,dd,4.0,8.8,H-9′a),3.90 (1H,d,9.3,H-7′),3.79 (1H,br d,8.2,H-9′b),3.76 (3H,s,H-3′-OCH3),3.73(3H,s,H-3-OCH3),3.05(1H,overlapped,H-9a),3.05(3H,s,H-7′-OCH3),2.89(1H,m,H-9b),2.41(1H,m,H-8′),1.60(1H,m,H-8).δC(200 MHz,DMSO-d6):147.7(s,C-3′),147.5(s,C-3),146.2(s,C-4′),145.7(s,C-4),133.6(s,C-1),130.9(s,C-1′),120.5(d,C-6′),118.7(d,C-6),115.2(d,C-5′),115.1(d,C-5),111.0(d,C-2′),110.4(d,C-2),85.4(d,C-7′),82.7(d,C-7),70.4(t,C-9′),60.4(t,C-9),55.8(q,C-7′-OCH3),55.7(q,C-3′-OCH3),55.6(q,C-3-OCH3),52.2(d,C-8),48.0(d,C-8′).
Erythro-2,3-bis-(4-hydroxy-3-methoxyphenyl)-3-methoxy-propanol(5):Yellow oil;C18H22O6;1H NMR(600 MHz,CD3OD):δH6.45-6.74(6H,m,ArH),4.47(1H,d,5.5,H-7),3.89 (2H,m,H-9),3.69,3.65(each 3H,s,H-3,3′-OCH3),3.15(3H,s,H-7-OCH3),2.85(1H,m,H-8).δC(150 MHz,CD3OD):148.5(s,C-3),148.3(s,C-3′),147.0(s,C-4),146.3(s,C-4′),133.3(s,C-1),132.4(s,C-1′),123.4(d,C-6),121.4(d,C-6′),115.7(d,C-5′),115.7(d,C-5),114.8(d,C-2′),111.9(d,C-2),85.1(d,C-7),64.5(t,C-9),57.2(q,C-11-OCH3),56.7 (q,C-3-OCH3),56.4 (q,C-3′-OCH3),56.2(d,C-8).
Threo-2,3-bis-(4-hydroxy-3-methoxyphenyl)-3-methoxy-propanol(6):Yellow oil;C18H22O6;1H NMR(600 MHz,CD3OD):δH6.46-6.74 (6H,m,ArH),4.31(1H,d,8.4,H-7),4.04 (1H,m,H-9a),3.88 (1H,m,H-9b),3.69(3H,s,H-3-OCH3),3.65(3H,s,H-3′-OCH3),3.25(3H,s,H-7-OCH3),3.03(1H,m,H-8).δC(150 MHz,CD3OD):148.7(s,C-3),148.5(s,C-3′),147.0(s,C-4),146.2(s,C-4′),132.8(s,C-1),132.8(s,C-1′),122.8(d,C-6),121.7(d,C-6′),115.8(d,C-5),115.6(d,C-5′),114.4(d,C-2),112.5(d,C-2′),87.5(d,C-7),65.1(t,C-9),56.9(q,C-11-OCH3),56.7(q,C-3-OCH3),56.5(d,C-8),56.3(q,C-3′-OCH3).
4 Conclusion
The genus Isodon contains more than 150 species worldwide.The diverse structures and potent biological activities of diterpenoids have attracted considerable attention.Among them,I.scoparius was special because this plant was a rich resource of diverse bicyclic diterpenoids and their interesting derivatives,such as meroditerpenoids and diterpenoid dimers.Up to now,a number of secondary metabolites involving 18 novel skeletons with significant biological activities,e.g.immunosuppressive activities[9],have been reported continuously from I.scoparius in our group.Herein,the diverse lignans of I.scoparius have been reported for the first time.The result suggested that I.scoparius is a“talented species”in the genus Isodon,and the cultivated I.scoparius is a reliable source for future in-depth research.
——李振声

