Metal coordination to a deep cavitand promotes binding selectivities in water
Yong-Qing Chen, Hu-Wei Gun, Kuppusmy Kngrj, Julius Rebek,b, Yng Yu,∗
a Center for Supramolecular Chemistry and Catalysis and Department of Chemistry, College of Science, Shanghai University, Shanghai 200444, China
b Skaggs Institute for Chemical Biology and Department of Chemistry, The Scripps Research Institute, La Jolla, CA 92037, United States
Keywords:Host-guest chemistry Metallo cavitand Hydrophilic small molecules Molecular recognition Water-soluble containers
ABSTRACT One goal of supramolecular chemistry is the creation of synthetic receptors that have a high affinity for hydrophilic molecules in water.We found that cavitands with upper rims extended by pyridyl groups coax hydrophilic guests into the cavity where they are shielded from the aqueous environment.The ability of Pd(II) to coordinate adjacent pyridyl groups leads to increased selectivity for highly hydrophilic solvent molecules such as acetone, 1,4-dioxane and tetrahydrofuran in water.Analysis of the binding behavior indicated that metal-coordination restricts the container entrance, shrinks the effective cavity volume and increases the energetic barrier to guest exchange.
Host/guest sequestration in water has become a popular research area as a probe for intermolecular forces involved in biological recognition processes [1].In the last decades, most attention has been paid to recognition of cations, anions and hydrophobic molecules in water [2,3].Macrocyclic receptors with preorganized binding sites such as crown ethers [4], cyclodextrins[5,6], cucurbit[n]urils [7], calix[n]arenes [8–10], pillar[n]arenes [11–13], naphthotubes [14], metal cages [15] and many others [16] have been well-developed.However, few synthetic receptors can recognize hydrophilic molecules in aqueous solution [17,18].
Deep cavitands can confine many small molecules and show applications in molecular recognition, sensing and biomimetic catalysis [19–23].Most of them are derived from shallow resorcinarenes that are deepened through covalently added aromatic walls.They typically exist in two conformations: a receptive vaselike shape and unreceptive kite-like shape.The kite shape is selfcomplementary and often dimerizes to a velcrand through solvophobic forces [24].Additional non-covalent interactions such as a cyclic array of intramolecular hydrogen bonds [25] and metal coordination have been used to stabilize the vase conformation and enhance guest binding [26].For small molecule guests, these cavitands can provide a hydrophobic surrounding, but the open end provides exposure to solvent.The in/out exchange rates of small molecule guests are often too fast to observe the signals for the bound guest in the1H NMR spectra [27].Metal coordination such as Pd(II) to a quinoxaline-rimmed cavitand can alter the shape of the cavity and constrict the size of its opening; this can enhance the binding of hydrophobic and even hydrophilic guests in water[28].Here we describe the synthesis of a deep cavitand 1 with benzimidazole rims extended four 3-pyridinyl functions.We find that metal coordination to the pyridyls enhances selective recognition of hydrophilic molecules in water.
Wepreparedthedeepcavitand1with2-(3-pyridinyl)benzimidazole arms from the well-known precursor,octanitro cavitand [29] through three reaction steps.The octanitro cavitand was reduced to octaamine cavitand and condensation with ylide (2-(pyridin-3-ylmethylene)malononitrile) to afford the 2-(3-pyridinyl)benzimidazole tethered cavitand [30].Water soluble cavitand 1 was obtained by attachingN-methyl imidazole groups to the feet (Scheme 1a and Scheme S1 in Supporting information).Cavitand 1 was characterized by analytical techniques such as1H,13C NMR and HRMS.The1H NMR spectra of cavitand 1 in D2O(Fig.1a) showed broad and undefined peaks which are attributed to the interconversion process between itsvaseandvelcrandconformations [31].
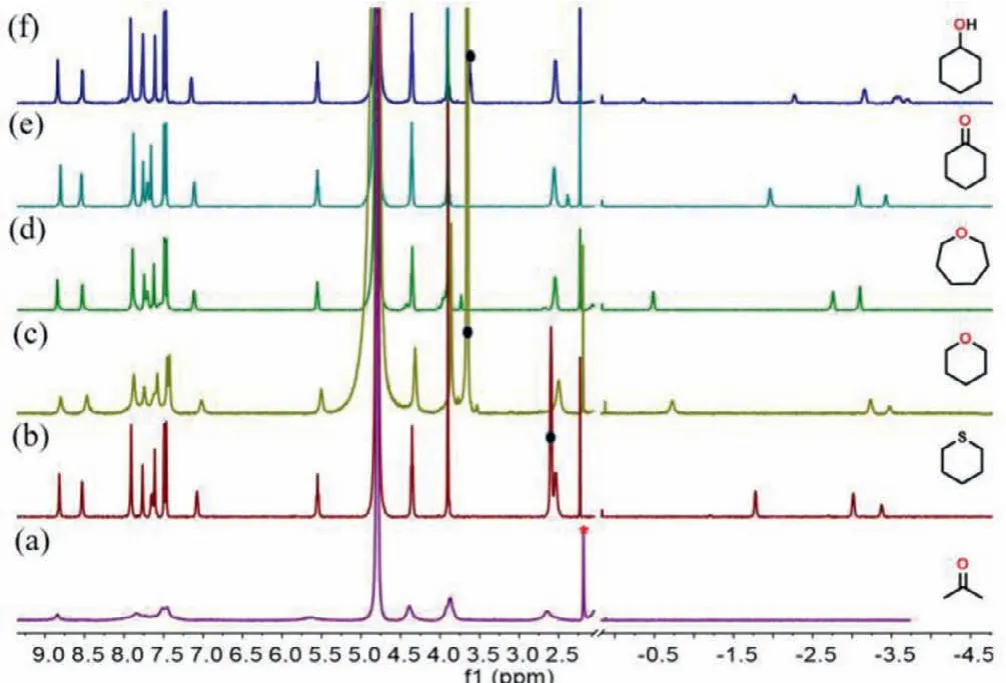
Fig.1 .Partial 1H NMR spectra of the cavitand 1 at 1 mmol/L in D2O.(a) Before the addition of guest, and in the presence of about 1 equiv.of (b) thiane, (c) tetrahydropyran, (d) hexamethylene oxide, (e) cyclohexanone and (f) cyclohexanol.Free acetone protons are marked with red asterisk symbol.Free guest proton signals are marked with black circular dots, and bound guest proton signals are shown in the upfield region between 0 and −4.0 ppm.
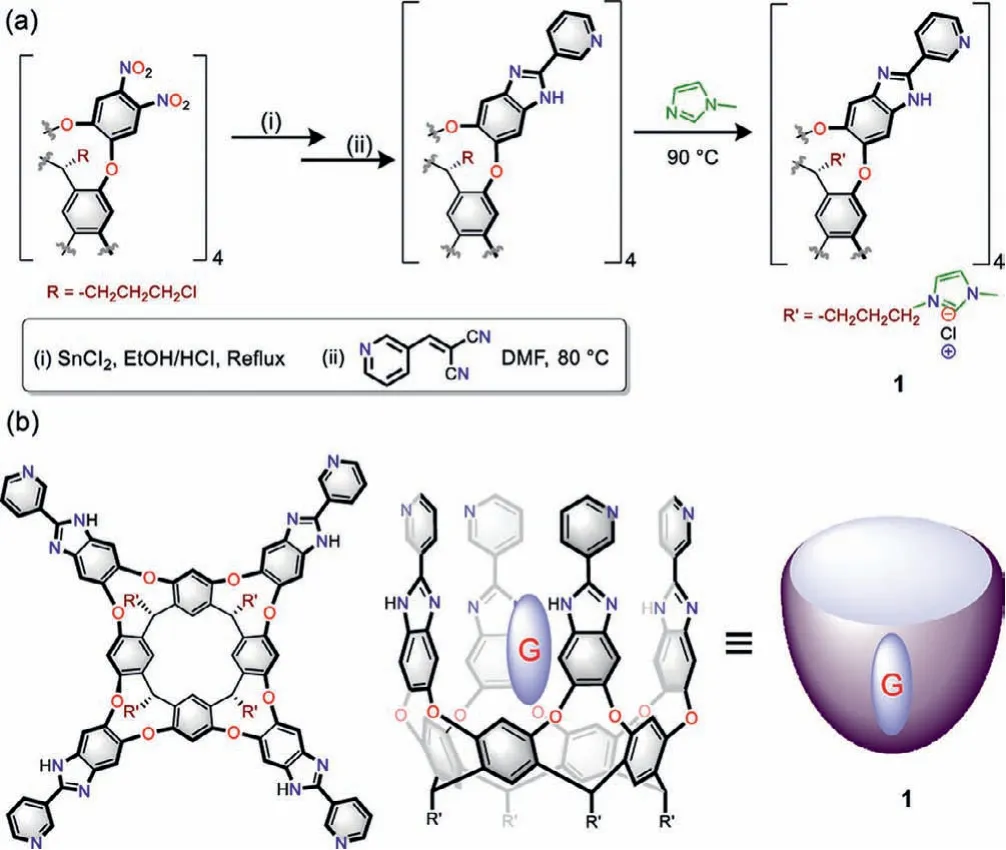
Scheme 1 .(a) Synthesis of the water-soluble cavitand 1 containing 2-(3-pyridinyl)benzimidazole.(b) Chemical structure and “vase” shape of 1 and cartoon representation of guest binding.
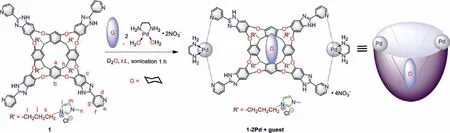
Scheme 2 .Schematic representation of 1–2Pd complex formation in water is in the presence of adaptive guest (G, Cyclohexane).
Cavitand 1 displays good affinity for some small amphiphilic molecules (Fig.1) and forms a kinetically stable complexes in“vase” conformations with these guests in D2O.Thevaseconformation is deduced from its methine proton’s characteristic signal observed around 5.5 ppm [32].We studied the binding of 1 with several cyclic, amphiphilic small molecules like thiane,tetrahydropyran (THP), hexamethylene oxide, cyclohexanone, and cyclohexanol by1H NMR in D2O (Figs.S9–S13 in Supporting information).The1H NMR integration indicated 1:1 (stoichiometric) complexes in all cases.The encapsulated guests’proton signals were shifted upfield and appeared between 0 and −4.0 ppm.The binding depth can be evaluated by comparing the changes in chemical shift (−Δδ) with respect to the free guests [33].The calculated maximum −Δδof these guests areca.−5.0 ppm when bound in the deepest part of the cavity and positioned near the aromatic panels.This is the case for the methylenes of these cyclic amphiphilic small molecules: the hydrophobic part of these guests is bound deep within the cavity, while the polar groups are directed toward the openings near the pyridyl rim.
However, highly hydrophilic molecules such as 1,4-dioxane, THF,1,4-dioxene and other small molecules (isopropanol and diethyl ether) were not observed inside the cavity (Figs.S33–S38 in Supporting information).
Our previous studies showed that metal coordination to Pd(II)with quinoxaline-rimmed cavitands can alter the shape and size of the cavity and enhance the binding properties in water [34].In cavitand 1 the donor nitrogens can be favorably arranged to chelate Pd(II), so we studied metallo cavitand formation and it’s effect on binding properties in water.
Monomeric metallo receptor complexes are known to assemble in the presence of adaptive guest molecules [35].The assembly process of cavitand 1 and Pd(II) precursor [Pd(EDA)(H2O)2·2NO3](EDA=ethylenediamine) was monitored by1H NMR titration in D2O (Fig.2) in the presence of cyclohexane.Upon addition of Pd(II)precursor (1–3 equiv.) to a solution of 1 and cyclohexane in D2O,new proton signals appeared in the upfield region (Fig.2b).When 2–3 equiv.of the Pd(II) precursor were added, the proton peaks became a set of well-resolved signal patterns (Fig.2c), indicating the formation of metallo cavitand 1–2Pd.The signals of the pyridyl H-atoms (d, e, f, g) shifted downfield, as expected for coordination of the pyridyl nitrogen with Pd(II) [36].The signals of the resorcinarene and benzimidazole H-atoms (a, b, c) appear as two doublets showing that complexation results in C2vsymmetry.The guest cyclohexane’s chemical shifts indicated it was deeper in the cavity in the presence of Pd(II) (Figs.2c and d).
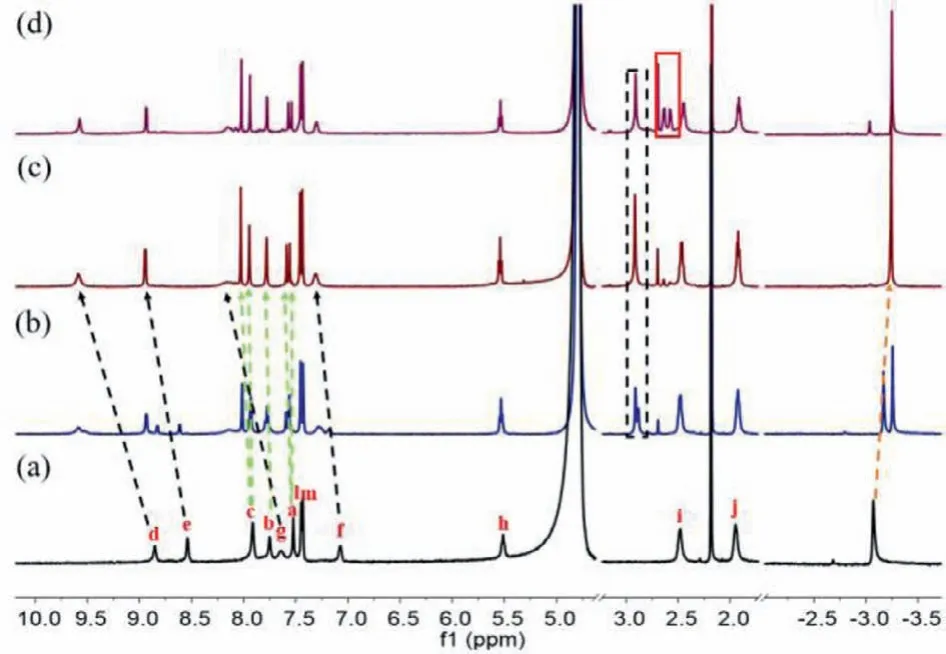
Fig.2 .1H NMR spectra of 1 and excess cyclohexane in the presence of increasing equivalent of Pd(EDA)(H2O)2·NO3 (a) 0 equiv., (b) 1 equiv., (c) 2 equiv., (d) 3 equiv.Black rectangles mark the chemical shift of Pd(EDA)(H2O)2·2NO3 coordinated to 1;the red rectangle shows the chemical shift of the free Pd(EDA)(H2O)2·NO3. Shifts of the proton peaks in 1 are shown, marked by arrows.Proton labeling is given in Scheme 2.
We also treated 1 with different quantities of the Pd(II) precursor in the presence of about 1 equiv.acetone, and sonicated the mixture for 1 h in D2O.Acetone is a very poor guest for 1 due to the high hydrophilic and small size, and the corresponding1H NMR spectrum was broad, indicating that cavitand 1 is flexible in D2O.But in the presence of 1 to 3 equiv.of the Pd(II) precursor,the cavitand 1 quickly shifts to thevaseconformation and takes up acetone (Fig.S18 in Supporting information).The new proton signal at −2.6 ppm indicates acetone bound within the cavity (calculated chemical shift, −Δδ=4.8 ppm).Apparently, Pd(II) coordination stabilizes the “vase” conformation of the cavitand which can better capture the guest.This implies that Pd coordination can shrink the cavity volume of host 1 and it simultaneously preorganizes the cavity to take up small hydrophilic molecules in water[37,38].
Several small solvent molecules are hazardous environmental pollutants and their detection and removal are highly desirable[39].We studied the competitive binding of small hydrophilic solvent molecules including acetone, isopropanol, DMF and diethyl ether to 1–2Pd (Figs.S20–S23 in Supporting information).The results show that acetone, diethyl ether and isopropanol bind better to 1–2Pd than does DMF.The sharp proton signals of aromatic protons and upfield shifted signals confirm that the complexes are stable at room temperature.
Cavitand 1–2Pd showed a good selective binding for hydrophilic molecules in water, forming 1:1 complexes with 1,4-dioxane, THF and sym–trioxane (Fig.3).Binding of THF to 1 results in a broad peak in the1H NMR spectra, but large upfield shifts and very sharp signals were observed when it binds to 1–2Pd.Likewise, the 1,4-dioxane rapidly tumbles or spins at room temperature in 1–2Pd,leading to an averaging of the chemical shifts to give only one sharp singlet in the1H NMR spectra in the upfield region [40].Cavitand 1–2Pd showed a low affinity for sym–trioxane-signals when competing with bound acetone.1,3-Dioxane, tetrahydropyran, cyclopentanol and others are also guests in 1–2Pd in water(Figs.S27–S32 in Supporting information).Binding constants (KA)of the guests to 1–2Pd were calculated from the1H NMR titrations(Table 1).TheKAvalues often reflect the kinetics of the guests in−out rates of complexation; highKAvalues often correspond toslow release of the guests by 1–2Pd [41].THP shows aKAvalue(5.53×103L/mol) of an order of magnitude larger than the binding to 1 (330 L/mol, Fig.S43 in Supporting information).
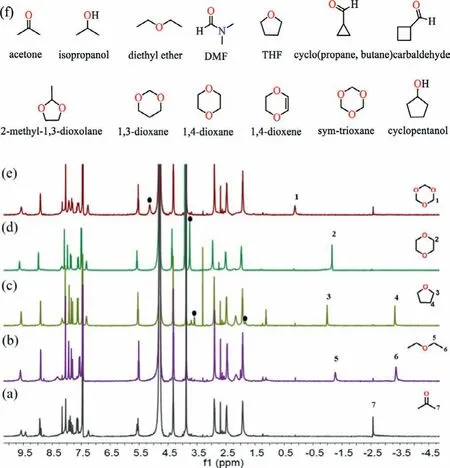
Fig.3 .1H NMR spectra of the cavitand 1–2Pd at 1 mmol/L in D2O (a) before the addition of guest, and in the presence of about 1 equiv.of (b) diethyl ether; (c)tetrahydrofuran; (d) 1,4-dioxane; (e) sym–trioxane.Signals arising from free guest protons are marked with black circular dots, and bound guest protons are shown in the upfield region between 0 and −4.0 ppm.(f) Chemical structures of the small molecules are studied as guests.
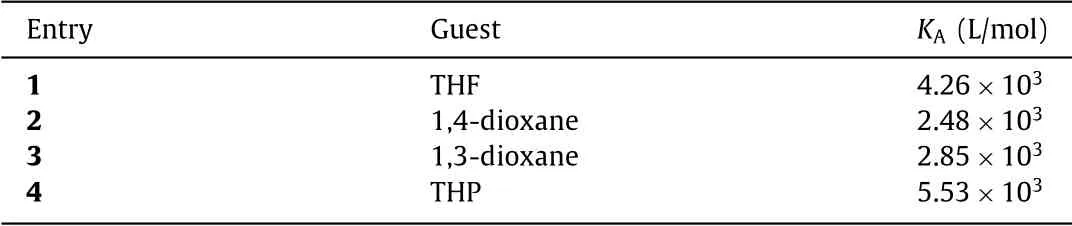
Table 1 Calculated binding constants (KA) for representative guests in 1–2Pd.a
In conclusion, we have shown a new method to introduce pyridyl groups to the upper rim of deep cavitand 1.Chelation of Pd(II) by the pyridyls gave 2:1 metal:cavitand derivatives 1–2Pd.The Pd(II) coordination holds the walls together and decreases the volume of the cavitand.Accordingly, small molecule guests experince more favorable packing coefficients [42].Even small hydrophilic solvent molecules show good binding affinity to 1–2Pd in aqueous solution.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.22071144 and 21801164) and Shanghai University (No.13-G210-20-201).Y.Yang thanks the Program for Professor of Special Appointment (Dongfang Scholarship) of the Shanghai Education Committee.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.039.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
