A bench-stable reagent for C-4 selective deuteriodifluoromethylation of azines
Junqing Ling, Lefeng Dong, Feng Qin, Yijin Kong, Mingxi Wng, Xioyong Xu,Xusheng Sho, Zhong Li,∗
a Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China
b Research Center of Analysis and Test, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237,China
Keywords:Reagent Deuterium Difluoromethylation C-4 selective Azines
ABSTRACT Deuteriodifluoromethyl (CF2D) is a challenging and important functional group due to difficult deuterium incorporation and lack of effective precursor reagents.Herein, we report a bench-stable reagent,deuteriodifluoromethyl phosphine (DDFP) from cheap deuterium source for selectivity deuteriodifluoromethylation of azines with a high deuterium incorporation yield.The late-stage modification of complex molecules further confirmed the potential of this reagent for practical applications.We expect that our reagent to find applications in synthesis of isotope-labelled molecules of interests for drug-discovery and related ilucidation of mechanism of action.
Deuterium-containing compounds find widespread applications in mechanistic elucidation, organic synthesis, analytic chemistry,optical materials and life sciences [1–7].In recent years, there is a growing interest in development of deuterium-containing pharmaceuticals (Fig.1a) [8–11].Therefore, efficient and practical methods for preparation of deuterated building blocks and pharmaceutical compounds, and natural products are warranted.
Fluoroalkylated azines are common drug candidates and agrochemicals [12–14].Among different azines, C-4 fluoroalkylation of pyridines are particularly hot research topic.In 2021, McNally and co-workers developed the fluoroalkyl phosphine, which could achieve one pot preparation of C-4 selective fluoroalkylated azines[15].Meanwhile, the difluoromethyl (-CF2H) has received great attention due to its unique biochemical and physical properties.It is a hydrogen bond donor, can be used as a biological isostere, and has high metabolic stability to biological thiols, amines and alcohols [16,17].The introduction of difluoromethyl (-CF2H) can improve potency and change the pharmacokinetic properties, such as lipophilicity, membrane permeability, and metabolic stability(Fig.1b).
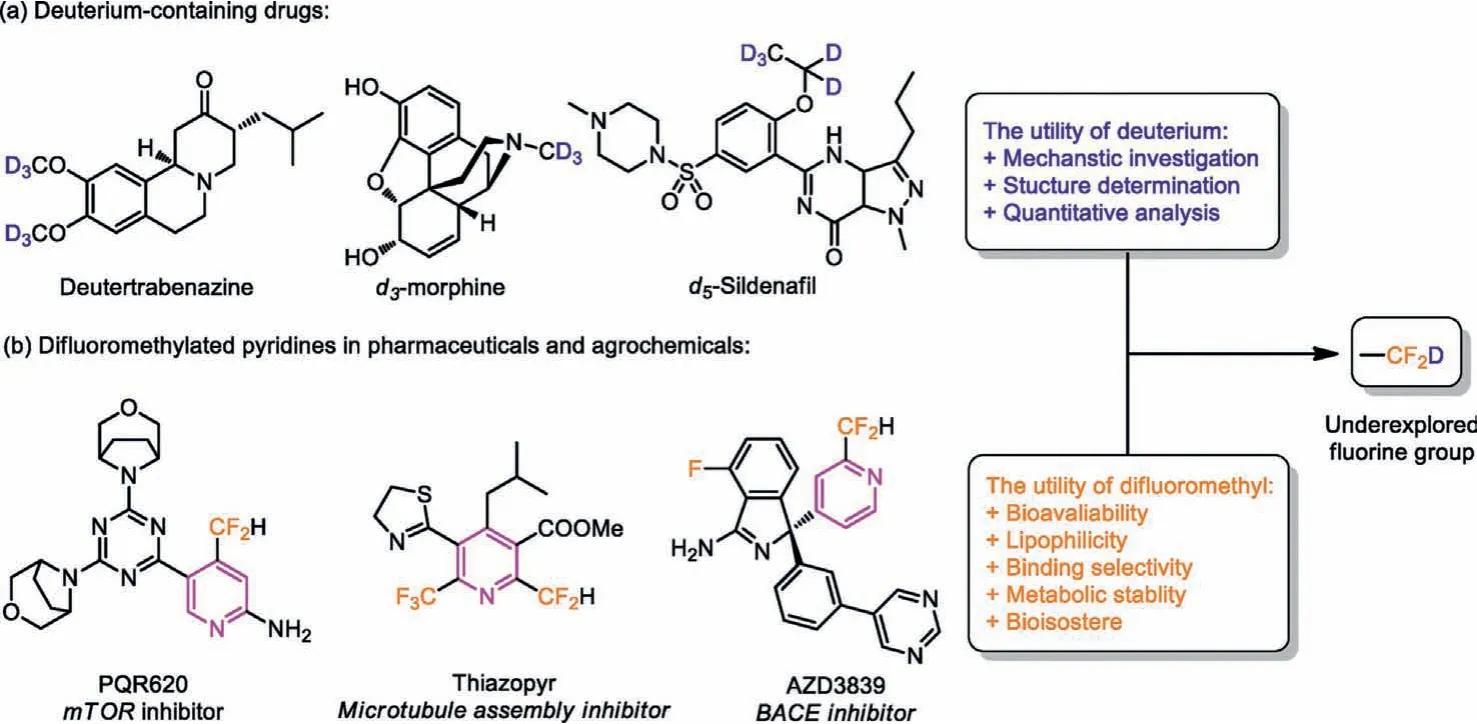
Fig.1 .Deuterium-containing and difluoromethylated drugs.
Due to the wide application of deuterium and difluoromethyl(-CF2H) in pharmaceuticals and agrochemicals, it is conceivable that the deuteriodifluoromethyl (-CF2D) group may be a potential functional group in drug development and modification.To the best of our knowledge, methods for synthesizing deuteriodifluoromethyl-containing compounds are scarce.In 2017, Colby and co-workers reported a synthesis of deuteriodifluoromethyl-containing ketones and sulfones by releasing trifluoroacetate from gem glycol (Scheme 1a) [18].Jamison and co-workers achieved deuteriodifluoromethylation in high yields with a high D-inclevel using the continuous-flow method in 2020(Scheme 1b) [19].Recently, Yan and co-workers reported thatt-BuOK catalyzed the H/D exchange reaction of difluoromethyl aromatic hydrocarbons in DMSO–d6solution, but the deuterated sites, numbers and radios are unruly (Scheme 1c) [20].In addition, (difluoromethyl-d)trimethylsilane (TMSCF2D), which was prepared from Ruppert-Prakash reagent, however, this reagent was confirmed to transfer deuterium atom instead of -CF2D group (Scheme 1d) [21].Overall, the direct introduction of deuteriodifluoromethyl group remains challenging and the lack of effective deuteriodifluoromethylation reagents has resulted to the deuteriodifluoromethylation developed slowly.Thus, the invention of a reagent to accomplish direct, high deuterium incorporation and selective deuteriodifluoromethylation under mild and environmentally friendly conditions is urgently needed.
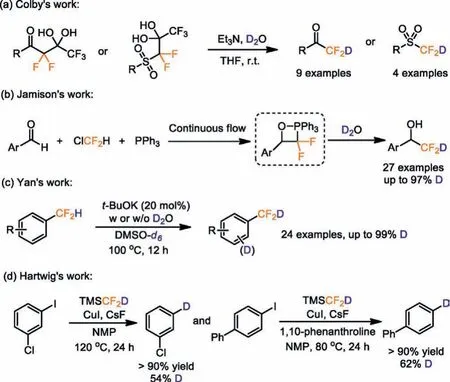
Scheme 1 .Strategies for the synthesis of deuteriodifluoro-methylated compounds.
Our group is committed to the development and application of effective deuteriodifluoromethylation reagents.More recently, we developed a novel deuteriodifluoromethylation reagent (CF2DSO2Na) based on sodium difluoromethanesulfinate(CF2HSO2Na) for deuteriodifluoromethylation at C-2 position of indoles (Scheme 2a) [22].However, the deficiency of CF2DSO2Na is its hygroscopicity.Herein, we report a new reagent,i.e., deuteriodifluoromethyl phosphine (DDFP), which inspired by the design principle of difluoromethyl phosphines reagent developed by McNally (Scheme 2b) [15].DDFP is prepared from bis(4-methoxyphenyl)phosphine oxide (1) on a gram-scale in two steps with an overall yield of 56% (Scheme 3).
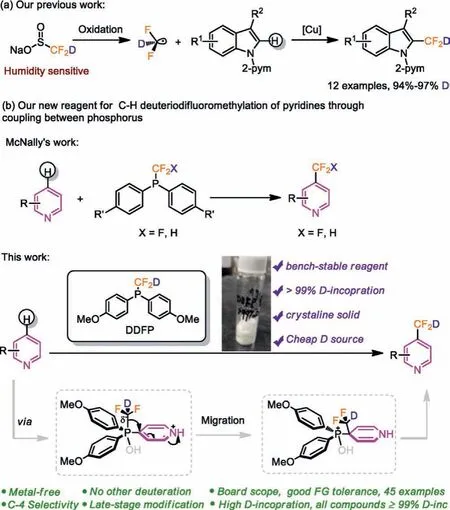
Scheme 2 .Our strategies for the development of deuteriodifluoromethylation reagents.
We explored the synthesis of the deuteriodifluoromethylated phosphine oxide (3) by reacting 1 with a difluorocarbene generatedin situ.Difluorocarbene reagents such as FSO2CF2COOH (2a),ClCF2COONa (2b), PDFA (2c), CF2BrP(O)(OEt)2(2d) and PhSO2CF2Br(2e) failed to yield the desired product (3) (Table 1).According to the Hu’s report [23], we speculated that TMSCF2Br (2f) was easy to generate: CF2due to the better leaving ability of the bromide ion under alkaline conditions, and then reacted with D2O to generate CF2D.Eventually, treatment of the compound 1 with TMSCF2Br (2f)generated 3 in a 75% yield with quantitative deuterium incorporation as a white solid.The reaction is readily scaled up to multigram scale.
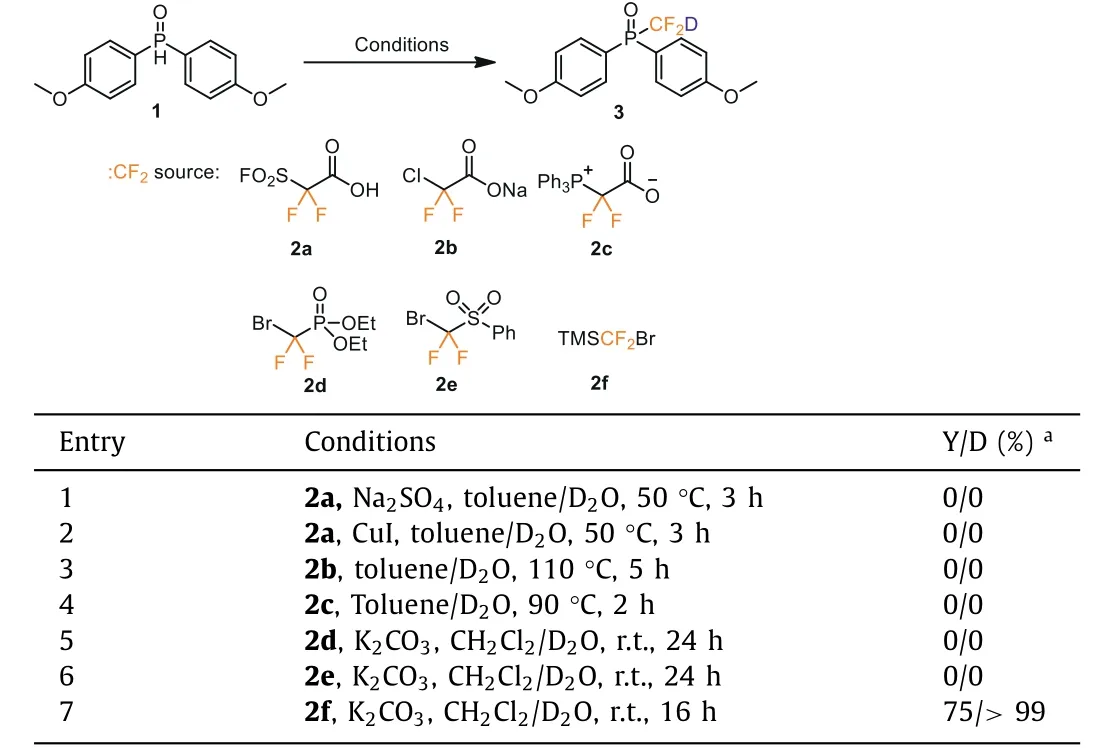
Table 1 Deuteriodifluoromethylation of 1 with various difluorocarbene reagents.
DDFP is stable to air and humidity at ambient temperature.Based on the1H/19F NMR, a batch of DDFP was found not to decompose in 5 months.The deuterium incorporation did not change as well.Differential scanning calorimetry (DSC) showed that the melting point of DDFP is 36.6 °C.In addition, thermogravimetric and derivative thermogravimetry (TGA) measurements that DDFP started to decompose at around 160 °C and was completely decomposed to 270 °C (details see Supporting information).
With this reagent in hand, we then explored the reactivity of DDFP with azines.Overall, all azines that react with DDFP could get the corresponding products with ≥99% deuterium incorporation.The reaction of 2-phenylpyridine with DDFP pro-ceeded smoothly under the standard condition (conditions optimization details see Supporting information), and obtained the -CF2D substituted 5 at the C-4 position in a 78% yield.Likewise,we screened the scope of azines with different substitutions.2-Substituted pyridines with various functional groups such as aryl(5–12), ester (13, 14) and (15, 16) were compatible with DDFP and furnished the corresponding products in moderate to good yields (51%−85%) (Scheme 4).2-Acetalpyridine was partially hydrolyzed under the standard condition, changing the condition to a mixture of tetra-n-butylammonium fluoride (TBAF) and HCl solved this problem, and obtained the desired product 17 in a 65% yield.The reaction of 2-substituted cycloalkane pyridines (18,19) also formed the products in moderate yields (51%−56%).3-Substituted pyridines were also afforded the corresponding products (20–23) in satisfactory yields (49%−78%).The reaction of multisubsituted pyridine proceeded smoothly to give the target compound 24 in a moderate yield.Additionally, we studied the deuteriodifluoromethylation of quinoline derivatives.Quinolines with electron-donating or electron-withdrawing groups could also react with DDFP to prepare the corresponding products (25–33) in good yields (50%−82%).The structure of compound 29 was confirmed by X-ray diffraction analysis (see Supporting information).Most remarkably, pyrimidine was also compatible with the reaction condition, giving the product 34 in 37% yield.
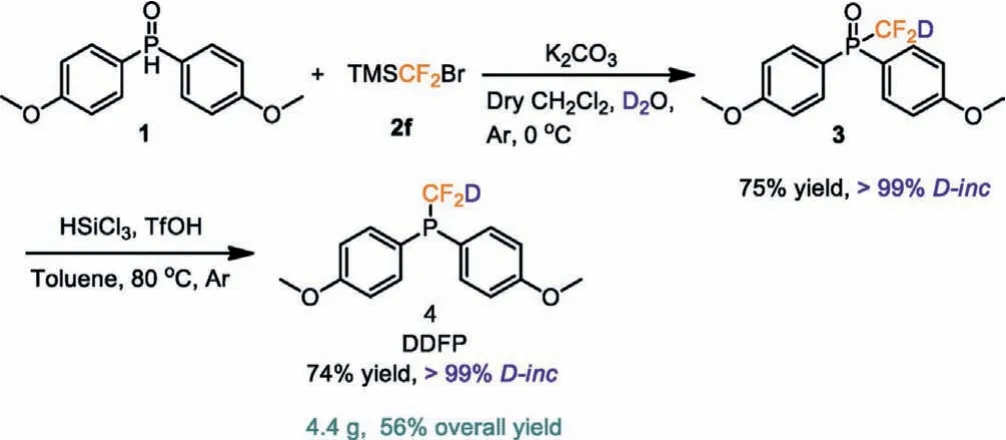
Scheme 3 .Preparation of DDFP.
Inspired by the introduction of the deuteriodifluoromethylation group at the C-4 position of azines and considering the application of deuteriodifluoromethylation group in mechanism studies of drugs [24], we tried to introduce deuteriodlfluoromethylation into complex azine-containing molecules.As illustrated in Scheme 4, we turned our attention to molecules that represent drug-like intermediates firstly.The results showed that the drug-like intermediates could be reacted under the standard condition to obtain the corresponding products 35 and 36 in good yield with 99% deuterium incorporation.Next, we introduced deuteriodifluoromethylation into molecules useful in material science, and obtained deuteriodifluoromethylated products 37–39 in a satisfactory yield with 99% deuterium incorporation.We further used DDFP in the subsequent modification of common natural products and drug molecules including Sesamol, Clofibrate, Pterostibene, Estrone,Bisacodyl, Ioratadine and Etofibrate.We obtained the corresponding products (40–46) in moderate to high yields with high levels of deuterium incorporation (up to>99%).
Finally, we set our sights on gram-scale preparation and subsequent transformation.As shown in Scheme 6, we synthesized the compound 30 on 5 mmol scale by using DDFP with 6-bromoquinoline.To our delight, we obtained the product in a 72%yield with 99% deuterium incorporation (Scheme 5a).Then, we carried out a 5 mmol preparation of drug modification, the yield of compound 44 decreased slightly (78%), and the deuterium incorporation remained unchanged at 99% (Scheme 5b).Meanwhile,quinoline derivatives not only exhibited good biopharmacological activities [25–29], but also have important applications in the field of fluorescent and phosphorescent probes [30,31].Hence, we had carried out subsequent transformations to compound 30, hoping to obtain more compounds containing deuteriodifluoromethylated quinoline moiety.For example, compound 30 could undergo with Sukuzi and Sonogashira coupling reactions, or oxidation reaction to obtain quinoline nitrogen oxide.These products could be obtained with high levels of deuterium incorporation (≥99%) (47–49).Additionally, compound 49 may be used as deuteriodifluoromethylated building blocks to synthesize more deuteriodifluoromethylcontaining molecules in the future.
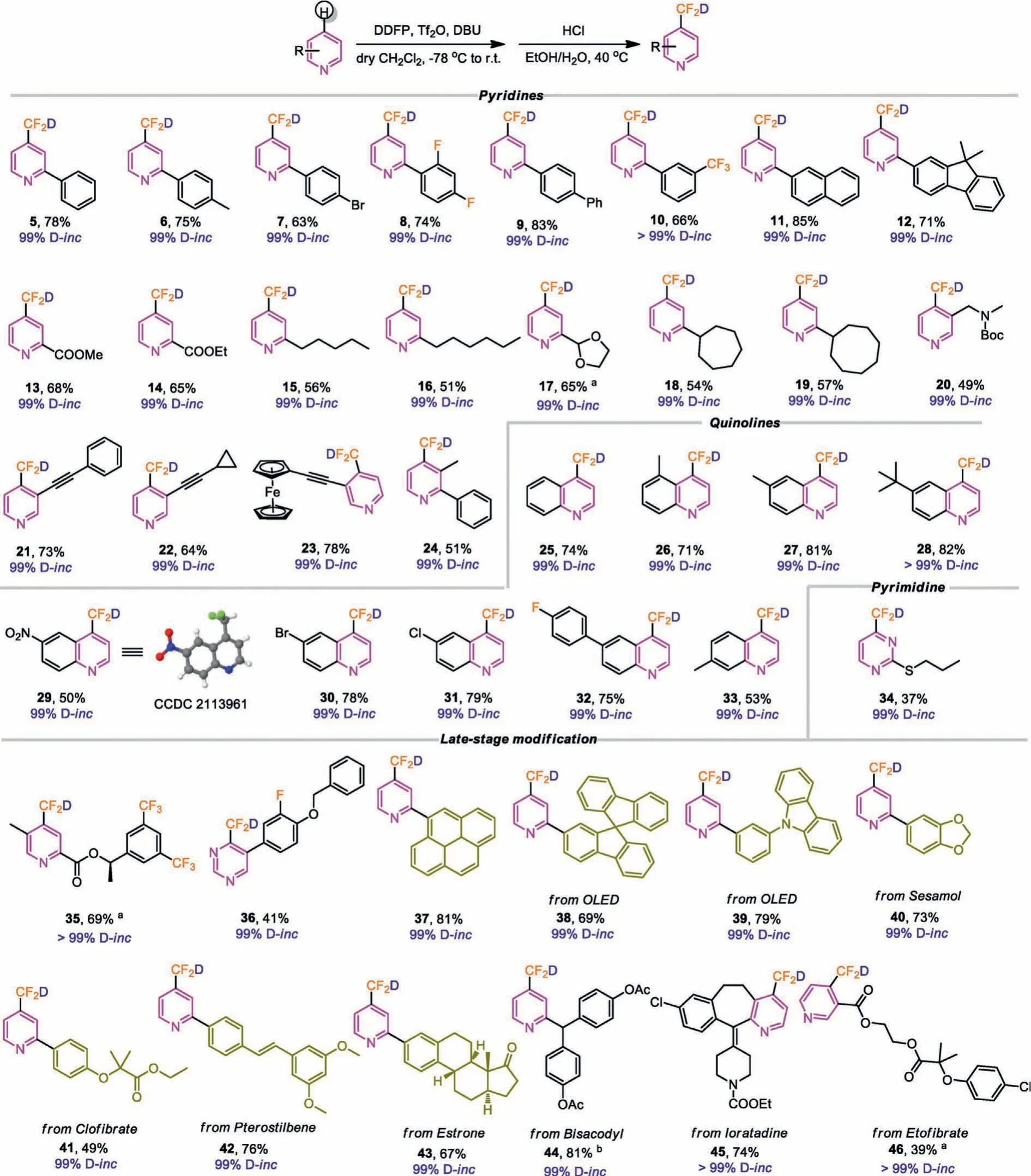
Scheme 4 .Reaction conditions: azines (0.5 mmol, 1.0 equiv.), DDFP (0.55 mmol, 1.1 equiv.), Tf2O (0.5 mmol, 1.0 equiv.), DBU (0.5 mmol, 1.0 equiv.), 4 mol/L HCl in dioxane(0.5 mmol, 1.0 equiv.), 40 °C, under Ar.Isolated yield, the D-inc was calculated based on the 19F NMR.a4 mol/L HCl in dioxane (0.5 mmol, 1.0 equiv.), TBAF (0.5 mmol, 1.0 equiv.), 60 °C.b4 mol/L HCl in dioxane (0.5 mmol, 1.0 equiv.), TBAF (0.5 mmol, 1.0 equiv.), 40 °C.
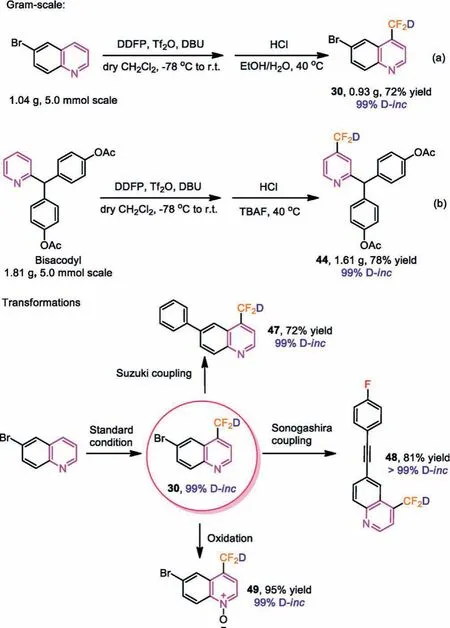
Scheme 5 .Gram-scale preparation and transformations of compound 30.
In summary, we developed a bench-stable reagent (DDFP) for deuteriodifluoromethylation, whose preparation used D2O as a cheap and convenient deuterium source.This reagent is suitable for introduction of deuteriodifluoromethyl group onto a variety of substituted azines and tolerates a wide range of functional groups.The deuterium incorporation of the products is consistently high(>99%).This reagent also can be used to introduce CF2D to complex azines such as drug-like intermediates, materials, drugs and natural products, providing a unique and powerful strategy for drug discovery and modification.We expect that our reagent to find applications in synthesis of isotope-labelled molecules of interests for drug-discovery and related ilucidation of mechanism of action.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
This work was financial supported by Innovation Program of Shanghai Municipal Education Commission (No.201701070002E00037).This work was also supported by National Key Research Program of China (No.2017YFD0200505) and the Fundamental Research Funds for the Central Universities.We thank Prof.Youjun Yang for the help of revised the manuscript.The authors thank Research Center of Analysis and Test of East China University of Science and Technology for the characterization of the help on HRMS analysis.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.085.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
