Palladium-catalyzed cyclization of 1-alkynyl-8-iodonaphthalene and double isocyanides for the synthesis of acenaphtho[1,2-b]pyrroles
Shangfeng Ren, Keke Huang, Jin-Biao Liu, Lianpeng Zhang, Min Hou,Guanyinsheng Qiu,∗
a School of Metallurgical and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China
b College of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, China
c International Joint Research Center for Biomass Materials, Southwest Forestry University, Kunming 650224, China
Keywords:[2+2+1] Cyclization Isocyanides Palladium catalysis Alkyne Pyrrole
ABSTRACT A palladium-catalyzed formal [2+2+1] cyclization of 1-alkynyl-8-iodonaphthalene with double isocyanides is developed herein.The transformation worked well to produce a series of 7H-acenaphtho[1,2-b]pyrrole with a broad reaction scope.Isocyanides play a dual role in the reaction.One is a C1 building block, and another is used as C1N1 component.In the process, the [2+2+1] cyclization involves imidoylation, regioselective addition of imidoylpalladium species into alkyne, double imidoylation, and another addition of the resulting imidoylpalladium species into imine bonds.
Isocyanides-based imidoylation has attracted ever-increasing attention of synthetic chemists [1–3].As we know, palladium catalysis was often used in isocyanides-based transformation, partially because of its ready coordination and ligand dissociation of palladium-isocyanides complexes [4–5].In the past decades,the established achievements on palladium-catalyzed imidoylation have proved isocyanides to be a powerful C1 synthons towards cyclic products from acyclic substrates [6–7].It is generally accepted that imidoylation under palladium catalysis represented a 1,1-insertion model of isocyanides, a reaction similar to carbonylation [8–11].Therefore, isocyanides often served as a replacement of carbon monoxide in organic synthesis.In recent years, isocyanides-based radical cyclization has been well documented as well [12–14].Many isocyano-containing dualfunctionalized synthons, including 2-vinylphenylisocyanides [15–17], 2-alkynylphenylisocyanides [18–20], 2-arylphenylisocyanides[21–23], and 2-isocyanophenylisocyanides [24–28]etc., have been recognized as efficient reaction partners.In these radical reactions,isocyanides could be installed into the cyclic products as C1N1 components.
Surprisingly, the use of simple isocyanides as C1N1 synthons remains rare in transitional metal-catalyzed annulation[29–31].In 2009, Tsukada and co-workers found that dinucleopalladium catalyst, derived from dinuclear ligandN,N’-bis[2-(diphenylphsphino)phenyl]formamidinate, enabled the use of isocyanides as C1N1 synthons in the reactions with terminal alkyne to release 2-amino-4-cyanopyrroles in high yields [29].In 2016,Zhu’s findings and Jiang’s findings showed that propargyl carbonate and propargyl bromide were efficient candidates to capture isocyanides for constructing fully substituted pyrroles, where isocyanides served as C1N1 synthons [30–31].Very recently, our group developed a palladium-catalyzed [2+2+1] cyclization of internal alkynes with double isocyanides for synthesizing various pyrrolo[3,2-c]quinolin-2-amines [32].
On the other hand, 1-alkynyl-8-iodonaphthalene is a versatile building block for the synthesis of various fluorescent compounds [33–36].Therefore, we would like to explore the reaction of 1-alkynyl-8-iodonaphthalene and isocyanides under palladium catalysis.Considering high ubiquity of pyrroles in medicine [37–40] together with our continuous interest in synthetic methodology development towards pyrroles [41–49], we envisioned that the palladium-catalyzed reaction of 1-alkynyl-8-iodonaphthalene and isocyanides could produce polycycles-fused pyrroles (Scheme 1), a core skeleton being always observed in many useful architectures.
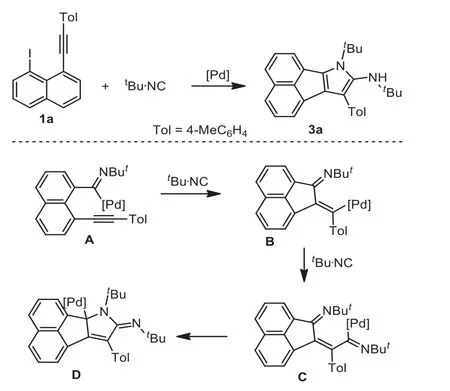
Scheme 1 .Proposed route for palladium-catalyzed cyclization of 1-alkynyl-8-iodonaphthalene and double isocyanides.
Theoretically, oxidative addition of Pd(0) species into carboniodo bond triggered the reaction.Subsequently, it is assumed that imidoylation is prior to the direct addition into alkyne, thus offering imidoylpalladium species A.A 5-exo-digcyclization happed to the resulting imidoylpalladium species A, with the formation of acenaphthylene-containing intermediate B.The intermediate B converted into intermediate C through another imidoylation.It is supposed that the addition of the resulting intermediate C into imino bond is of high importance to form pyrrole ring.According to the results from Tsukada in 2009 [29] and our findings in 2020 [32], it seems reliable that organopalladium species added into intramolecular imino bonds.Supposing the intermediate D was formed, isomerization and protonation finally provided the products 3.
Initially, the reaction of 1-alkynyl-8-iodonaphthalene 1a andtert–butyl isocyanides 2a was employed as a model reaction.A preliminary result showed that the use of 5 mol% Pd(PPh3)4in toluene enabled the aforementioned formal [2+2+1] cyclization to deliver 7H-acenaphtho[1,2-b]pyrrole 3a in 73% isolated yield, and delightfully, the by-products, which was probably derived from intermediate B and C, were not observed.Encouraged by these positive results, other result-affecting factors including solvent, palladium source, base, ligand, and loading of substrateetc.were explored accordingly.The results were presented in Table 1.
Initial trials were focused on screening various solvents.As presented in Table 1, DCE was the best choice due to higher safety score [50], although leading to the desired 7H-acenaphtho[1,2-b]pyrrole 3a in a similar yield (entry 3) to that of the reaction in toluene (entry 1).The use of other solvents did not give rise to better outcomes (entries 4 and 5).
Then, the effect of palladium catalyst was also explored accordingly.From the results, the use of Pd(OTf)2improved the reaction efficiency, leading to the desired product 3a in 79% yield (entry 7).To further improve reaction efficiency, the ligand effect was also examined.Pleasingly, the use of dicarbonylO,O-ligand L3enabled the reaction yield to be increased to 84% (entry 12).Other monophosphine ligands and biphosphine ligands did not enhance the reaction yields.Various bases were also screened.From the results, the reactions using other bases including K2CO3,tBuOK,K3PO4, DABCO and Et3N provided inferior results (entries 15–19).The decrease of isocyanides to 3.0 equiv.suppressed the reaction,leading to the final product 3a in 66% yield (entry 20).Increase of isocyanide loading or palladium catalyst loading did not make significant impact on the reaction efficiency.
With the optimized conditions (entry 12, Table 1) in hand,we then explored the generality of our method.The results were illustrated in Scheme 2.From the results, an ar-ray of 7H-acenaphtho[1,2-b]pyrrole 3 was achieved in moderate to good yields accordingly.For example, the reaction of 1-(4-methoxylphenylalkynyl)-8-iodonaphthalene 1d under standard conditions provided the corresponding product 3d in 75% yield, while the reaction of steric substrate 1e 1-(2-methoxylphenylalkynl)-8-iodonaphthalene gave rise to corresponding product 3e in a similar yield.Particularly, the sensitive groups such as chloro, bromo, and aldehyde were survived in the reaction, and the corresponding products 3i, 3j, and 3l were obtained in 69%, 70%, and 73% yields, respectively.
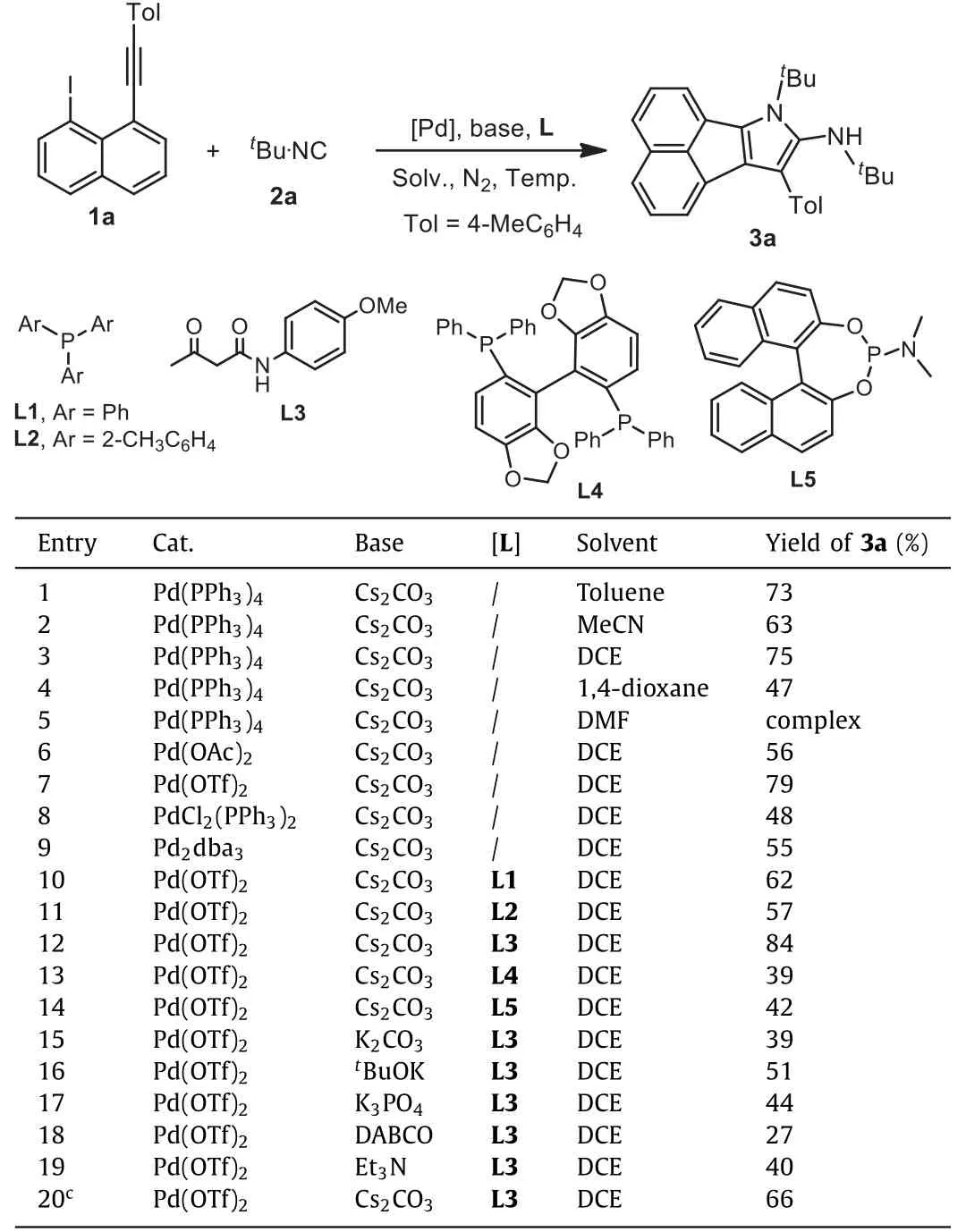
Table 1 Initial studies for the reaction of [2+2+1] cyclization of 1-ethynyl-8-iodonaphthalene 1a and isocyanides under palladium catalysis.a
From the results noted in Scheme 2, the heteocyclic alkyneconnected substrates were compatible for the reactions.For instance, the reaction of 1-(2-thiophenylalkynl)-8-iodonaphthalene 1m under standard conditions provided the corresponding product 3m in 51% yield as expected.Moreover, the substrates with alkyllinked alkynes were also efficient reaction partners.The reactions of 1n and 1o offered the expected products 3n and 1o in moderate yields.
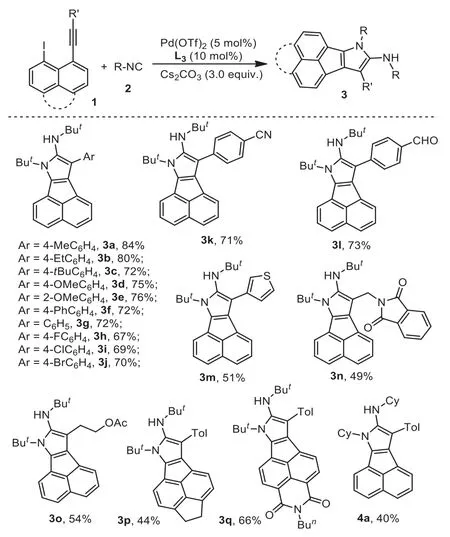
Scheme 2 .Generation of 3 through the reaction of [2+2+1] cyclization of 1-ethynyl-8-iodonaphthalene and isocyanides.Reaction conditions: 1-ethynyl-8-iodonaphthalene 1 (0.2 mmol), Pd(OTf)2 (5 mol%), isocyanides (4.0 equiv.), Cs2CO3(3.0 equiv.), DCE (2 mL), 90 °C, overnight.Isolated yields based on 1-ethynyl-8-iodonaphthalene 1.
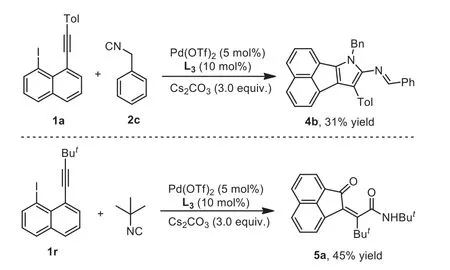
Scheme 3 .Generation of 4b and 5a through the reaction of [2+2+1] cyclization of 1-ethynyl-8-iodonaphthalene and isocyanides.Reaction conditions: 1-ethynyl-8-iodonaphthalene 1 (0.2 mmol), Pd(OTf)2 (5 mol%), isocyanides (4.0 equiv.), DCE(2 mL), 90 °C, overnight.Isolated yields based on 1-ethynyl-8-iodonaphthalene 1.
Interestingly, substituted 1-ethynyl-8-iodonaphthalenes 1p and 1q were good reaction partners.The reactions of 1p and 1q gave rise to the corresponding multi-cyclic products 3p and 3q in 44%and 66% yields, respectively.
Subsequently, various isocyanides were also screened accordingly.As presented in Scheme 2, the reactions of 1-ethynyl-8-iodonaphthalene 1a and cyclohexyl isocyanide 2b proceeded smoothly, resulting in a desired product 4a in 40% yield.To our surprise, the reaction of benzyl isocyanide 2c produced an unexpected product 4b (Scheme 3).Compared to the structure of product 4a, the product 4b was generated through a de-hydrogenation process.This information probably provided a hint on the reaction mechanism.
In particular, the reaction of 1-(tert–butylalkynyl)-8-iodonaphthalene 1r under standard conditions did not offer the desired product, but form acenaphthylenone 5a in 45% yield(Scheme 3).It is reasoned that carbonyl group in product 5a was ascribed to hydrolysis oftert–butylimido group, and the product 5a was probably attributed to reductive elimination of the acenaphthylene-containing imidoylpalladium intermediate C(noted in Scheme 1) with H2O.
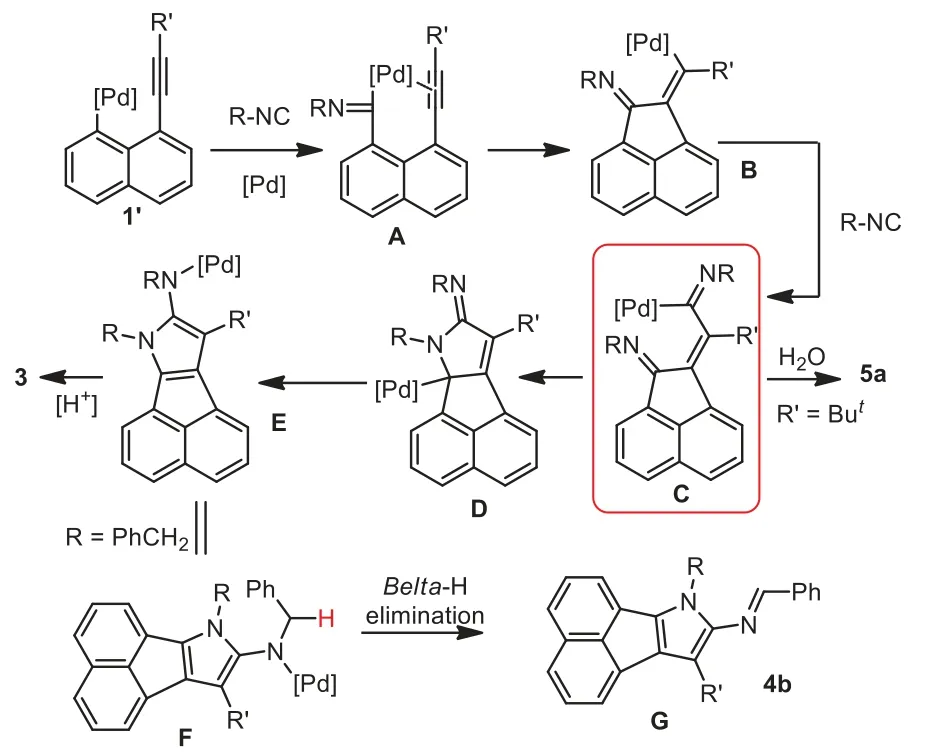
Scheme 4 .Plausible mechanism.
To improve the practicality of this [2+2+1] cyclization, a millimole scale reaction of 1a (1 mmol) andtert–butylisocyanide(4 mmol) was carried out, and 247.6 mg of the desired product 3a was obtained in 78% yield.
Considering the fluorescent properties of this skeleton, the photophysical properties of products 3a, 3p and 3q were investigated.The UV–vis absorption spectra and fluorescence emission spectra of these compounds were measured in EtOH (0.1 mmol/L) (Fig.S1 in Supporting information).Although 3a showed relatively weak fluorescence emission at about 590 nm, 3p and 3q showed strong fluorescence emission at about 590 nm and 530 nm, respectively.
In light of the aforementioned results and our previous findings, a plausible mechanism was proposed in Scheme 4.As illustrated in Scheme 4, a formal [2+2+1] cyclization of 1-ethynyl-8-iodonaphthalene and isocyanides was triggered by oxidative addition of Pd species into C-I bonds.Followed by the first imidoylation, the 1-alkynylnanaphthalene-containing palladium species 1'converted into an imidoylpalladium species A.It is noteworthy that the formation of the intermediate A was ascribed to the priority of imidoylation of 1'to direct addition into alkyne.A sequentialcisaddition of the intermediate A into intramolecular triple bond released an acenaphthylene-containing vinylpalladium intermediate B.The second imidoylation happened to the intermediate B with the formation of acenaphthylene-containing imidoylpalladium intermediate C.It is believed that the intermediate C was a key intermediate in the reaction which enabled the divergent synthesis as noted in Schemes 2 and 3.Whentert–butyl–linked substrate 1r was used, the intermediate underwent a reductive elimination with H2O, probably due to steric hinderance, to produce a distinctive product 5a.
Otherwise, the intermediate C went through an addition into intramolecular imino group to form an intermediate D.Followed by isomerization, the intermediate D converted into intermediate E.Protonation of the intermediate E finally provided the desired products 3.Supposing R group in intermediate E was a benzyl group, aβ-hydride elimination tool place to release 4b.
In conclusion, we have developed a palladium-catalyzed formal[2+2+1] cyclization of 1-ethynyl-8-iodonaphthalenes with double isocyanides.The transformation worked well to produce a series of 7H-acenaphtho[1,2-b]pyrroles with high efficiency and a broad reaction scope.It is noteworthy that isocyanides play a dual role in the reaction.One serves as C1 sources, and another is used as C1N1 building blocks.The use of isocyanides as C1N1 building blocks orπ-component is ongoing in our lab, and the results will be reported in due course.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial supports from the Natural Science Foundation of Zhejiang Province (No.LY22B020010), the National Natural Science Foundation of China (Nos.21772067, 21801096 and 22161043) are gratefully acknowledged, and The Youth Jinggang Scholars Program in Jiangxi Province.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.028.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
