Gastrointestinal microbiota: A predictor of COVID-19 severity?
Maria Adriana Neag, Damiana-Maria Vulturar, Diana Gherman, Codrin-Constantin Burlacu, Doina AdinaTodea,Anca Dana Buzoianu
Abstract Coronavirus disease 2019 (COVID-19), caused by a severe acute respiratory syndrome coronavirus 2 infection, has raised serious concerns worldwide over the past 3 years. The severity and clinical course of COVID-19 depends on many factors (e.g., associated comorbidities, age, etc) and may have various clinical and imaging findings, which raises management concerns. Gut microbiota composition is known to influence respiratory disease, and respiratory viral infection can also influence gut microbiota. Gut and lung microbiota and their relationship(gut-lung axis) can act as modulators of inflammation. Modulating the intestinal microbiota, by improving its composition and diversity through nutraceutical agents, can have a positive impact in the prophylaxis/treatment of COVID-19.
Key Words: Gut microbiota; COVID-19; Prognostic biomarkers; Gut-lung axis; Probiotics;Nutraceuticals
INTRODUCTION
Coronavirus disease 2019 (COVID-19), first reported as a new infectious disease in December 2019 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, can result in acute respiratory syndrome and has raised serious global concerns[1]. The clinical course of COVID-19 ranges from asymptomatic and mild to life-threatening forms[2]. Interindividual variability influences clinical symptoms and disease outcomes and is related to varying genetic profiles of the host immune response and angiotensin converting enzyme 2 (ACE2) binding affinity of SARS-CoV-2[3,4].
Disease severity and clinical course of COVID-19 depends on the patient’s associated comorbidities,including cardiovascular disease, hypertension, diabetes, chronic pulmonary disease, age, and smoking status[5]. The hyperreactivity of the host immune responses caused by SARS-CoV-2 infection, known as“cytokine storm”, leads to a massive and uncontrolled activation of proinflammatory pathways, which ultimately results in multiorgan failure and mortality[6,7]. Evolving research data suggests several conventional serum biomarkers are correlated with disease onset and disease severity, including white blood cells, D-dimers, fibrinogen, C-reactive protein (CRP), procalcitonin, lactate dehydrogenase, serum ferritin, interleukin 6 (IL-6), alanine aminotransferase, aspartate aminotransferase (AST) and total bilirubin[8].
The human microbiota is a complex microecosystem composed of bacteria, viruses, fungi and archaea within the oral cavity, gut, lung, skin and vagina, with the highest abundance and diversity in the gut[9]. The role of microbiota in health and disease conditions has been recently shown in experimental and clinical setting[10]. The involvement of gut microbiota and its associated metabolites in maintaining body homeostasis includes regulation of host immunity, influencing physiological functions, such as digestion and nutrition and biosynthesis of vitamins and numerous active compounds[10-12]. Gut bacterial compounds play a critical role in regulating disease pathogenesis and recovery and providing promising therapeutic targets for stroke[13], neurodegenerative diseases[14], cardiovascular dysfunctions[15] and cancer-related diseases[16].
Targeting inflammatory responses triggered by SARS-CoV-2 infection by modulating the gut-lung axis represents a promising therapeutic target[17]. A clinical perspective of gut bacterial changes associated with disease severity and outcomes might provide predictive fecal and serum biomarkers with prognostic and diagnostic value[18]. Metabolomic and microbiome profiling research studies depicting bacterial changes during and after COVID-19 may provide a better understanding of the role of gut microbiota in COVID-19 pathogenesis[19,20].
This narrative review aimed to provide new insight into the involvement of gut microbiota in COVID-19 patients by modulating inflammatory responses and disease severity. For a better understanding of the translational relevance of gut microbiota as disease modifying therapy in COVID-19 disease, we summarized the most common changes in gut composition abundance of commensal and pathogenic species in relation to disease onset and severity.
MATERIALS AND METHODS
This narrative review aimed to provide an overview of the current knowledge of the involvement of gut microbiota in COVID-19 patients. We performed an electronic search in the databases of Medline(PubMed, PubMed Central) by using different term combinations of “COVID-19” or “Sars-Cov-2” and“microbiota”, “airway microbiota”, “lung microbiota”, “gut microbiota”, “dysbiosis” and “leaky gut”.
SHORT OVERVIEW OF SARS-COV-2 INFECTION: FROM ORIGINS TO RECENT DATA
It is known that in the last two decades three coronaviruses have been described to cause lifethreatening severe infection in humans: SARS-CoV, Middle East respiratory syndrome-CoV and SARSCoV-2[21-23]. SARS-CoV, originated in China generated a global pandemic in 2002, having a mortality rate of 10%[21]. Middle East respiratory syndrome-CoV-2, was first reported in Saudi Arabia in 2012 and caused another transmissible disease impacting the public health sector[22]. The most recent pandemic declared was the COVID-19 pandemic, first reported in Wuhan China with a quick spread around the world[23].
The newly acquired infection is continuing to spread because of the mutations that occur in the genome and leads to an intensive viral replication with a high risk of reinfection, reducing the antibodies produced by vaccination or previous infections[24]. Being an RNA virus, SARS-CoV-2 has an important potential to adapt to new hosts, developing mutations and leading to different variants with different characteristics. To identify the new variants genomic sequencing is used. At the beginning of the pandemic, the mutation, D614G, was very contagious but not very dangerous with severe manifestations[24-26].
After this mutation, another large variety of variants have been found and named variants of concern.A variant of SARS-CoV-2 infection is a variant of concern when it impacts the public health sector.Variants of concern are linked to high transmissibility, virulence, decreased effectiveness to vaccines or medical treatment and with the capacity to evade detection.
These mutations with a high transmissibility have an increased hospital admission rate and mortality rate[27]. The five variants found to be variants of concern since the beginning of the pandemic,according to World Health Organization, are illustrated in Table 1[28]. The initial step of the infection is the recognition of the receptor, which is the key to tissue tropism[29]. The affinity of the spike glycoprotein to bind to the ACE2 receptor influences the replication and the severity of the disease[29-31].
The spike protein is formed by two subunits: The S1 subunit, which contains the receptor-binding domain and recognizes ACE2 on the host cells; and the S2 subunit, which mediates the fusion of the viral and cellular membrane. Mutations that appear in the receptor-binding domain lead to a higher viral replication and contagion. They allow the virus to not respond to vaccine-elicited antibodies[32,33]. The viral protein is cleaved by transmembrane serine protease 2, a host cell molecule involved in viral entry[34]. It has been shown that the expression of ACE2 and transmembrane serine protease 2 is increased in the nasal and oral mucosa, airways, lungs and intestine[35,36].
GUT MICROBIOTA IN HEALTH AND DISEASE
The human gut microbiota is a complex ecosystem composed of all microorganisms (1013to 1014) at this level, including bacteria, viruses, fungi and archaea[9]. The human microbiota, known as the “hidden organ” is composed of all the microorganisms in the oral cavity, gut, lung, skin, vagina,etc, but the greatest diversity and abundance is in the gut[37]. At this level, there are two dominant phyla,Firmicutes and Bacteroidetes, in healthy adults. For each of the two phyla, there are several dominant genera:Lactobacillus, Faecalibacterium, Clostridium, Enterococcusfor Firmicutes; andBacteroidesand
Prevotellafor Bacteroidetes[38].
The microbiota has an important role in maintaining body homeostasis, can modulate host immunity,and has the ability to influence physiological functions[11]. Communication between the gut microbiota and the immune system is mainly carried out through mediators. Short chain fatty acids (SCFAs) are included in this category[39]. These mediators (SCFAs), represented by acetate, propionate and butyrate, play important roles through interactions with host immune cells and represent an important carbon source for colonocytes[40].
THE INVOLVEMENT OF GUT MICROBIOTA IN COVID-19 PATHOGENESIS
Intestinal microbiota and intestinal permeability have an important role both in regulating the transition of beneficial elements (e.g.,nutrients) and in stopping the penetration and transfer of harmful particles from the intestinal lumen into the circulation[41]. It has been shown that probiotics can regulate the composition of the microbiota and thus contribute to maintaining the body’s homeostasis[42].Management of COVID-19 by administration of probiotics or other nutraceutical agents that can modulate the microbiota has not been a mainstay in the pandemic. However, the influence of COVID-19 by modulating the microbiota was not completely neglected during that period[43].
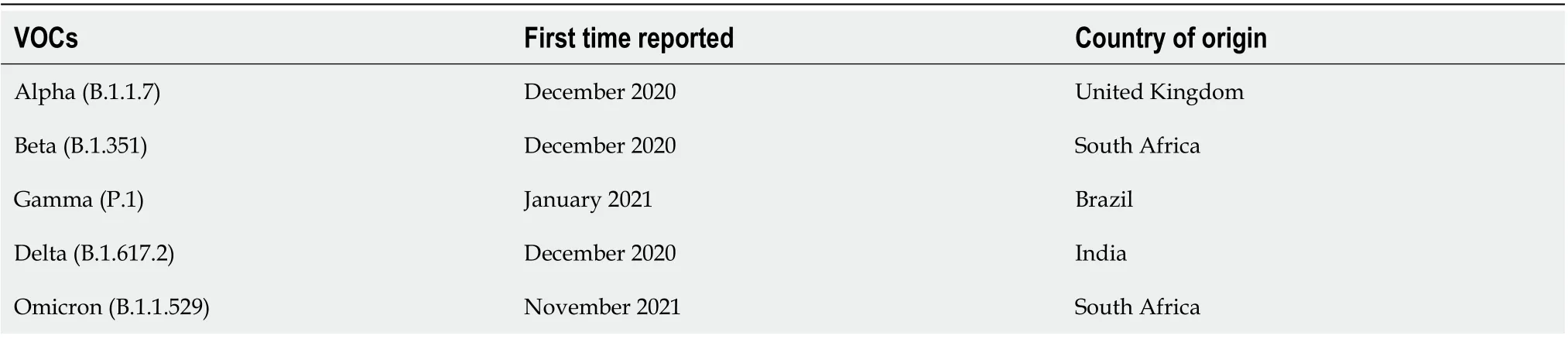
Table 1 Variants of concern reported for coronavirus disease 2019
THE GUT-LUNG AXIS IN COVID-19
Gastrointestinal symptoms account for frequent complaints of patients with SARS-CoV-2 infection[44,45]. Mounting preclinical and clinical evidence pointed out the relationship between pulmonary injury and intestinal dysfunction within viral lung infection[46], influenza A virus infection[47], bronchial asthma[48], chronic obstructive pulmonary disease[49] and cystic fibrosis[50].
Though distinguished by their functional and compositional microflora,i.e.,species and density, both the gut and lung microbiota systems contribute to host homeostasis by mediating local and systemic inflammatory responses, forming the so-called “gut-lung axis”[51]. Immunomodulatory effects of the gut-lung axis are mediated by mucosal-related immune systems, consisting of gut-associated lymphoid tissue and bronchial-associated lymphoid tissue[52-54].
In healthy conditions, a similar signature of microbial phyla is shared between gut and lung microbiota being provided by Firmicutes, Actinobacteria, Bacteroidetes and Proteobacteria, with Fusobacteria and Verrucomicrobia only found in the intestinal microbiota[55,56]. However, there is a distinctive pattern in the compositional bacterial genera of gut and lung microbiota, withLactobacillus,Clostridium, Bacillus, EnterococcusandPrevotelladominant in gut microbiota andStreptococcus, Veillonella,FusobacteriumandHaemophilusdominant in lung microbiota[55,56].
The crosstalk between the gut and lung microbiota is bidirectional, with dysbiosis of either tract influencing each other[57]. Therefore, once gut microbiota are dysregulated an enrichment of blood flow with microbiota-derived products will result in a systemic inflammatory state, affecting multiple organ systems, including the lungs[58]. Several pulmonary diseases have been associated with altered samples of gut microbiota, including asthma, chronic respiratory dysfunction and pulmonary allergic responses[59,60]. However, pulmonary dysfunction during acute and chronic inflammatory lung diseases trigger intestinal changes by altering intestinal permeability and promoting bacterial translocation[61-63].
Differences in gut microbiota diversity have been reported in multiple pulmonary diseases[64].Among 43 patients with chronic pulmonary dysfunction, an overgrowth of Proteobacteria,i.e.,Haemophilus spp., and Firmicutes with a decreased proportion of Bacteroidetes,i.e., Prevotella spp., have been shown[64]. Environmental factors, such as dietary fibers, antibiotics and pre/probiotics, impacted gut microbiota, providing therapeutic insights into the microbiota-associated gut and lung dysfunction to re-establish the homeostasis in the gut-lung axis[65]. A high-fiber diet has been associated with bacterial changes in the gut-lung axis by increasing the abundance of Bacteroidaceae and decreasing the ratio of Firmicutes/Bacteroidetes in both the feces and lungs[17]. Moreover, in an experimental model of allergic lung inflammation, mice treated with a high-fiber diet or propionate showed changes in the bacterial composition of the gut and lung microbiota and an enhanced capacity of bone marrow to generate macrophage and dendritic cell precursors[17]. Moreover, in an asthma mouse model, a SCFA and fiber diet increased the phagocytic capacity of dendritic cells in the lungs and regulated T helper 2 cell-promoting inflammatory responses[17]. This experimental finding suggested that dietary fermentable fibers and SCFAs might regulate immunological tolerance in atopic asthma patients[17].
Microbiota-derived metabolites mediate the immune cross-talk between gut and lung microbiota[66,67]. SCFAs, the most dominant microbiota-derived metabolites, derived from dietary fermentable fibers in anaerobic conditions, are represented by fatty acid molecules, which are formed by chains of up to six carbon atoms, consisting of acetate, propionate or butyrate[68-70]. SCFAs play an essential role in maintaining the integrity of the intestinal epithelial barrier and mitigating inflammatory events within the gut and respiratory tract by regulating the expression of G-protein coupled receptors or histone deacetylases[68,69,71]. Circulatory acetate or propionate stimulate bone marrow hematopoiesis and enhance airway immunity by activating the differentiation of T helper cells and monocytes and increasing the expression of various chemoattractant molecules on immune cells, thereby promoting immunomodulatory mechanisms against respiratory tract infections[72,73].
The development of asthma has been shown to be influenced by the synthesis and secretion of bacterial-derived metabolites[74]. The correlation between SCFAs, specifically acetate concentration in feces, and the risk of asthma in 319 pediatric subjects demonstrated the link between dysregulation of bacterial metabolites and pulmonary dysfunction[74]. The unstable state of gut microbiota associated with an increased risk of asthma in a cohort of pediatric patients was driven by a decrease inFaecalibacterium, Veillonella, LachnospiraandRothia[74].
Increasing experimental models ascertained the role of microbiota metabolites in immune cell differentiation. In anin vivomodel of experimental colitis, butyrate promoted the differentiation of regulatory T cells and alleviated the development of colitis[67]. Butyrate enhanced IL-10 synthesis and decreased the production of IL-6 by binding GPR109a on dendritic cells and macrophages. Several expression targets were activatedviainteraction with SCFAs. For instance, in healthy conditions, butyrate could activate peroxisome proliferator-activated receptor gamma[75].
In gut microbiota analysis, Gironet al[76] showed that a systemic inflammatory response was linked to elevated serum markers of tight junction permeability markers and microbial translocation.Regarding the lung microbiome, Ruecaet al[18] found the absence ofBifidobacteriumandClostridiumspecies in the nasal/oropharyngeal samples of COVID-19 patients. An outgrowth ofProteobacteriaandFirmicuteshas been reported during respiratory diseases[77]. Growing clinical evidence showed an altered profile of the gut microbiome in stool samples of COVID-19 patients. A recent research study reported that changes in bacterial microbiota in COVID-19 patients could be driven by an active replication of SARS-CoV-2 within the gut[78]. In a functional analysis study of gut microbiota, a study demonstrated an increase in bacterial proliferation ofCollinsella tanakaei,Collinsella aerofaciens,Morganella morganiiandStreptococcus infantis, with a high metabolic rate for de novo synthesis of amino acid and nucleotides[78].
The differences in gut microbial species between diseased and healthy control patients have been correlated with disease severity and complications[20,79]. We provided a synopsis of the most common gut bacterial species changes during COVID-19 in Table 2. More studies to analyze the metabolomic and microbiome profiling data on large cohorts of COVID-19 patients to further depict the role of gut-lung axis in COVID-19 pathogenesis are needed.
CLINICAL COURSE OF SARS-COV-2 INFECTION
Several comorbidities associated with a higher risk of infection, such as cardiovascular disease,hypertension, diabetes, chronic pulmonary disease, age and smoking, have been reported. These factors can modulate the expression of ACE2[5]. The association between ACE2 expression and advanced age and being male are controversial[90]. Smoking led to an upregulation of ACE2 with an increased risk of severe disease[91]. Another condition that predicts severe outcomes is obesity[92]; it increased intensive care unit (ICU) admission and the requirement of invasive mechanical ventilation[93]. Also, attention should be taken in psychiatric patients (with anxiety and depression) because some possible associations between these comorbidities and sleep problems were reported[94]. Regarding the complications developed in the context of the infection, one meta-analysis showed that acute respiratory distress syndrome, shock and acute kidney injury are conditions associated with a worse prognosis and with a higher rate of ICU admission[95]. There are other complications associated with high severity like disseminated intravascular coagulation, superinfections, arrhythmias and cardiac trauma.
Several feasible circulation biomarkers used to assess disease severity included lymphocyte count,thrombocytes, serum ferritin, lactate dehydrogenase, CRP and D-dimer levels[96]. It has been shown that lymphopenia is an important and useful predictor for the severity as it was reported in those with a bad prognosis[97]. In a study of 52 critically ill COVID-19 patients, 80% reported lymphopenia[98],whereas another study of 99 patients reported a rate of only 25% in those with mild COVID-19 infection[99]. These results suggest that lymphopenia can be used as an important marker in the diagnosis of the new coronavirus infection in the evaluation of disease severity. It shows that a high number of immune cells, especially T lymphocytes, are consumed, and the immune function is inhibited[100].
In the context of COVID-19 pneumonia, a cytokine storm can be released, and the cytokines (IL-6,tumor necrosis factor-α) stimulate hepatocytes to produce CRP. It has been demonstrated that CRP is correlated with COVID-19 progression and severity[101-103]. In addition, the chronic conditions associated with hyperinflammation such as metabolic syndrome, atherosclerosis and hypertension can predict worse outcomes[104]. A study by Alamdariet al[105] on 459 patients with high body mass index demonstrated that high levels of CRP, lymphopenia, hypomagnesemia and creatinine at admission were associated with a higher mortality.
High levels of serum ferritin: High levels of serum ferritin are observed in many inflammatory diseases and is considered a biomarker in different conditions such as rheumatologic disorders or different cancers[106]. In the context of SARS-CoV-2 infection, due to the inflammatory process, the cytokines,and in particular IL-6, stimulate hepcidin production, which is involved in the regulation of ferritin[107-109].
The studies showed that high levels of ferritin were observed in COVID-19 patientsvscontrols, and those with severe or critical disease had increased levels of ferritin than those with mild or moderate disease. Moreover, it was shown that non-survivors had increased levels of serum ferritin than survivors. One meta-analysis showed that the sensitivity of serum levels of ferritin in predicting theseverity of the disease is about 91% with a cutoff level of > 548.5 ng/mL[96].
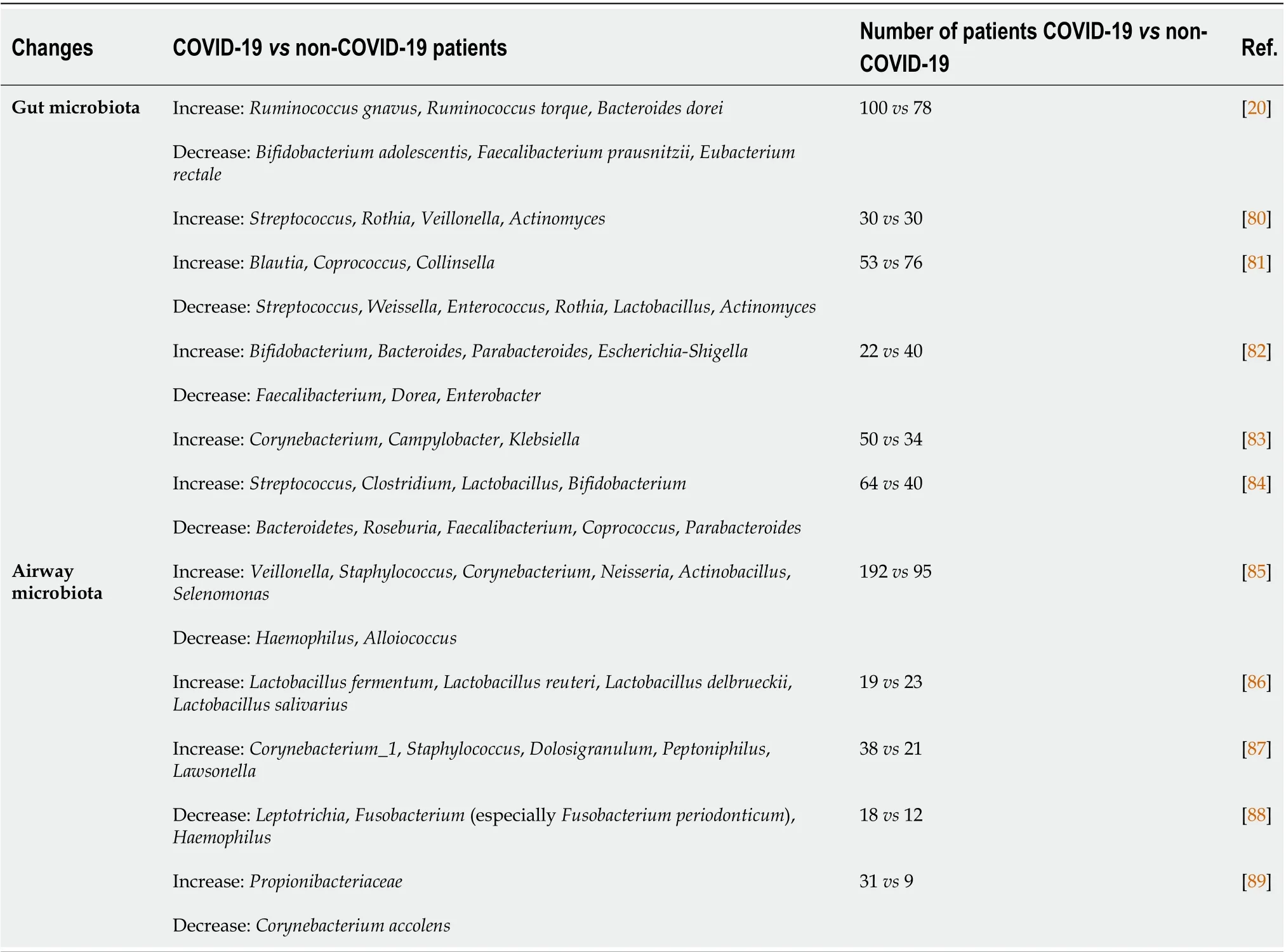
Table 2 Changes in gut and airway microbiota bacterial species during coronavirus disease 2019
D-dimers: D-dimers are one of the fragments produced when plasmin cleaves fibrin to break down clots. They are assessed as an algorithm in the thrombosis exclusion, but any pathologic or nonpathologic process that increases the production or disruption of fibrin can lead to high D-dimer levels[110]. Infections, venous thromboembolism and deep vein thrombosis are the most common causes of increased D-dimer levels[111].
A study by Yaoet al[112] on 248 patients revealed that increased D-dimers at hospital admission for SARS-CoV-2 infection, after excluding pulmonary embolism and deep vein thrombosis, were associated with increased severity and with in-hospital mortality. Also, they showed a significant correlation between D-dimer levels and COVID-19 severity classified by lung involvement on computed tomography (CT) scan, oxygenation index and clinical staging according to the Novel Coronavirus Pneumonia Diagnosis and Treatment Guideline (6thedition) by the National Health Commission of China. It was highlighted that D-dimers are a useful marker to assess the severity even before the thoracic CT scan.
The hepatic injury with increased liver enzymes was reported. There are some potential mechanisms through which the liver is affected: Direct liver injury; associated inflammatory responses; congestive hepatopathy; hepatic ischemia; drug-induced liver injury; and muscle breakdown. The levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and total bilirubin were elevated and increased in a disease progression manner. The AST level was correlated with disease severity.According to Moonet al[113], AST levels had the highest correlation with mortality rate compared with other circulating markers, reflecting the involvement of liver injury in disease progression. An overview of circulating biomarkers that were correlated with COVID-19 infection is provided in Table 3.
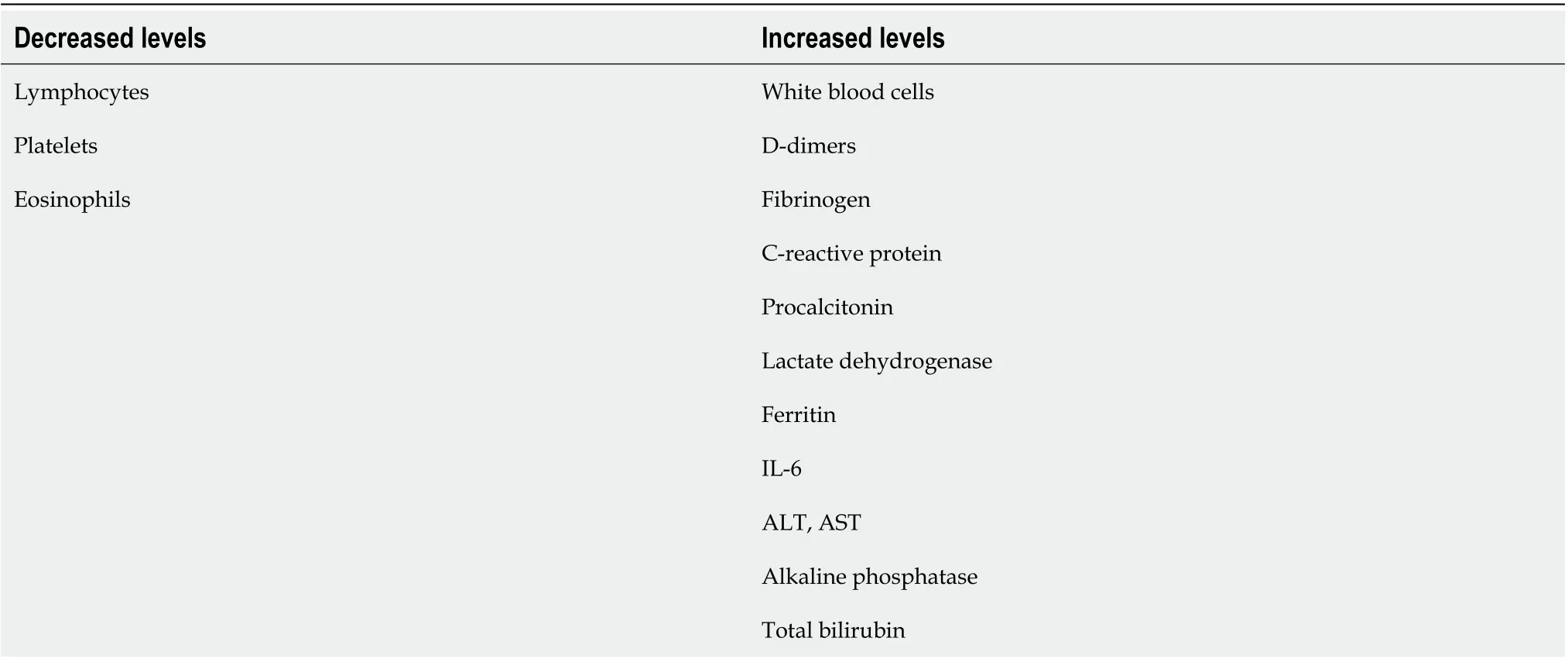
Table 3 Biomarkers associated with coronavirus disease 2019
DIAGNOSTIC TOOLS FOR THE ASSESSMENT OF SARS-COV-2 INFECTION AND DISEASE SEVERITY
Chest imaging in the diagnosis and monitoring of COVID-19 pneumonia plays a significant role, and all available methods should be used. Chest radiography may show no abnormalities at symptom onset,with considerable findings visible only 10-12 d later[114]. Similarly, thoracic CT scans performed in the first 5 d after symptom onset may reveal isolated ground-glass opacities or consolidations in limited distribution. The full extent of the acute pulmonary manifestations increases over the 1stwk, with a peak on day 10[115]. The available imaging tools are chest radiography, chest CT and lung ultrasound (LUS).
Chest radiography
Chest X-ray (CXR) is commonly used as the first imaging examination when pneumonia is suspected[116]. Despite not being a substitute for real-time polymerase chain reaction test or chest CT, CXR can provide a prompt and cost-effective diagnosis in a small percentage of patients (approximately 15%)[117]. Chest radiography shows low sensitivity, as low as 25%, and high specificity, estimated at 90%, in detecting COVID-19 abnormalities, thus it should not be used as a screening method[118]. Frequent chest radiographic findings are airspace opacification, pulmonary consolidation and ground-glass opacification. Pneumonia in COVID-19 tends to be bilateral, with a pattern involving predominantly peripheral lung regions and lower lobes[1]. A proposed radiological scoring of pneumonia severity describes four disease degrees based on the percentage of lung involvement as it follows: Mild if < 25%;moderate if 25%-50%; severe 50%-75%; and critical if > 75%[119].
Chest CT
CT is the most sensible imaging examination and is best correlated with the severity of the disease. To reduce the patient’s exposure time to radiation, imaging tools should be performed in patients with moderate to severe symptomatology and in those with progressive alteration of respiratory parameters[120]. Frequent findings in COVID-19-positive patients are ground-glass opacities, consolidations,interlobular septal thickening and crazy paving. Additional findings consist of the reverse halo sign, air bronchogram sign, tree in bud, pleural or pericardial effusions and mediastinal lymphadenopathies.The ground-glass opacities reflect the parenchymal involvement, and they represent the most consistent feature, being found in almost all affected patients, symptomatic or asymptomatic[6]. Compared to other types of pneumonia, COVID-19 pneumonia presents with multifocal and multilobar involvement of both lungs, with a subpleural and basal distribution[121].
Disease severity can be appreciated by different scoring systems. The percentage of the overall parenchymal involvement may predict mild (< 25% lung involvement), moderate (25%-50%), severe(50%-75%) or critical (> 75%) forms of disease. Another score uses the visual estimation of the surface affected of each of the five lung lobes, with each lobe being given a score from 0 to 5, 0 meaning no involvement, 1 involving < 5%, 2 involving < 25%, 3 involving < 50%, 4 involving < 75% and 5 involving> 75% of the lobar surface. The total score obtained is the sum of the scores attributed to each lobe, and it varies from 0 to 25[122]. In a retrospective study, Bernheimet al[123] proposed a similar score by assessing the degree of involvement of each of the five lobes. The only difference from the previous score is that involvement of 1%-25% was attributed 1 point resulting in a score from 0 to 4 for each lobe.The total CT severity score ranged from 0 to 20. In a study of 739 patients, the authors proposed a semiquantitative scoring system in order to predict the outcome of infected patients. They visually appreciated the pulmonary involvement by assessing each of the five lobes separately for ground-glass opacities and consolidations. Each lobe had a score varying from 0 to 7, and the maximum total score was 35[124].
Despite its high accuracy, CT may be unsuitable for critical ICU patients who may not be able to undergo transfer to the radiology department in order to perform a CT scan[120,125,126]. Moreover,there is a potentially increased risk of disease transmission to CT technicians and other patients who require imaging investigations in the same department[127]. For monitoring ICU patients, portable CXR or LUS are preferable.
LUS
LUS is a widely accessible, non-invasive, non-irradiating and cost-effective tool that can be used in the initial assessment and monitoring of symptomatic patients[128,129]. The main advantages of this examination reside in the possibility of being performed in children and pregnant women and at the patient’s bedside. It is portable and offers replicable examination for follow-up[130,131]. Portable ultrasound devices can be used in ICU departments, both by radiologists and clinicians, offering realtime information about a patient’s evolution.
Numerous authors stated that LUS can offer similar diagnostic information to chest CT in the evaluation of COVID-19 pneumonia[126,132,133]. Gibbonset al[132] concluded that LUS compared to portable CXR had a higher sensitivity for detection of viral pneumonia. LUS findings in COVID-19 patients include multiple B lines below the pleural surface, subpleural consolidations, pleural thickening and irregularity[134]. The B lines depict the interstitial involvement and represent the most common ultrasonographic pattern found in patients with COVID-19[129,132,135]. Despite having a high sensitivity in detecting COVID-19 pneumonia in subpleural lung regions, the deep pulmonary parenchyma remains inaccessible to LUS due to air interposition leading to an underestimation of the disease extent[136]. LUS findings are not distinctive for viral cases of pneumonia, but just as it was previously discussed in the case of chest radiography and chest CT scans, the bilateral and predominantly basal distribution is a strong indicator for COVID-19 pneumonia rather than influenza or bacterial pneumonia[137]. Nonetheless, LUS has a low specificity, since it cannot distinguish from other pulmonary and cardiac conditions such as acute respiratory distress syndrome, heart failure and subpleural lung masses[125,136].
TRANSLATIONAL RELEVANCE OF GUT MICROBIOTA IN MODIFYING DISEASE SEVERITY AND OUTCOMES OF COVID-19 PATIENTS
The involvement of gut microbiota in modifying disease outcomes and therapeutic responses of COVID-19 patients might represent a promising therapeutic strategy. Emerging clinical studies suggested that dysfunctional immune response triggered by gut microbiota dysregulation upon SARSCoV-2 infection might influence the severity and the course of COVID-19[20,138].
The proinflammatory state triggered by the host immune response against SARS-CoV-2 infection promotes changes in gut commensal microflora, leading to dysbiosis, which will further result in alteration of the intestinal epithelial barrier[51,138,139]. Once the integrity of the intestinal barrier is disrupted, the high permeable state of the intestine creates the most favorable conditions for entering into the circulation of bacterial products and toxins, activating a systemic inflammatory response[140].
Evolving data examined predictive biomarkers of disease severity from serum samples of COVID-19 patients, which were correlated with inflammatory response and disease severity[20,138]. Compared to controls, the serum samples of COVID-19 patients exhibited high levels of fatty acid-binding protein 2,peptidoglycan and lipopolysaccharide, markers of gut permeability, suggesting the unstable state of the intestinal barrier within these patients[138].
Significant dysbiosis in 146 COVID-19 patients has been reported by Prasadet al[138]. The phylogenetic changes in the serum microbiome of COVID-19 patients consisted of enrichment ofActinobacteria spp.and underrepresentation ofBacteroides spp.,with an increased ratio of Firmicutes to Bacteroidetes[138]. Therefore, the unstable state of gut microbiota in COVID-19 patients is reflected by a decrease in beneficial bacteria,i.e., Bifidobacterium, and an increase in deleterious bacteria related to bacteremia or sepsis,i.e., BrevibacteriumandPantoea[138].
By RNA and DNA sequencing of blood and stool samples of COVID-19 patients, Yeohet al[20]depicted a distinct signature of gut microbiota composition in 100 positive subjects. A decreased abundance of gut commensals such asFaecalibacterium prausnitzii,Eubacterium rectaleandBifidobacterialhave been reported up to 30 d after disease course in 87 hospitalized COVID-19 patients, suggesting the long-term dysregulation of gut microbiota[20].
At the species level, the authors identified a significant association between the compositional abundance of gut microbiota and disease severity. The microbial species,Faecalibacterium prausnitzii,Bifidobacterium bifidum,Bifidobacterial adolescentisandEubacterium rectalenegatively correlated with disease severity, the mean value of the gut microbial composition decreased compared to non-COVID-19, and mild COVID-19 samples correlated to the lowest value compared to the severe and critical patients[20].
Analysis of gut microbial samples might provide valuable prognostic serum markers, which could predict disease severity and outcomes[20]. Correlational analysis between different microbial taxa and cytokine and chemokine levels suggested the role of gut microbiota in regulating the magnitude of immune response and modifying disease severity of COVID-19 disease[20].
Thus, the decrease in the abundance ofBifidobacterium adolescentis, Faecalibacterium prausnitziiandEubacterium rectalein COVID-19 patients was associated with elevated cytokine levels of tumor necrosis factor-α, IL-10, C-C motif chemokine ligand 2 and CXCL10[20]. In identifying the microbial species associated with disease severity, Schultet al[79] analyzed gut microbial profiles and observed a different bacterial composition in COVID-19 patients with a low risk of complications, with a predominance ofFaecalibacterium prausznitzii, and high risk complications, in whichParabacteroides spp.Dominates. The changes in the abundance of microbial species were more pronounced in patients with severe associated conditions, such as acute kidney injury and acute respiratory distress syndrome, followed by a lesser microbiota change in acute cardiac events and venous thromboembolism. Moreover, the authors proposed 12 gut microbial species as cocktail biomarkers with an accuracy of 0.94 for predicting the progression of disease and the severity of COVID-19 patients[79]. Thus, the abundance ofRuthenibacterium lactatiformans,Clostridium innocuumandAlistipes finegoldiiwas correlated with inflammatory blood markers, such as white blood cells, CRP and procalcitonin, and disease progression. In severe and fatal cases, the microbial profile of the gut exhibited depleted levels ofBlautia luti,Faecalibacterium prausnitzii,Alistipes putredinis,Dorea longicatenaandGemmiger formicilis[79].
Aging, diet and comorbidities, such as obesity, diabetes and cardiovascular diseases, have a significant impact on the microbial profile of the gut, leading to dysbiosis[141-144]. Age and comorbidity-related changes in the gut microbial profile of COVID-19 patients influence immune regulatory mechanisms, which might explain the severe forms of disease and the associated complications in older and comorbid patients[145].
In relation to the mechanistic data mentioned, patients with severe forms of COVID-19 faced more pronounced gastrointestinal symptoms, suggesting the association between clinical symptoms and disrupted gut microbiota during COVID-19 disease[146,147]. Another study revealed a depressed state of bacterial composition species, consisting of lower levels of beneficial symbionts and higher levels of opportunistic pathogens, such asStreptococcus,Rothia,ActinomycesandVeillonella[80]. The authors proposed five gut microflora biomarkers, includingIntestinibacter,Erysipelatoclostridium,Actinomyces,FusicatenibacterandRomboutsiawith diagnostic value to distinguish between COVID-19 patients and healthy controls[80].
The long-term dysregulated effects of SARS-CoV-2 have been revealed in fecal samples of COVID-19 patients, with persisted gut dysbiosis after clearance of the virus[148]. Prognostic valuable bacterialbased markers includeCoprobacillus,Clostridium ramosumandClostridium hathewayi, which were associated with COVID-19 severity. In a murine gut model, some beneficial bacterial species, includingBacteroides dorei,Bacteroides thetaiotaomicron,Bacteroides massiliensisandBacteroides ovatus, downregulate ACE2 expression level, negatively correlating with the viral load of SARS-CoV-2[148].
At the basis of immune interactions between lung and gut microbiota are microbiota-derived metabolites that modulate host immune cells in a direct or indirect manner[66,149]. Some bacteria species, such asAnaerostipes butyraticus,Faecalibacterium prausnitziiandRoseburia intestinalis, display enzymatic systems to digest the complex carbohydrates, which resulted in SCFA products[70,150]. By analyzing oral microbiota, Firmicutes, Actinobacteria and Bacteroidetes were enriched in the COVID-19 group compared with healthy controls. Moreover, the oral microbiota exhibited fewer levels of butyric acid-producing bacteria and more lipopolysaccharide-producing bacteria in the positive patients[77].
Changes in the metabolomic profile of fecal samples of COVID-19 patients have been correlated with different microbial composition profiles[19]. A better understanding of metabolic changes in serum or fecal COVID-19 samples will further provide new insight into the gut-lung axis and propose putative prognostic markers in COVID-19. Up to 20 metabolites were changed in the fecal sample of COVID-19 patients, including monosaccharides,i.e.,D-allose, D-glucose and D-arabinose, nucleotides,i.e.,hypoxanthine, pseudouridine and inosine, and amino acids,i.e.,l-tyrosine and l-tryptophan, and were associated with bacterial species modifications[19].
Targeting severe immune responses in COVID-19 represents the main therapeutic approach[151].Recent studies pointed out the role of a high-fiber diet and probiotics as disease-modifying therapy in COVID-19[151,152]. The role of nutraceutical compounds, consisting of vitamins, dietary supplements and pro/prebiotics in COVID-19, have been reported to improve the clinical course and severity of COVID-19 disease (Table 4). Several clinical trials investigating the role of probiotics enriched with different types of beneficial species are in progress (NCT04854941, NCT05080244, NCT04390477).
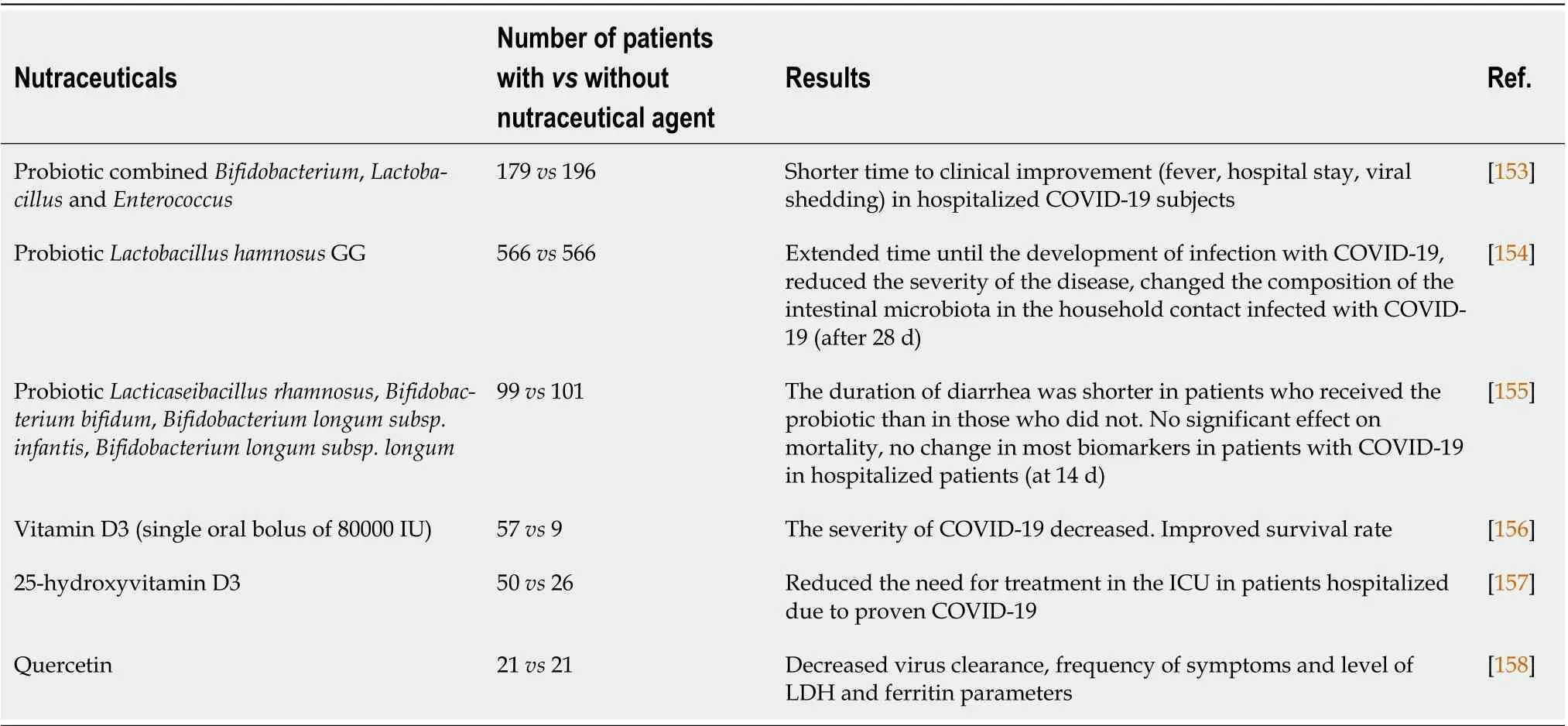
Table 4 Nutraceuticals used to improve disease severity and outcomes of coronavirus disease 2019 patients
CONCLUSION
There is evidence that changes in gut microbiota are an important factor in the pathogenesis of COVID-19. An important role in this disease is also played by the relationship between the gut and the lungs,known as the “gut-lung axis”. Modulating gut microbiota to increase diversity and abundance can positively influence the severity of COVID-19. Further studies are needed to explore the microbiota in COVID-19 patients with varying degrees of severity, in post-COVID-19 patients and their medical history with nutraceutical agents.
FOOTNOTES
Author contributions:Neag MA, Vulturar DM Gherman D and Todea DA performed the research; Neag MA,Vulturar DM, Burlacu CC analyzed the data and wrote the manuscript; Burlacu CC contributed the new reagents and analytic tools; Buzoianu AD designed the research study.
Conflict-of-interest statement:All authors report having no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Romania
ORCID number:Maria Adriana Neag 0000-0002-3904-2852; Damiana-Maria Vulturar 0000-0002-6805-1619; Codrin-Constantin Burlacu 0000-0002-2017-3086; Doina Adina Todea 0000-0003-1306-2350; Anca Dana Buzoianu 0000-0002-3511-2788.
S-Editor:Wang JJ
L-Editor:Filipodia
P-Editor:Wang JJ
 World Journal of Gastroenterology2022年45期
World Journal of Gastroenterology2022年45期
- World Journal of Gastroenterology的其它文章
- COVID-19 drug-induced liver injury: A recent update of the literature
- The mononuclear phagocyte system in hepatocellular carcinoma
- Deep learning based radiomics for gastrointestinal cancer diagnosis and treatment: A minireview
- Best therapy for the easiest to treat hepatitis C virus genotype 1b-infected patients
- Endoscopic mucosal resection-precutting vs conventional endoscopic mucosal resection for sessile colorectal polyps sized 10-20 mm
- Meta-analysis on the epidemiology of gastroesophageal reflux disease in China
