Study of the mechanism of nitrogen doping in carbon supports on promoting electrocatalytic oxygen reduction reaction over platinum nanoparticles
SUN Xue-qin ,GAO Xin-hua ,WANG Ying-yong ,TONG Xi-li
(1. State Key Laboratory of Coal Conversion, Analytical Instrumentation Center, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2. University of Chinese Academy of Sciences, Beijing 100049, China;3. State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering,Ningxia University, Yinchuan 750021, China)
Abstract: Nitrogen-doped carbons (Nano-NC) are often employed as functional supports for boosting oxygen reduction reaction (ORR) over Pt-based catalysts, however, the mechanism of N doping on the adsorption and activation of molecular oxygen on Pt active sites is still not clear. Herein, Nano-NCs as the supports were prepared by a facile NH3 antipyretic method, which allowed to tune the kinds of nitrogen species in carbon matrix and their contents by adjusting the NH3 antipyretic temperatures. With such an exquisite control, the Pt nanoparticles loaded on the as-obtained Nano-NC showed an optimal Pt particle size (2.10 nm), a higher content of Pt0, a large electrochemically active surface area, and fast electron transport ability. As a consequence, the Pt/Nano-NC-800 catalyst with the optimal N-doping showed an outstanding ORR performance with half-wave potential of 0.80 V vs. RHE, limit diffusion current of 5.37 mA/cm2 and improved methanol/CO anti-poisoning, which is superior to the commercial Pt/C catalyst (20%, JM), and most of previously reported Pt-based catalysts. This work may pave a way for the design of the advanced supports for Pt-based catalysts for the ORR applications.
Key words: N-doped carbon;nanoparticle size;oxygen reduction reaction;mechanism
With the aim to mitigate the consumption of fossil fuels and alleviate related environmental pollution issues, fuel cells have attracted extensive interest due to their great potential to provide clean and sustainable energy conversion pathways[1-4]. As the cathode reaction in fuel cells, oxygen reduction reaction (ORR)has become the bottleneck for its sluggishly kinetical process derived from higher overpotential[5-7]. Up to date, only Pt and its alloys are widely used as the efficient electrocatalysts for ORR in acidic electrolytes.However, their high cost, susceptibility to carbonaceous and CO poisoning severely impede their practical applications[8-10].
To address this issue, several approaches[11-17]including designing nanostructures and constructing dual active sites for tandem reaction, etc, have been proposed to improve the ORR performances of Ptbased catalysts. Among these potential approaches, the design of suitable carbon supports to better anchor or disperse Pt nanoparticles has attracted more and more attention, because the good dispersion of Pt nanoparticles (NPs) on carbon supports (CNTs[18,19],graphene[20-22], and porous carbon, etc.[23,24]) can optimize the electronic structures of Pt sites to improve their ORR activity and stability remarkably, which is relatively simple, scalable, and cost-effective[25,26].However, carbon supports are prone to be oxidized into CO or CO2during the ORR. These oxidation products are stably adsorbed on the surface of Pt catalyst at a lower potential, causing the poisoning of Pt catalysts[27-30]. To solve this problem, further boosting the support role of carbon materials to enhance the performances of Pt-based catalysts is highly desirable.
The modification of the carbon material by means of heteroatom doping, especially nitrogen doping, has been widely investigated[31-33], which was concentrated on the exploration of nitrogen doping to introduce new electrochemical active sites, for example, the change of atomic charge density distribution of adjacent carbon atoms due to the electronegativity of the nitrogen and carbon atoms is regarded as the reason for the formation of ORR active sites[34,35]. However, the research about nitrogen-doped carbons applied as the advanced supports for Pt catalysts in the ORR and others is still deficient. It has been well demonstrated that the strong electronegativity difference between the nitrogen and carbon could enhance the metal-support interaction to stably anchor Pt nanoparticles[36,37].Besides, the optimal content of nitrogen doping could improve the electrical conductivity of the carbon material for facilitating electron transport during the ORR process[38,39]. However, the mechanism of the ORR over Pt nanoparticles derived from nitrogen-doped carbon supports is still not clear, which is highly pursuit for the design of advanced electrocatalysts for the ORR and beyond.
Herein, nitrogen-doped carbon (Nano-NC)supports were prepared by a facile NH3antipyretic method. It is easy to achieve controllable nitrogen doping by tuning the temperature. In addition, different N-doped compositions in the supports have been observed to play an impact on the size of Pt nanoparticles and the content of Pt0, which could boost the ORR performances. Among them, the Pt/Nano-NC-800 catalyst with the appropriate size (2.10 nm) and higher content of Pt0showed a superior ORR activity(e.g., limit diffusion current of 5.37 mA/cm2and Tafel slope of 82.0 mV/dec) to the counterparts, even better than the commercial Pt/C catalyst (20%, JM) under the same conditions.
1 Experimental
1.1 Material
All chemicals were analytical reagent and used without further purification. Chloroplatinic acid hexahydrate (H2PtCl6·6H2O) was obtained from Sinopharm Chemical Reagent Co. Ltd. (Beijing,China). Ethanol, ethylene glycol (EG) and perchloric acid (HClO4) were purchased from Kermel Reagent Co. Ltd. (Tianjin, China). 5% Nafion solution was bought from Dupont company (USA). 20% Pt/C was bought from Johnson Matthey Co. High purity argon and ammonia atmospheres were provided from Taiyuan Taineng Gas Co.LTD. (Taiyuan, China).
1.2 Synthesis
The synthesis of Nano-NC:In a typical synthesis,200 mg of carbon nanoparticle (reported in our previous paper[40]) was placed in a high-temperature tubular furnace and heated from 20 to 700 °C at a rate of 5 °C/min under argon atmosphere, then it was maintained for 3 h under NH3atmosphere. After that,the sample was cooled naturally down to room temperature under argon atmosphere. The obtained product was labeled as Nano-NC-700. Under the same reaction conditions, except that the treatment temperature was changed to 800 and 900 °C, the obtained samples were denoted as Nano-NC-800 and Nano-NC-900, respectively.
The synthesis of Pt/Nano-NC catalysts:Pt/Nano-NC catalysts were synthesized by an ethylene glycol reduction method. First, 30 mg of Nano-NC and 10 mL of H2PtCl6·6H2O (2 mg/L) were mixed in 20 mL of ethylene glycol. Then, the mixture was heated up to 150 °C in oil and remained undisturbed for 3 h. The precipitates were then collected and washed with distilled water and ethanol repeatedly, which were further dried at 80 °C under vacuum for 6 h.
1.3 Characterization
The surface area and pore volume of the supports were measured through the Brunauer-Emmett-Teller(BET) method. X-ray diffraction (XRD, Bruker D8 Advance) with Cu-Kα radiation (λ=1.5406 Å) was conducted to the crystallographic structures.Transmission electron microscopy (TEM, FEI Tecnai G2 F20) was performed at an acceleration voltage of 200 kV to characterize the morphologies and microstructures. X-ray photoelectron spectroscopy (XPS,Kratos Axis Ultra DLD) with Al-Kα source (hʋ=1846.6 eV) was analyzed to the surface chemical composition and chemical state of the catalysts. Inductively coupled plasma optical emission spectrometry (ICP-OES,ThermoiCAP 6300) was detected the content of platinum on the catalysts.
1.4 Electrochemical measurements
The electrochemical workstation (CHI760E,Shanghai Chenhua Instrument Co., Ltd, China) was used to measure oxygen reduction reaction (ORR) in a standard three-electrode system. The Ag/AgCl electrode and the Pt electrode were used as the reference electrode and the counter electrode,respectively. In this work, all the potentials were converted into the reversible hydrogen electrode (RHE)according to the Nernst equation:

The working electrodes were prepared as follows:5 mg of Nano-NC-700 catalyst powder was dispersed in 1 mL of ethanol and 20 μL of 5% Nafion mixed solution, and then sonicated for 30 min. Then 5 μL of the catalyst ink was coated onto a glassy carbon disk electrode (5 mm in diameter,S=0.196 cm2) and dried at room temperature. Other catalysts were prepared in the same way. The Pt loading amount of Pt/Nano-NC-700,Pt/Nano-NC-800 and Pt/Nano-NC-900 are 4.1, 3.8 and 3.7 μg, respectively. The Pt loading amount of JM Pt/C is 5 μg for the comparison.
The cyclic voltammetry (CV) was swept at a scan rate of 50 mV/s from 0.056 to 1.256 V (vs RHE) in Ar and O2-saturated solution of 0.1 mol/L HClO4. The linear sweep voltammetry (LSV) curves were recorded in O2-saturated 0.1 mol/L HClO4at a scan rate of 5 mV/s from 0.056 to 1.056 V with a rotating speed from 400 to 2025 r/min. The electrochemical impedance spectroscopies (EIS) were carried out on a Zahner workstation (Germany) at 0.756 V (vs RHE).The frequency range was from 100 kHz to 0.01 Hz and the amplitude was 5 mV. The electron transfer number per oxygen molecule involved in oxygen reduction can be determined by the Koutechy-Levich equation[6,41,42].
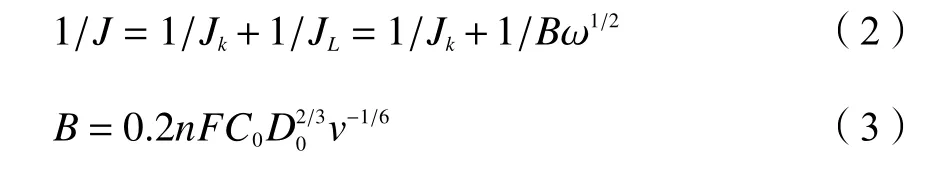
whereJ,JkandJLare the measured current density,kinetic current density, and diffusion-limited current density, respectively,ωis the electrode rotating rate,nis the electron transfer number,Fis the Faraday constant (96485 C/mol),C0is the concentration of dissolved O2in solution (1.2×10-6mol/cm),D0is the diffusion coefficient of O2in 0.1 mol/L HClO4(1.93×10-5cm2/s),vis the kinematic viscosity (0.01 cm2/s).
The hydrogen adsorption/desorption charge method was used to estimate the electrochemically active surface area (ECSA) of Pt for each sample[43-45].Chronoamperometry was used to investigate the durability, CO poisoning, and the methanol tolerance of catalysts at the potential of 0.756 V (vs RHE) in O2-saturated 0.1 mol/L HClO4at 1600 r/min.
2 Results and discussion
2.1 Characterization of the supports and catalysts
The Nano-C shows a network structure composed of carbon nanoparticles with a particle diameter of about 30 nm, which exhibited a high degree of graphitization with few defects based on clear lattice fringe (Figure S1(a)-(b)). After the heat treatment under NH3atmospheres, Nano-NC-800 kept the nanoparticle with the same diameter, yet displayed the rough surface derived from nitrogen-doped into carbon structure, resulting in the amorphous structure with many defects (in Figure S1(c)-(d)), similar to the previous report[46]. From the XRD patterns (Figure S2),the intensity of diffraction peaks ascribed to carbon(002) decreases with the increasement in heat-treated temperature, suggesting that the amorphous structure of carbon is formed, in line with the TEM observation[47].In addition, it is found that the BET surface area and the average pore size are slightly improved when the treated temperature is gradually increased (in Table S1).
To further evaluate the defects in carbon supports,Raman spectra were measured before and after NH3antipyretic treatments. As shown in Figure 1(a), two peaks around 1320 and 1580 cm-1were observed,which are ascribed to the characteristic peaks of the D band and G band of carbon material, respectively.Furthermore, the intensity ratios of the D band to G band (ID/IG) of Nano-NC-700, Nano-NC-800, and Nano-NC-900 are calculated to be 1.44, 1.45, and 1.47,respectively, indicating that the surface defects of carbon nanoparticles gradually increased with the increasement in NH3antipyretic temperature.
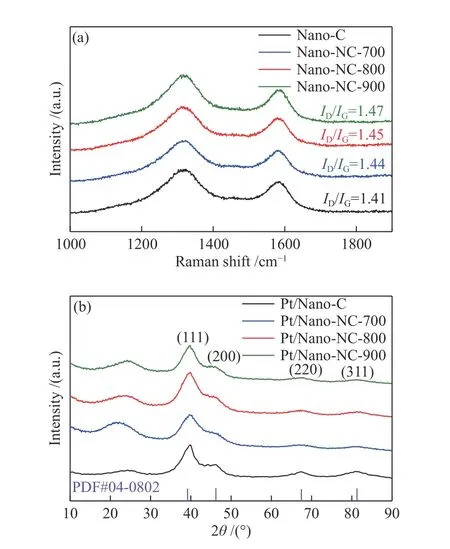
Figure 1 (a) Raman spectra of carbon nanoparticles, Nano-NC-700, Nano-NC-800, and Nano-NC-900; (b) XRD patterns of Pt/C, Pt/Nano-NC-700, Pt/Nano-NC-800, and Pt/Nano-NC-900
To clarify the phase composition and crystal structures of catalysts after depositing Pt nanoparticles on the different carbon support, X-ray diffraction (XRD)tests were performed. As illustrated in Figure 1(b),the four catalysts have similar XRD patterns, and the broad peaks at about 24° are assigned to the supports of carbon nanoparticles. The diffraction peaks at the 2θvalues of 39.76°, 46.24°, 67.45°, and 81.28° are indexed to the (111), (200), (220) and (311) plane of crystalline Pt nanoparticles (PDF# 04-0802),respectively[25]. Interestingly, it is also found that the full width at half maximum of Pt (111) in Pt/Nano-NC-700, Pt/Nano-NC-800, and Pt/Nano-NC-900 are 4.52°,3.98°, and 2.66°, respectively, suggesting that the size of platinum nanoparticles increases with the increasement in NH3antipyretic temperature based on the Scherrer equation[48].
From the TEM images in Figure 2(a)-(c), it is clear that the Pt nanoparticles are well dispersed on the Nano-NC supports. And high-resolution transmission electron microscopy (HRTEM) images exhibited clear lattice fringes with a spacing of 0.22 nm, corresponding to the (111) plane of fcc Pt (insets in Figure 2(a)-(c)).In the case of pristine Nano-C, Pt nanoparticles were uniformly dispersed, and the average diameter was about 1.8 nm (in Figure S3(a)-(b)). After treated under NH3atmosphere, the average Pt particle diameter of Pt/Nano-NC-700, Pt/Nano-NC-800, and Pt/Nano-NC-900 catalysts was calculated to be 1.9, 2.1, and 3.1 nm,respectively (in the Figure 2(d)-(f)), indicating the size of platinum nanoparticles increased with the increasement in NH3antipyretic temperature, which are consistent with the XRD results. It is found that the content of pyrrole nitrogen in nitrogen doping carbon is enhanced when the NH3antipyretic temperature increased. Pyrrole nitrogen as an electron donor can act as the active sites for the nucleation and growth of Pt nanoparticles. When more pyrrole nitrogen content is formed in the surface, Pt will tend to deposit on pyrrole nitrogen sites, which results in the increase of the size of Pt nanoparticles[49]. Due to the size effect of Pt towards the ORR, the particle size in Pt/Nano-NC-800 catalyst (2.10 nm) is close to the ideal value from the literature[50,51].

Figure 2 TEM images of Pt/Nano-NC-700, Pt/Nano-NC-800, and Pt/Nano-NC-900 ((a)-(c)) and the corresponding size distribution of Pt nanoparticles of ((d)-(f)) The inset of ((a)-(c)) is the HRTEM image of three catalysts
To further clarify the influence of the kinds of nitrogen species and their contents on the chemical state of Pt, the Pt/Nano-NC catalysts were analyzed by XPS. As shown in Figure 3(a), it can be seen that nitrogen is successfully doped into carbon materials,and the C, N, and Pt elements are detected based on their binding energy positions. For the Pt/Nano-NC-700, Pt/Nano-NC-800, and Pt/Nano-NC-900 sample (in Figure 3(b)-(d)), all N 1sXPS spectra could be deconvoluted into major 5 types of nitrogen ascribed as pyridinic-N (398.6 eV), pyrrolic-N (400.2 eV),graphitic-N (401.1 eV), oxidized pyridinic nitrogen(402.0 eV) and Pt-N (399.4 eV)[52-54], but the content of each type of nitrogen is different when treated at different temperature. As shown in Table 1, the content of pyridinic-N and Pt-N (40.3%) and graphite nitrogen(34.6%) in Pt/Nano-NC-800 is higher than those of Pt/Nano-NC-700 (39.9%, 33.0%), and Pt/Nano-NC-900 (39.3%, 30.6%). It is well known that the high content of pyridinic-N and graphitic-N active sites in the catalyst is beneficial for the ORR, due to the conjugation effect of the nitrogen lone pair electrons on the nitrogen and graphenep-system[36,55].
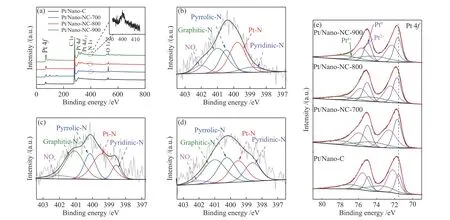
Figure 3 XPS survey spectra (a) and HR-XPS spectra of (b), (c), and (d) N 1s obtained from Pt/Nano-NC-700, Pt/Nano-NC-800, and Pt/Nano-NC-900, and HR-XPS spectra Pt 4f (e) of Pt/Nano-C, Pt/Nano-NC-700, Pt/Nano-NC-800, and Pt/Nano-NC-900 catalysts

Table 1 Results of the fits of the N 1s XPS For each single component, the binding energy (eV) and amount (%) values are given
All Pt 4fspectra show the double peaks of Pt 4f5/2and Pt 4f7/2(in Figure 3(e)), which can be divided into three doublets indexed to the Pt0, Pt2+, and Pt4+. Take the Pt/Nano-NC-800 for example, the double peaks of located at 71.8 and 75.1 eV, 72.4 and 75.7 eV, 74.6 and 77.9 eV are attributed to metallic Pt0, Pt2and Pt4+state,respectively. In addition, the Pt0peaks in Pt/Nano-NC-700 (71.7 and 75.0 eV), Pt/Nano-NC-800, and Pt/Nano-NC-900 (71.6 and 74.9 eV) exhibited slightly positive shifts in comparison to that in the Pt/Nano-C(71.5 and 74.8 eV), indicating that the strong interaction occurred between Pt nanoparticles and Ndoped carbon supports, which would improve the stability for Pt catalysts[56-59].
As shown in Table S2, the content of Pt0(37.8%)in Pt/Nano-NC-800 is higher than those of Pt/Nano-NC-700 (35.2%), Pt/Nano-NC-900 (33.8%), and Pt/Nano-NC(24.0%), which would optimize the catalytic activity for the ORR in the Pt/Nano-NC-800,as the Pt0was extensively regarded as the active site[60-63].
2.2 Electrocatalytic performance of the catalysts
To test the electrocatalytic activity of the prepared catalysts, the LSV measurements were carried out in O2-saturated 0.1 mol/L HClO4electrolyte using a rotating disk electrode (RDE) at a rotation speed of 1600 r/min. As shown in Figure 4(a), Pt/Nano-NC-800 exhibits an excellent ORR activity with a much more positive onset potential (1.02 V vs RHE) and half-wave potential (0.80 V vs RHE), and a higher limiting diffusion current density (5.37 mA/cm2), which are superior to Pt/Nano-C (1.02 V, 0.75 V, 4.37 mA/cm2),JM Pt/C (1.00 V, 0.78 V, 4.75 mA/cm2), Pt/Nano-NC-700 (1.01 V, 0.76 V, 4.88 mA/cm2), Pt/Nano-NC-900(1.02 V, 0.77 V, 4.96 mA/cm2) and other reported platinum-based catalysts (in Figure S4 and Table S3).Besides, the Pt/Nano-NC-800 exhibits the higher mass activity (279.6 mA/mgPt) and specific activity (0.24 mA/cm2) at a potential of 0.9 V (vs RHE) than the counterparts (Figure S5 and S6). The excellent ORR activity of Pt/Nano-NC-800 is due to the fact that the nitrogen doped carbon support can transfer electrons from the nitrogen species to the unfilled orbit of platinum, which is beneficial for the adsorption of O2molecule and O-O dissociation[64].

Figure 4 (a) RDE voltammogram of the Pt/Nano-NC-800, Pt/Nano-C, and JM Pt/C catalysts recorded in O2-saturated 0.1 mol/L HClO4 electrolyte at a scan rate of 5 mV/s at 1600 r/min, (b) corresponding Tafel plots, (c) LSVs in O2-saturated 0.1 mol/L HClO4 at different RDE rotation rates of Pt/Nano-NC-800, (d) The corresponding K-L plots of Pt/Nano-NC-800
In order to clarify the ORR kinetics on those catalysts, the Tafel curves were measured. The Tafel slope of Pt/Nano-NC-800 catalyst was 82.0 mV/dec,smaller than that of JM Pt/C (92.6 mV/dec) and Pt/Nano-C (116.8 mV/dec) (Figure 4(b)). This result indicated that Pt/Nano-NC-800 catalyst has excellent ORR kinetics derived from the optimal nitrogen doping in the carbon supports. In addition, the number of electron transfer (n) is an important feature of ORR to explore the reaction pathway. Thenvalue was calculated according to the Koutecky-Levich (K-L)equation. Thenvalue of the Pt/Nano-NC-700,Pt/Nano-NC-800, and Pt/Nano-NC-900 catalysts were found to be 3.8, 4.0, 4.0, respectively, higher than those of the JM Pt/C (3.7) and Pt/Nano-C (3.5). This emphasized that the ORR on the Pt/Nano-NC catalysts proceeds mainly through a four-electron mechanism via nitrogen doping (in Figure 4(c), (d) and Figure S7).In addition, the RRED test of Pt/Nano-NC-800 and JM Pt/C were also carried out in O2-saturated 0.1 mol/L HClO4electrolyte. As shown in Figure S8(a), the Pt/Nano-NC-800 still showed excellent ORR electrocatalyst performance. The H2O2% of Pt/Nano-NC-800 was 5%,lower than of JM Pt/C (30%), which further verifies that the ORR on Pt/Nano-NC-800 follows a fourelectron-transfer mechanism (Figure S8(b)).
To further reveal the ORR kinetics of those catalysts, Nyquist plots were fitted via the Randles equivalent circuit model, where the semicircle illustrates that the charge transfer at the interface is the rate-determining step of the ORR. The charge transfer resistance (Rct) of the Pt/Nano-NC-800 (294 Ω) is smaller than that of the Pt/Nano-C (4892 Ω) and JM Pt/C (1675 Ω) catalysts, which reveals desirable electron transport and catalytic kinetics of the Pt/Nano-NC-800 (Figure 5(a)). Because nitrogen doping on carbon is conducive to the formation of localized donor states close to the Fermi level, the carbon materials have metal properties and improve the conductivity of the materials[65].
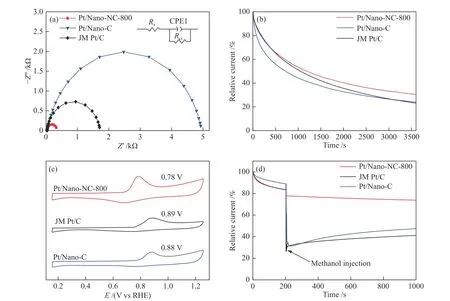
Figure 5 (a) Nyquist plots of the Pt/Nano-NC-800, Pt/Nano-C, and JM Pt/C catalysts and equivalent circuit model; (b) Endurance test of three catalysts tested at 0.756 V in O2-saturated 0.1 mol/L HClO4; (c) CO stripping experiments on three catalysts at 10 mV/s;(d) the addition of 4 mL of 3 mol/L methanol in O2-saturated solution at 1600 r/min
To evaluate the electrochemical durability of the samples, the ORR was performed by chronoamperometry at 0.756 V for the time of 3600 s(Figure 5(b)). Under the identical conditions, the Pt/Nano-NC-800 catalyst still maintained 31.84% of its initial activity, better than the Pt/Nano-C catalyst(24.16%) and the JM Pt/C catalyst (22.38%).Furthermore, theE1/2of the Pt/Nano-NC-800 only negatively shifts 52 mV after a 5000-cycling endurance test is smaller than that of Pt/Nano-C (101 mV) and JM Pt/C (69 mV) (Figure S9). Simultaneously, the specific current density of the Pt/Nano-NC-800 remained 0.16 mA/cm2, significantly greater than Pt/Nano-C(0.06 mA/cm2) and JM Pt/C (0.08 mA/cm2) catalysts(Figure S11). This higher stability of Pt/Nano-NC-800could be attributed to the stronger interaction between the Pt nanoparticles and the nitrogen species in the Ndoped carbon support in comparison to that of Pt/Nano-C and JM Pt/C, which could protect Pt nanoparticles from aggregation. This could be confirmed from the TEM images of the recovered catalyst (Figure S9). And after a 10000-cycling CVs test, theE1/2of the Pt/Nano-NC-800 was negatively shifted 150 mV, which is close to JM Pt/C (145 mV) ( Figure S10).
It is well documented that CO poisoning is the main cause for the deactivation of the Pt-based catalyst[29,66,67]. Therefore, the CO stripping evaluation was carried out for these catalysts. The position of CO stripping peak of Pt/Nano-NC-800 was 0.78 V, which is more negative than that of Pt/Nano-C (0.88 V) and JM Pt/C (0.89 V) (Figure 5(c)), indicating a higher ability for anti-CO poisoning on the Pt/Nano-NC-800.The presence of nitrogen in carbon supports will promote the formed Pt-OHads, which will facilitate CO oxidation to CO2and then improve the ability of Pt catalysts for anti-CO poisoning.
In addition, the methanol resistance for these catalysts was also explored, which is one of the key parameters for the application of Pt/C (Figure 5(d)). It is clear to see that the ORR current density of the Pt/Nano-C and JM Pt/C catalyst decreased sharply by 60% after adding 4 mL of 3 mol/L methanol while only a slight decrease of current density on Pt/Nano-NC-800 under the same conditions. This observation demonstrated that Pt/Nano-NC-800 has superior methanol resistance due to the preferential adsorption of O2over methanol on the Pt site derived from the influence of nitrogen-doping carbon supports[56,62].
3 Conclusions
As shown above, the ORR performance of Pt nanoparticles was remarkedly improved by loading them on the Nano-NC supports, which optimized the chemical state of surface Pt sites by controlling the heat-treated temperature under NH3atmospheres. The Pt/NC-800 catalyst exhibited the higher content of pyridinic-N and graphitic-N than its counterparts,which resulted in higher conductivity and more active Pt0as well as optimal particle size (2.10 nm). Hence,the Pt/Nano-NC-800 catalyst shows higher ORR activity in acidic electrolytes than the commercial JM Pt/C and most of previously reported carbon-supported Pt catalysts. Besides, the Pt/Nano-NC-800 catalyst also exhibited stronger long-term durability and better ability for CO and methanol anti-poisoning compared with the other catalysts such as Pt/Nano-C and JM Pt/C. In summary, this work offers a strategy for designing the high-performance support to boost the ORR for Pt-based catalysts.

