CuBr-promoted domino Biginelli reaction for the diastereoselective synthesis of bridged polyheterocycles: Mechanism studies and in vitro anti-tumor activities
Mengting Zeng, Ying Xue, Yunn Qin, Fen Peng, Qun Li,∗, Ming-Hu Zeng,c,∗∗
a Hubei Collaborative Innovation Center for Advanced Organic Chemical Materials, Ministry-of-Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Hubei University, Wuhan 430062, China
b Sichuan Center for Disease Control and Prevention, Chengdu 610041, China
c Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin 541004, China
Keywords:Multicomponent reaction Domino reaction Biginelli reaction Bridged polyheterocycles Reaction mechanism Anti-tumor activities
ABSTRACT The low-cost CuBr-promoted domino Biginelli reaction among readily available ketones, salicylaldehyde derivatives and 3-amino-1,2,4-triazole was studied under solvothermal conditions, giving the novel bridged polyheterocycles bearing two or three stereocenters depending on the starting ketones.This multicomponent reaction proceeded with high diastereoselectivity (dr > 20:1) based on a combined 1H NMR,crystallographic and supercritical fluid chromatographic (SFC) analysis of the product.Time-dependent high-resolution mass spectrometry (HRMS) was performed to track the reaction process, and several key intermediates were identified, leading to the drawing of a plausible reaction mechanism.Density functional theory (DFT) calculation was supplemented, and two reaction pathways were differentiated.Moreover, in vitro antitumor activity was evaluated using HeLa and HepG2 cell lines, and two of these polyheterocycles demonstrated promising activities against HepG2 cells with EC50 down to 10 μmol/L.Additionally, ESI-MS/MS studies on all the polyheterocycles suggest a common fragmentation pathway (loss of one molecule of amino-triazole) they shared, providing the first-hand fragmentation rules for future rapid structural identification of them.The multicomponent domino reaction presented here may offer prospects for future design of more efficient strategies to access medicinally important bridged polyheterocycles.
Developing highly efficient chem or stereoselective reactions to access functional molecular scaffolds such as medicinally important compounds or other additional valued chemicals, and in particular, understanding the mechanism of these reactions for a better rational design of matter transformation process represents the holy grail in the research area of modern synthetic chemistry[1–3].One of the most intriguing aspects in this vein is the multicomponent domino reactions that can transform all the readily available starting materials into the final complexed chemical entities in a one-pot manner, offering exceptional prospects to shape our knowledge of the chemical bond formation and valence theory and the way we fabricate functional chemicals [4–7].In this context, exploring stereoselective methods for synthesizing bridged compounds particularly the bridged polyheterocycles with multiple stereocenters have attracted widespread interest due to the broad bioactivities of these compounds (Scheme 1a) [8–11].Conventional methods of preparing these bridged polyheterocycles involve various [n+m] cycloaddition reactions, molecular rearrangement, free radical reactions, transition-metal-catalyzed cyclization reactions,etc., which requires the preparation or adoption of appropriate precursors bearing particular electronic or steric features enabling the subsequent molecular folding or atomic arrangement occurred in a predesigned pathway [12–15].However, the multicomponent domino reaction may offer an alternative and greener strategy over others because of its outstanding atomic economy,and there is no requirement for the preparation of specific synthons or separation of intermediates during the reaction.
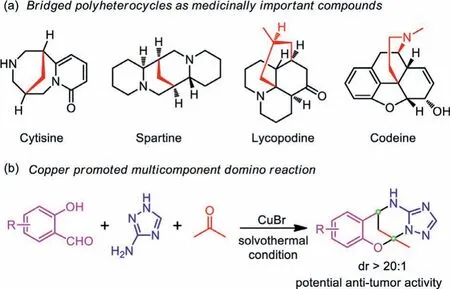
Scheme 1 .(a) Examples of bridged polyheterocycles as medicinally important compounds; (b) Copper-promoted stereoselective multicomponent domino strategy employed in this work to access bridged polyheterocycles.
Of all the multicomponent domino reactions, the Biginelli reaction has been utilized extensively for the synthesis of medicinally important multi-functionalized dihydropyrimidines [16–19].Moreover, the asymmetric Biginelli reaction was also investigated,and several organocatalytic or lanthanide catalyzed methods were reported to access dihydropyrimidines containing a single stereocenter with high enantioselectivity [20,21].Concerning the atomic economy, molecular diversity and challenging stereochemistry encountered for the synthesis of those bridged polyheterocycles,there is still plenty of room for chemists to improve and extend the current multicomponent domino reactions.To our best, using low-cost transition metals to promote domino Biginelli reaction to access bridged polyheterocycles bearing multistereocenters has rarely been reported [22–25], although many solid-phase protocols have been investigated [26].Importantly, the mechanism, particularly the stereochemistry associated with the ring fusion during the reaction necessitates a detailed investigation for better control over the stereoselectivity towards efficient preparation of these compounds.
As our long term and continuous endeavors in coordination directed sequential reaction towards functional molecular materials [27–30], we herein report copper promoted diastereoselective Biginelli domino reaction to piece up readily available acetone, salicylaldehyde derivatives and 3-amino-1,2,4-triazole affording bridged triazole fused tetrahydroazolopyrimidines bearing multi-stereogenic centers which often prove challenging to install efficiently in the ring (Scheme 1b).Efforts were spent on understanding the reaction mechanism with a combination of crystallography, high-resolution mass spectroscopy, and DFT calculations, thus allowing us to disclose the origin of diastereoselectivity for this domino process.Thein vitroanti-tumor activity of these compounds was also evaluated, and the preliminary results showed that compounds 2b and 2d possess relatively high activity against HepG2 tumor cells even at very low concentrations(10 μmol/L).Moreover, tandem mass-spectroscopy was employed to analyze the fragmentation rules of these novel polyheterocycles,providing first-hand information for future possible bioanalytical purposes of rapid identification of these compounds.
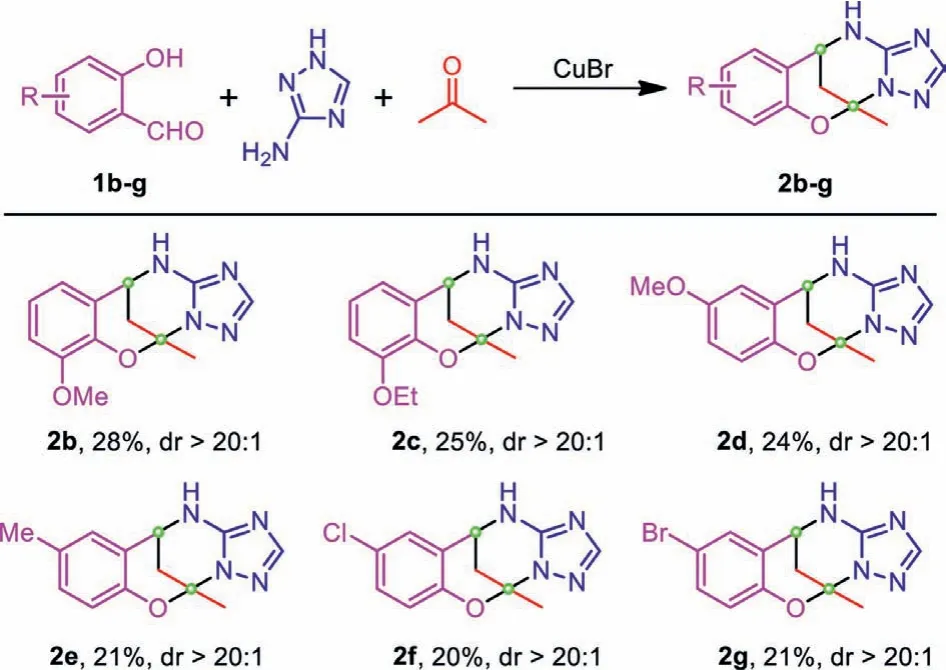
Scheme 2 .Expansion of substrate scope when acetone was used as the reactant and solvent.
Generally, the domino synthesis of target triazole fused tetrahydroazolopyrimidines was firstly performed under solvothermal conditions, after which the mixture was treated directed with Na2S·9H2O to cleave the copper ion that bound to the product.Hence, the solvothermal reaction conditions were initially optimized regarding the type of metal salts, the amount of metal salts,reaction temperature, and time (Table 1).Screening of different copper salts revealed that CuBr could afford the desired product 2a with a higher yield (entries 1–3).Thus CuBr was employed for the subsequent reactions.The reactions performed at elevated temperatures from 80 °C to 160 °C suggests that the highest yield canbe achieved at 120 °C (entries 3–7).Investigation on reaction time shows that the product yield increased as the time elongated, but the yield reached its maximum when the reaction was performed for 72 h, and the yield was not improved further for an even longer reaction time (entries 8–11).The metal salts loading was also studied, and it was surprisingly found that the product yield could be improved by reducing the amount of metal salts (entries 12–14),and the optimal salts loading was set to be 30 mmol%.This unexpected relationship between metal salt loading and yield may be ascribed to the coordination of copper with the triazole compound, which may inhibit the reaction.Meanwhile, a Schiff base intermediate is believed to be formed initially between the aldehyde and triazole compound, thus a small amount of HOAc was added to catalyze the formation of the Schiff base.Indeed, in this case, the final product yield could be further increased to 29% (entry 15).Therefore, the optimal reaction condition could be obtained as: CuBr (30 mmol%) for the metal salt, HOAc as the additive, temperature for 120 °C, and the reaction time for 72 h.
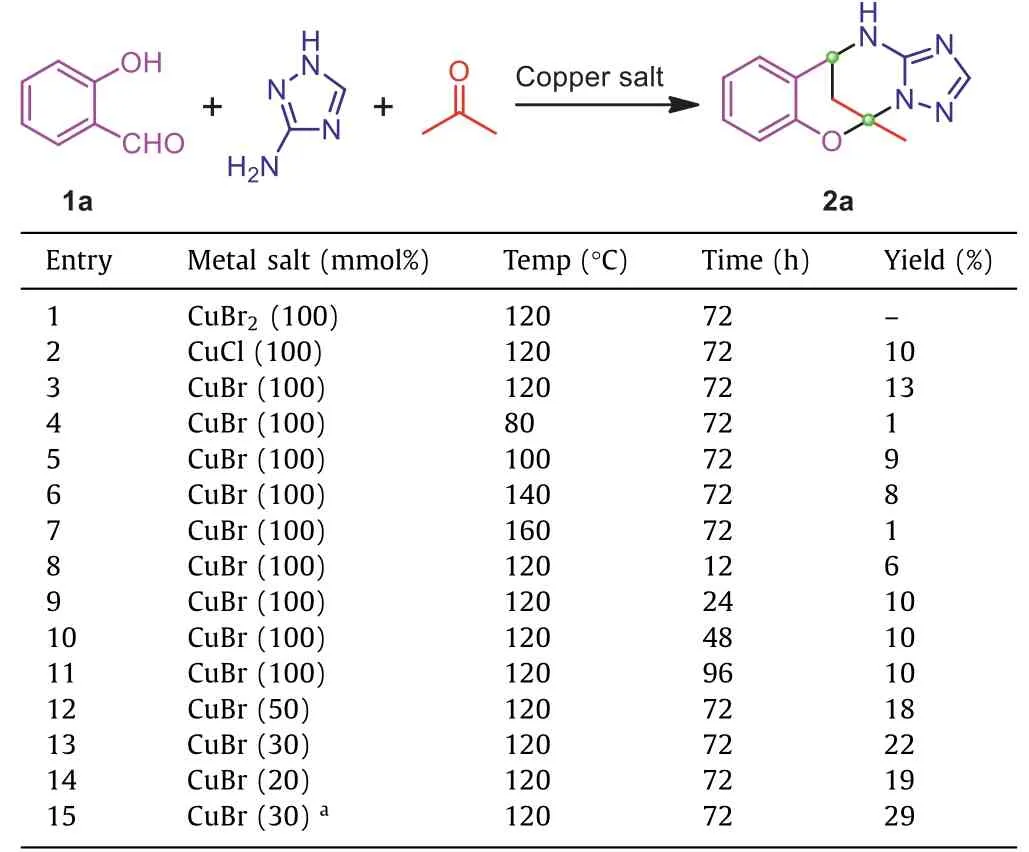
Table 1 Optimization of the reaction condition.
With the optimal solvothermal reaction condition in hand, we next attempted to expand the substrate scope for this domino reaction.To this end, several readily available aldehydes were employed and subjected to the domino reaction (Scheme 2).It was found that the aldehyde substrate with electron-donating substitution atorthoorparaposition gave a relatively higher yield (2b-2d)than electron-withdrawing ones atparaposition (2f and 2g).Noticeably, two stereogenic centers were formed during the synthesis of all these heterocycles, and four stereoisomers were thus expected from the reaction.Nevertheless, the1H NMR spectra of these heterocycles have shown only one set of signals, and only two chromatographic peaks were detected with CO2supercritical fluid chromatography (SFC) (Figs.S6–S12 in Supporting information).All these data strongly suggest that each heterocycle was obtained as a racemic mixture, and thus this domino reaction is assumed to be a highly diastereoselective process (dr>20:1).Delightfully, crystals of several heterocycles were also obtained, and all the crystallographic data confirmed the structures of these heterocycles, particularly for the assignment of stereochemistry (Figs.S1 and S2, Tables S1–S14 in Supporting information).
At this stage, our results demonstrated that a domino diastereoselective process could be achieved using cheap and readily available chemicals as starting materials, and a series of triazole fused tetrahydroazolopyrimidines bearing two stereogenic centers could be obtained.
To further expand the potential of this domino reaction to access heterocycles with high structural diversity, the solvent and reactant acetone was replaced with 3-pentanone, and it was assumed that more stereogenic centers would be formed after the reaction.Indeed, following the similar reaction procedure to the acetone reaction system, the reaction involved 3-pentanone afforded a similar skeleton of heterocycle but with an additional stereogenic center as revealed by X-ray crystallography (Scheme S1,Fig.S3, Tables S15–S18 in Supporting information).Again, the aldehyde substrate with electron-donating substitution atorthoorparaposition gave a relatively higher yield than electron-withdrawing ones atparaposition (Scheme S1).However, the yield for these products is significantly reduced, perhaps due to increased steric hindrance from two methyls of 3-pentanone.Moreover, the1H NMR spectra of all the products now become more complicated,and two sets of signals appeared.Furthermore, SFC analysis unambiguously shows that four isomers are now present in the product(Figs.S13–S19 in Supporting information), suggesting a diastereoselective nature of this reaction.
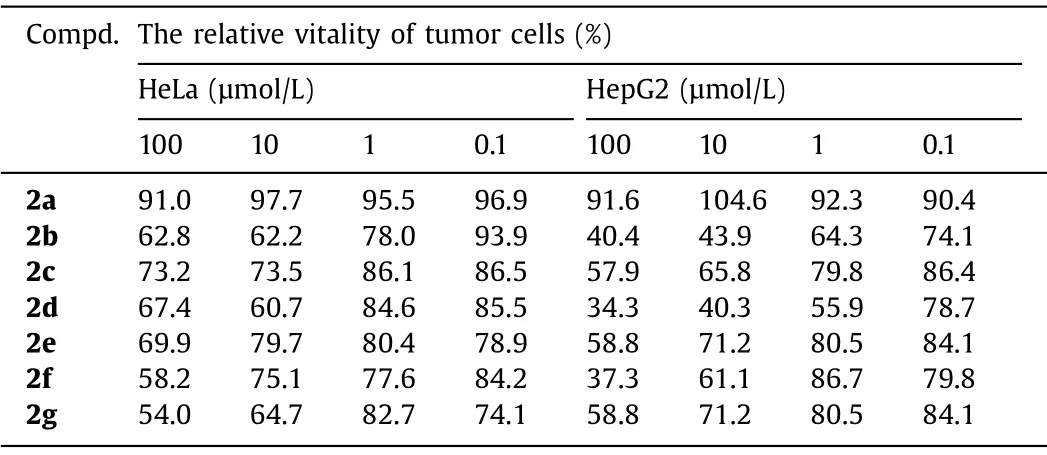
Table 2 In vitro anti-tumor activity of polyheterocycles.
To gain more insight into this multicomponent domino reaction, the time-dependent HRMS technique was utilized to monitor the reaction process [31,32].As shown in Fig.1, at the earlier stage,a peak atm/z189 appeared, and it could be assigned as the Schiff base product being from the condensation of aldehyde and triazole amine.As the reaction proceeded, the intensity of this peak declined gradually, suggesting the consumption of this Schiff base.Meanwhile, the peak atm/z247 which was tentatively assigned as three possible intermediates intensified, reaching its maximum at two hours followed by a gradual decrease of signal intensity.Thepeak atm/z229 that was assigned as the product showed its maximum intensity at two hours, then decreased gradually and leveled off until the end of the reaction.Thus a plausible mechanism was proposed based on the time-dependent HRMS studies on the reaction process (Scheme 3).The Schiff base A was formed at the initial stage.Because the reaction starting from the Schiff base A and acetone could also generate the product in a separate control experiment, validating the key intermediate role of Schiff base A.From the intermediate D, two reaction pathways were possible:triazole N-addition followed by phenolic O-addition or phenolic Oaddition followed by triazole N-addition with respect to the carbonyl in acetone moiety (Scheme 3).However, DFT calculation suggests the former one is highly disfavored due to the higher energy of H and higher activated energies of transition state TS3 and TS4(Fig.S20 in Supporting information).Instead, the phenolic oxygen was found to be prone to undergo intramolecular carbonyl addition (TS5), and then dehydration occurs assisted by the acidic N-H bond (TS6), followed by a rapid cyclization (TS7) to give the final bridged polyheterocyclic product.Concerning the diastereoselectivity of this domino reaction, the conformation of TS7 provides a clear hint that the configuration of the first imine carbon will dictate the direction from which the subsequent N-addition occurs,i.e., the proton on the imine carbon always favors asynconformation with respect to the methyl group.
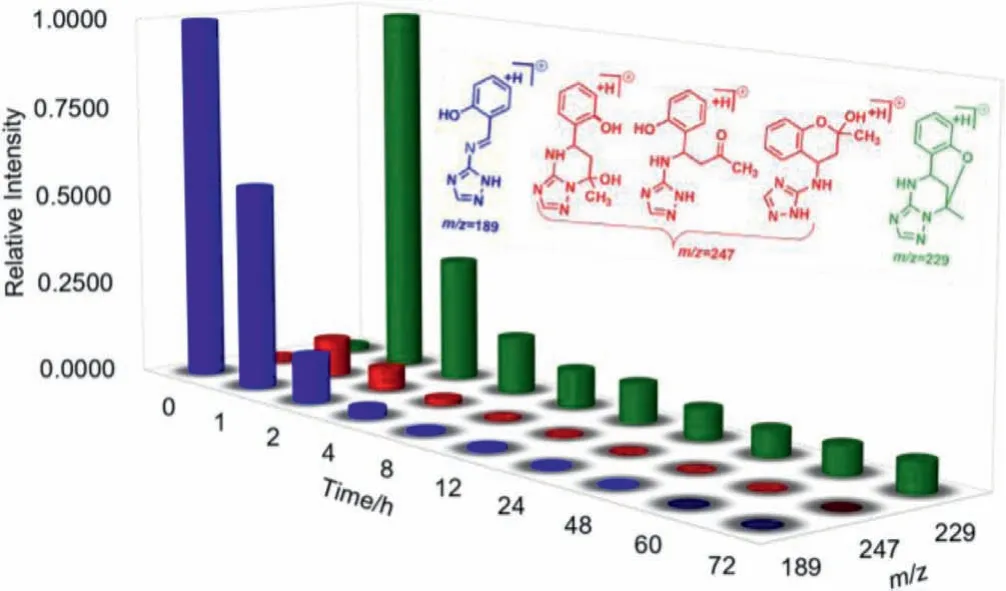
Fig.1 .Time-dependent ESI-MS to monitor the reaction process.
The successful obtainment of these novel bridged polyheterocylces prompted us to evaluate their potentials in biomedicine,thus we performed the initialin vitroanti-tumor activities of them.To this end, two classical tumor cell lines HeLa and HepG2 were incubated with each compound at different concentrations,and the cell vitality was measured after 24 h incubation time with MTT method.As shown in Table 2, for HeLa tumor cells,all the compounds demonstrated medium to low activities within all the concentration ranges (0.1–100 μmol/L).Nevertheless, compounds 2b, 2d and 2f showed great potential as anti-tumor agents based on their activity against HepG2 cells.More than 50% of the cell death was observed when the concentration was 100 μmol/L for these compounds.In particular, compounds 2b and 2d still showed robust activity even after their concentration was reduced to 10 μmol/L.Concerning the high activity against HepG2 for compounds 2b and 2d, future attempts may be carried out to further establish a comprehensive structure-property relationship and the origin of the anti-tumor activity,e.g., which stereoisomer serves as the actual effective one.
Finally, ESI-MS/MS analysis was performed on all the heterocycles, gaining the information of their fragmentation under the mass spectroscopic condition and thus providing the first-hand fragmentation rules for future rapid structural identification of them when potential biopharmaceutical analysis is needed.It was found that all the heterocycles showed very strong signals for[M+H]+in the mass spectrum at positive ion mode.Furthermore, MS/MS analysis showed that two C-N bonds between aminotriazole and chromane moiety were labile and could be easily cleaved into characteristic 4H-chromene ionsviathe loss of one molecule of amino-triazole (Scheme 4, Table S20, Figs.S21–S48 in Supporting information).Meanwhile, the cleavage of two labile C-N bonds from MS/MS results also offers a perspective for us to conduct a retrosynthetic analysis of these novel heterocycles, enhancing our knowledge of the theory of chemical bond-forming and scissoring of them.
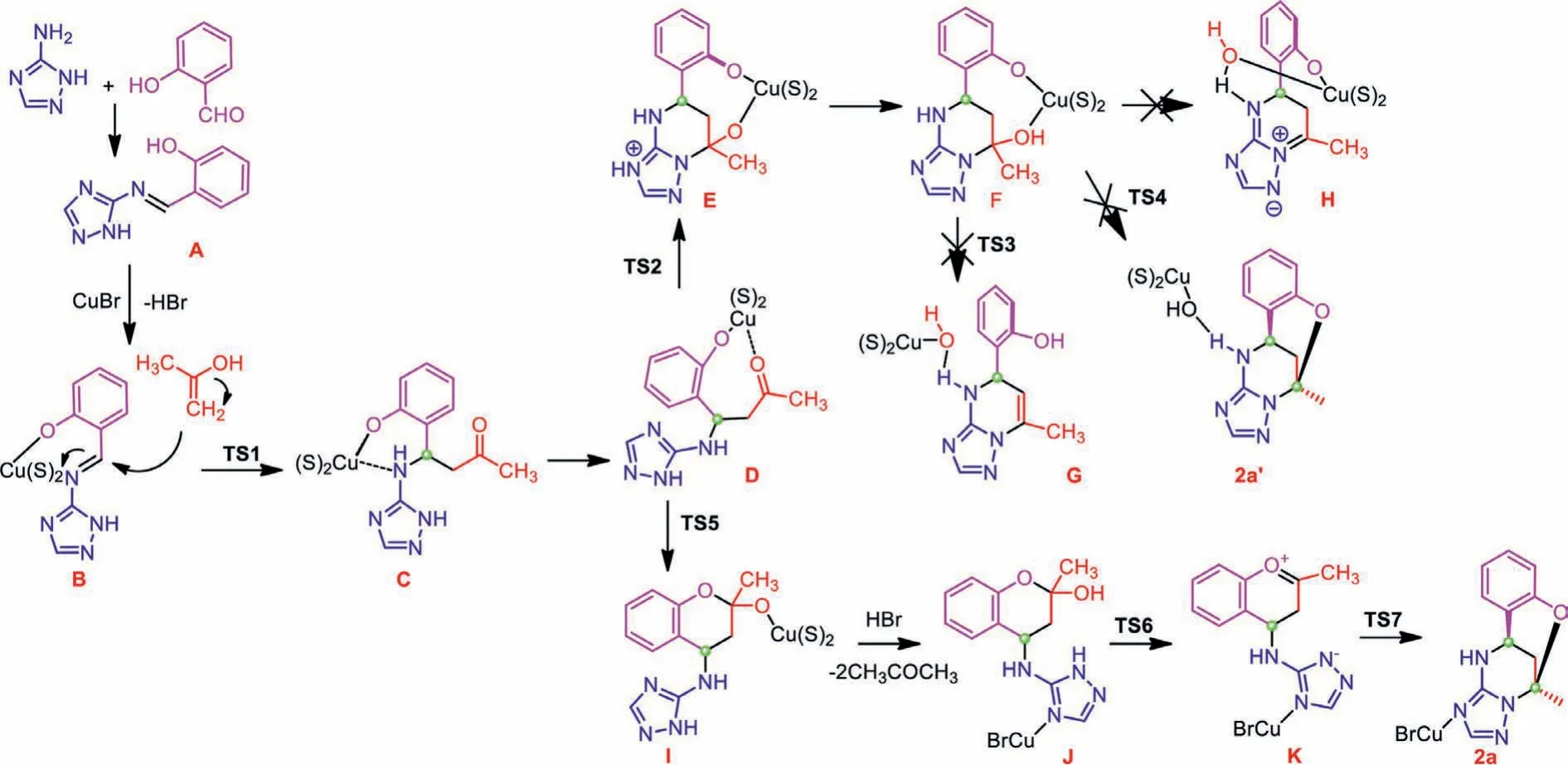
Scheme 3 .Proposed reaction mechanism.S=solvent (acetone).
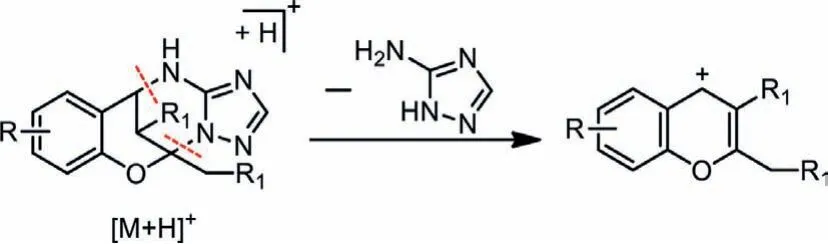
Scheme 4 .Proposed fragmentation pathway of the heterocycles in positive ion mode.
In conclusion, an efficient copper-promoted Biginelli-type domino strategy was successfully developed to access triazole fused tetrahydroazolopyrimidines with high diastereoselectivity.Four new bonds (2C-N, C-O and C-C) and two stereocenters at the bridge site were formed.Although the total yield is not high,considering the multiple steps (7 steps) involved in the domino process, the yield for each single step reaction can be as high as 84%.To our best, this is the first time using 3d metal to promote the Biginelli reaction.The one-pot operation of this “black-box”solvothermal reaction among readily available chemicals further guarantees its great potential in green synthesis of novel heterocyclic compounds.The mechanism of this domino reaction was investigated with a combination of MS and DFT calculations, revealing a preferred sequence of intramolecular nucleophilic addition.The initialin vitroanti-tumor activity tests on these compounds suggest that compounds 2b and 2d possess the highest activity against HepG2 tumor cells, offering prospects for future extensive medicinal chemistry research.ESI-MS/MS analysis on these heterocycles indicates two C-N bonds are easily cleaved with the loss of amino-triazole fragment, providing very useful fragmentation rules for future rapid identification of these novel heterocycles.The line of research shown here may ignite chemists’interest in developing more efficient multicomponent domino strategies for synthesizing medicinally important bridged polyheterocycles.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21901067, 22171075), Starting Grant from the Ministry of Human Resource and Social Security of China (Q.L.), the NSFC of Guangxi Province (Nos.91122032, 2017GXNSFDA198040),and the BAGUI talent program (No.2019AC26001).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.075.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
