铜基串联催化剂电催化CO2还原的研究进展
石永霞,侯曼,李俊俊,李丽,张志成
天津大学理学院化学系,天津市分子光电科学重点实验室,天津 300072
1 Introduction
Since the industrial revolution, the combustion of fossil fuels and other human activities have caused massive emissions of CO2gas. The concentration of CO2has reached 0.042099% in May 2022, which is higher than the safe limit of 0.035%1–3.After CO2in the atmosphere absorbs ground radiation, it will radiate longer wavelength radiation to the ground, which has a thermal insulation effect on the ground4–6, increasing the greenhouse effect and leading to global warming7–12. Against this grim backdrop, there is an urgent need to develop and utilize renewable clean energy to contribute to the goal of carbon neutrality. Electrochemical CO2reduction reaction (CO2RR) has been considered as an effective approach to obtain high valueadded chemicals and fuels, which can store intermittent renewable energy and realize an artificial closed carbon cycle,reducing the use of fossil energy such as coal, oil, and natural gas to a certain extent13–17. Meanwhile, due to the mild reaction conditions, tunable product selectivity, simple and easy operation of the device, electrochemical CO2RR has received considerable interests18–20.
Currently, Cu is the only metal in the periodic table that can efficiently reduce CO2to hydrocarbons and oxygenates, which was firstly demonstrated by Hori21. However, the poor stability,low product selectivity, and high overpotential of pure Cu hinder the production of industrial-grade multi-carbon products22,23.Thus, various strategies have been developed to enhance the catalytic performance of Cu, such as crystal facet24–28,morphology29–31and size32–36regulation; oxidation state regulation37–42; ligand modification43–45; surface doping46,47;defect engineering48–53and interface engineering54–57, etc.Furthermore, electrochemical CO2RR involves multiple electron-proton transfer steps in which intermediates and reaction pathways also intersect, resulting in an extremely complex mechanism58,59. Currently, there are no catalysts that can directly reduce CO2to ideal fuels or high value-added chemicals60, instead further reduction of*CO intermediate is usually required to generate multi-carbon products with high efficiency. After extensive research in the past, it is widely believed that*CO is a key intermediate in the electrochemical CO2RR61–65. Cu has suitable adsorption energy for*CO and can further reduce*CO to multi-carbon products66. It is widely believed that the higher the coverage of*CO on the surface of Cu can facilitate the formation of C2+products. For example,Kanan and co-workers67reported that in CO-saturated alkaline aqueous solution, oxide-derived Cu electrodes can catalyze the production of multi-carbon products with the Faraday efficiency(FE) as high as 57% at a medium potential. This investigation also revealed that the activity and selectivity of the catalyst towards multi-carbon products can be improved by increasing the coverage of*CO on the Cu surface. Gao et al.68revealed by operando Raman spectroscopy that in CuAg bimetallic catalysts,CO intermediates are formed at Ag sites, 95% of which would escape to Cu nanowires for further hydrogenation to multicarbon products. In view of this, a highly selective catalyst for CO can be combined with Cu to achieve efficient and sequential tandem catalytic reduction of CO2.
Although there are many reaction steps in the tandem catalytic system, they can be carried out in an ordered manner. The intermediate products synthesized in the previous step can directly participate in the next reaction, which is very favorable in terms of thermodynamics69,70. In recent years, tandem catalysis has become a research hotspot for electrochemical CO2RR. To date, although several literatures have reviewed the wide application of tandem catalysis in the field of chemistry71–76,there is still a lack of comprehensive and systematical review on the application of Cu-based catalysts in electrochemical CO2RR.In this review, we first introduce the reaction routes and tandem mechanism of electrochemical CO2RR. Then, we systematically summarize the recent research progress of Cu-based tandem catalysts for electrochemical CO2RR, including Cu-based metallic materials (alloys, heterojunction, and core-shell structures) as well as Cu-based framework materials, carbon materials, and polymer-modified materials. Particularly, the preparation methods of various Cu-based tandem catalysts and their structure-activity relationship towards CO2RR are discussed in depth. Finally, the challenges and opportunities towards the rational design and controllable synthesis of advanced tandem catalysts for electrochemical CO2RR are proposed.
2 Mechanism of electrochemical CO2RR
Mastering the mechanism of electrochemical CO2reduction is crucial for accurately improving the activity and selectivity of catalysts and obtaining highly reduced multi-carbon products.Current electrocatalysts cannot exhibit high activity in an extremely large potential window range, thus, the kinetic exploration of CO2reduction is still shallow77,78.
2.1 Reaction routes of electrochemical CO2RR
In the electrode reaction, different numbers of electrons are transferred, and the CO2at the cathode can be converted into different products. Involving 2, 4, 6, 8, 12 electrons, CO2can be reduced to high value-added chemicals and fuels such as C1products (e.g., CO, CH4, CH3OH, HCHO, HCOOH), C2products (e.g., C2H4, C2H6, C2H5OH, CH3CHO, CH3COOH) and C3products (e.g., propionaldehyde, n-propanol)79,80. The relevant half-reactions in the aqueous solution are as follows:
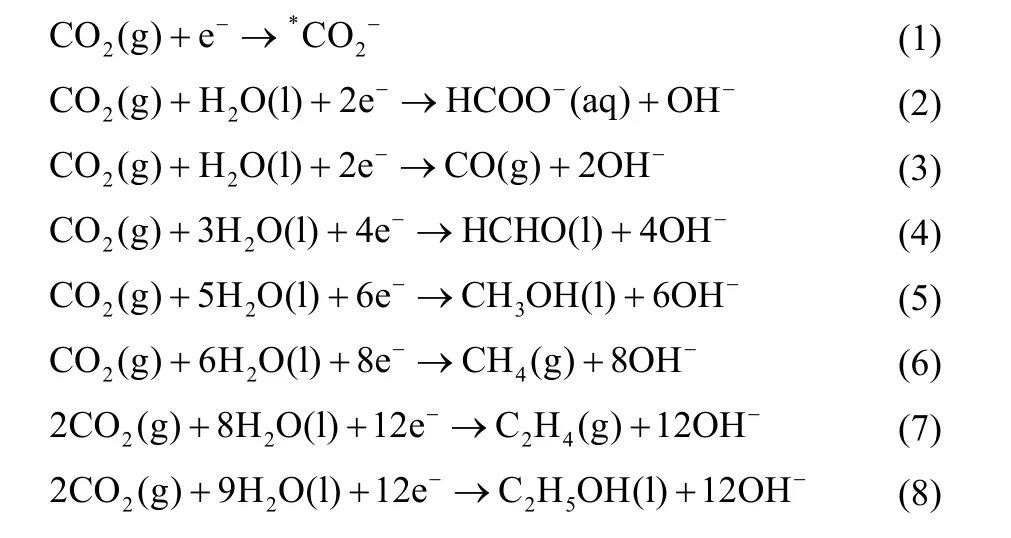
Electrode process is a continuous reaction with multiple basic processes, in order: liquid mass transfer, adsorption,electrochemical reaction, desorption, liquid mass transfer, which obeys the kinetic laws of general heterogeneous catalytic reactions81. The interface properties and area, mass transfer kinetics, and new phase formation kinetics all affect the reaction rate82. In electrochemical CO2RR, the adsorption strength of intermediates and the next reaction path are affected by many factors83, including the morphology and size of the catalyst, the composition and pH of electrolyte, etc. Birdja et al.84discussed the process of initial activation of CO2and C-C bond formation on electrocatalysts, showing that the intricate interactions between surface structure, electrolyte effects and mass transport conditions could affect CO2RR. They also summarized the reaction routes of electrochemical CO2RR to different products,as shown in Fig. 1. The first step in CO2RR is to activate the CO2molecules. By forming chemical bonds between CO2and the catalyst, electrocatalysts can stabilize*CO2−intermediates,resulting in a lower negative redox potential. Therefore, the selection of a suitable electrocatalyst allows the reduction of CO2to CO or HCOOH at low overpotentials85,86.
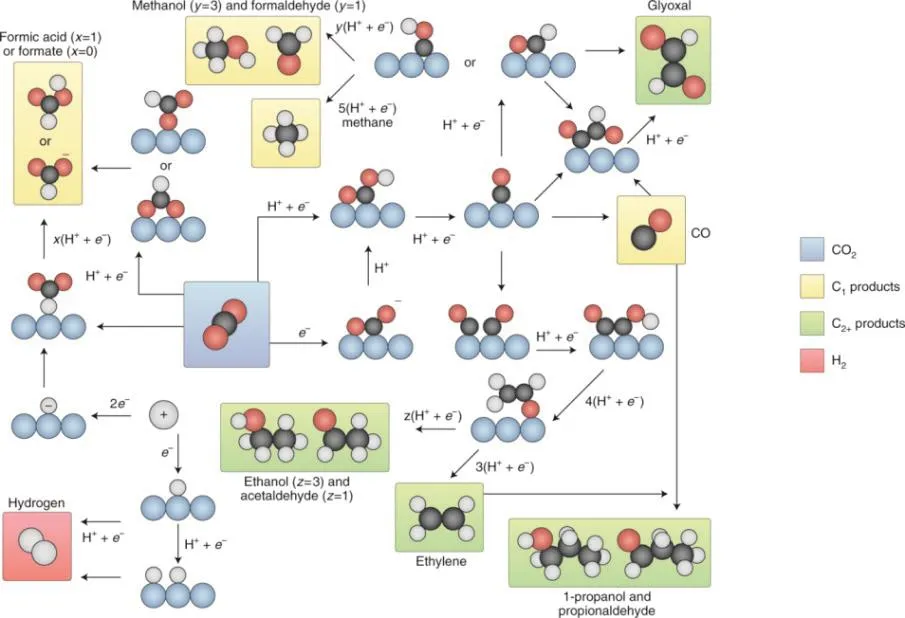
Fig. 1 Overview of the possible reaction pathways for CO2RR towards different products. Black spheres, carbon; white spheres,hydrogen; red spheres, oxygen; blue spheres, catalyst. The orange arrows indicate proton or electron transfer 84.
It is generally accepted in the literatures that the*COOH is the first intermediate for the formation of CO, while*OCHO is the intermediate for the production of HCOOH. Interestingly, in the field of molecular electrocatalysis, the initial activation of CO2seems to be viewed slightly differently.*CO2−intermediate,rather than*COOH, is the form of CO2activation.*CO2−is usually bound to the center of the metal catalyst. In addition, a similar mechanism was proposed on Au catalysts towards CO2RR87. On the Cu-based electrode, the CO2RR process can form one or more C-C bonds. For C2H4, there are two different production pathways: the first path requires a higher overpotential, and there is a shared intermediate with CH4pathway, which usually reacts on Cu(111) facets; the second path can proceed at a lower overpotential and produce none C1products, more inclined to Cu(100) facets. The Cu(100) pathway is widely considered to be preferable88. The CO dimer intermediate is critical in the C2pathway, and the more negative potential is unfavorable for CO dimerization. As an alternative reaction, the*CO and*CHO coupling pathway requires a lower activation energy22,89. Both experiments and theories suggest that the C2pathway is a pH-sensitive response and that alkaline electrolyte contributes to the production of C2species90. In addition to C2H4, the C2products obtained from the reduction of CO2catalyzed by Cu include C2H5OH and CH3CHO, with a shared intermediate*CH2CHO with C2H4pathway, which is further reduced by hydrogenation to obtain CH3CHO, followed by C2H5OH91. The structural sensitivity of the intermediate determines the type of C2products92,93. Furthermore, several C3products can also be observed on Cu during CO2RR, such as propanol and propionaldehyde. Among these, n-propanol can be produced on 0D Cu nanocrystal aggregates via C-C coupling between CO and the precursors of C2H494. However, relatively little research has been carried out on the mechanism of the formation of C3+products.
It has been widely demonstrated that*CO is a key reaction intermediate for electrochemical CO2RR. Moreover, following the formation of*CO, CO2reduction is believed to share similar pathways to those of electrochemical CO reduction reaction(CORR)95,96. In a customized electrochemical cell, Wang and colleagues78conducted a sensitive and precise study on the electrochemical reduction of CO by polycrystalline Cu, and drew the following conclusions: (a) the low solubility of CO in the electrolyte results in low activity on the surface of polycrystalline Cu; (b) the C2+products are always generated at a lower overpotential than the C1products, indicating that the C2+pathway and the C1pathway intersect and then separate at the initial stage; (c) when the overpotential is more negative,C2H4and C2H5OH come to be the main products, but their partial current densities gradually plateau due to the limitation of CO mass transfer; (d) the partial current density of aldehydes decreases when alcohol begins to form, which demonstrates that aldehydes may be further reduced to alcohol.
2.2 Mechanism of tandem catalysis
In electrochemical CO2RR, based on the multiple electronproton transfer steps of CO2reduction, in order to obtain highly reduced C2+products, a suitable adsorption energy for the intermediate*CO is needed for the catalysts97. The adsorption energy of Cu for the intermediate product*CO (ΔE*CO) is moderate, while the adsorption energy for*H (ΔE*H) is weak.Therefore, Cu can inhibit the hydrogen evolution reaction (HER)to a certain extent, and strengthen the hydrogenation of*CO intermediates or C-C coupling, which can be further reduced to obtain C2+products. In addition, the reduction of CO2to CO also requires a catalyst with suitable adsorption energy for the intermediate*COOH or*CO2−98–100.
It is difficult to control the adsorption strength of the two intermediates at the same catalyst site101. Designing tandem catalysts, artificially splitting CO2RR into two independent reactions: CO2→*CO,*CO→C2+, regulating the adsorption strengths of the intermediates separately on the two catalytically active sites, can achieve efficient conversion of CO2to multicarbon products. A simulated CO2RR mechanism on tandem catalysts is schematically illustrated in Fig. 2.
Recently, Zhang et al.102proposed a possible tandem reaction mechanism in CO2RR. The reduction process of CO2can be split into two consecutive steps. First, a highly selective catalyst (such as Au, Ag, Zn, etc.) is used as a substrate to reduce CO2to*CO,and then a catalyst (Cu) supported on the substrate is used to reduce*CO to the desired chemical product. The aforementioned two processes can be optimized separately. The calculation model is shown in Fig. 3a, where the surface along the direction from Ag (Au) to Cu is divided into eight regions. The researchers have progressively demonstrated the tandem mechanism. Firstly,they proved that the*CO generated from the substrate can spontaneously migrate to the Cu catalyst by comparing the binding energy of*CO of these eight regions (Fig. 3b). Then, the reduction of*CO on Ag-Cu surface was investigated theoretically. As can be seen in Fig. 3c, the hydrogenation of*CO to*CHO is the most difficult reaction in the reaction path from CO to CH4, but still with a ΔG≠< 0.75 eV. This result indicates that the whole process is thermodynamically downhill and has a very small free energy barrier, suggesting that it is also kinetically feasible. According to the guidance of computational chemistry conclusions, the researchers further verified the feasibility of this model through electrochemical experiments.Initially, the CO partial current of the Cu-Ag electrode increases with increasing potential, similar to that of pure Ag, while as the potential becomes more negative, the CO partial current drops sharply and at the same time, CH4starts to be produced in large quantities on the surface. The CO generated by the reduction of CO2by the substrate Ag is consumed and reduced to CH4by a large amount of Cu on the surface, and the tandem reaction mechanism has been proved. In addition, the researchers further confirmed the mechanism by operando attenuated total reflectance surface enhanced infrared absorption spectroscopic(operando ATR-SEIRAS) (Fig. 3d, e). At a lower potential, on the Ag-Cu surface of the composite, new bands appear at 2048 and 1847 cm–1, denoting the deconvoluted C≡O stretching band and the bridging CO band, respectively, which are absent in either bare Ag or bare Cu. This demonstrates the tandem mechanism spectroscopically and also shows that the tandem catalysts are not a simple superposition of two metals. This work clarifies the feasibility of the tandem reaction mechanism in the electrochemical CO2RR by designing density functional calculations and the corresponding experimental model,accompanied by operando ATR-SEIRAS for surface characterization. It provides new ideas for the research of reaction mechanism, catalyst design and reactor optimization in the field of CO2RR.
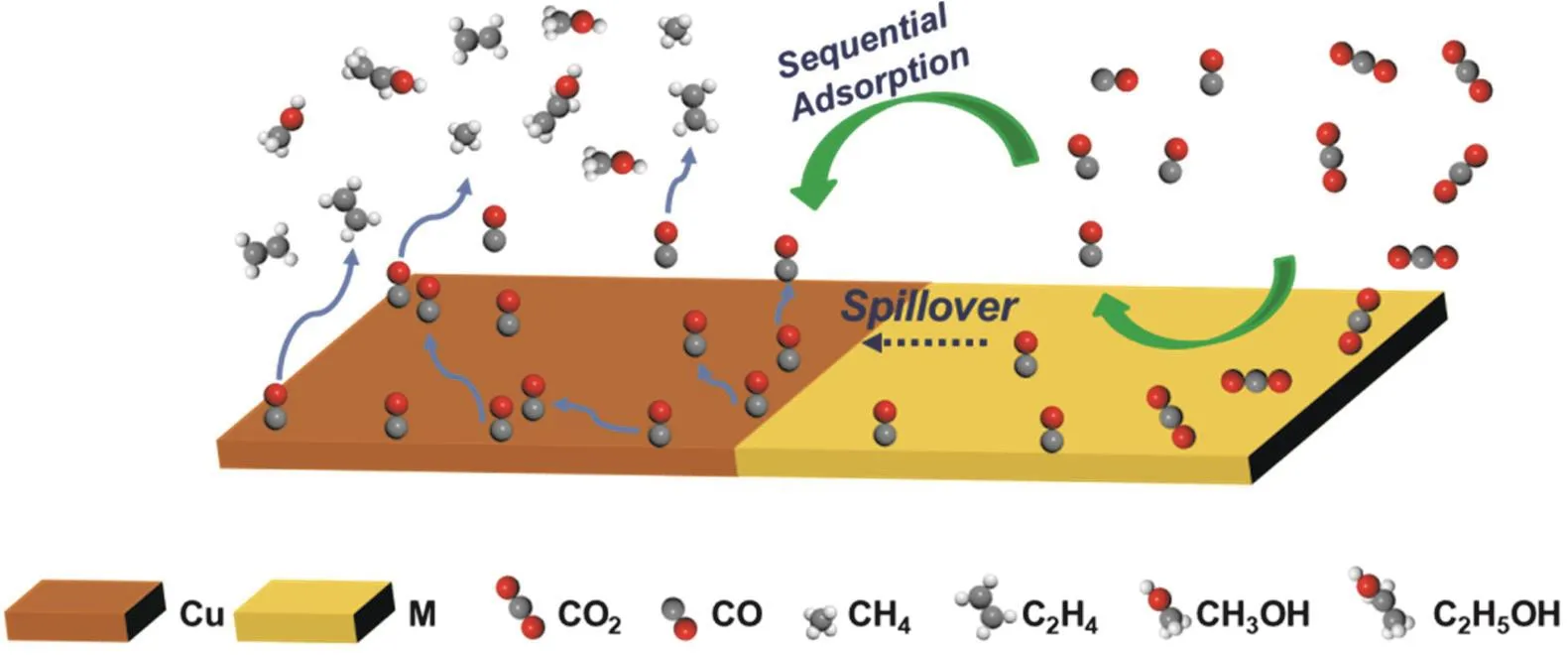
Fig. 2 Schematic illustration of the tandem mechanism towards CO2RR on Cu/M (M = Au, Ag, Zn, etc.) bimetallic catalysts.
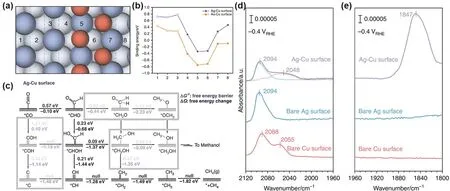
Fig. 3 (a) Top view of the calculation model (orange: Cu atoms; blue: top layer Ag or Au atoms; light blue: bottom layer Ag or Au atoms),the numbers represent the position of the binding sites; (b) binding energy for CO on Ag-Cu surface (blue) and Au-Cu surface (orange),hollow bullets indicate the sites nonadjacent to Cu surface; (c) kinetics and free energy diagrams of all possible pathways for the reduction of CO to C1 products on the Ag-Cu surface; (d, e) studies of CO adsorption on Cu, bare Ag and Ag-Cu thin films by Operando ATR-SEIRAS 102.
3 Cu-based tandem catalysts
Tandem catalysts, with multiple active sites, can sequentially reduce CO2molecules into high value-added chemicals and fuels. During tandem catalysis, the intermediate products synthesized in the previous step can be directly used as the reactant in the next step. Three basic principles should be followed in designing tandem catalysts. First, the arrangement order of two or more active sites of the catalyst is fixed, i.e., the reaction proceeds in a certain order; second, there should be a short distance between the active sites to ensure that the occurrence of tandem catalysis; third, the design of tandem catalysts should pay attention to the compatibility of different sites to avoid interactions between sites that interfere with tandem catalysis63. A series of recent studies have reported Cubased tandem catalysts in electrochemical CO2RR, including Cu-based metallic materials (alloys, heterojunction, and coresshell structures) as well as Cu-based framework materials,carbon materials, and polymer-modified materials.
3.1 Cu-based metallic materials
Metallic materials are widely used in electrochemical CO2RR due to the stable chemical properties. Until now, Cu has been considered as the only metallic material capable of electrochemically reducing CO2to hydrocarbons and oxygenates with appreciable efficiency. However, the high reduction overpotential and low product selectivity hinder the commercialization of this craft. Nørskov and co-workers101pointed out that Cu-based bimetallic catalysts can break this scaling relationship to stabilize the intermediate*CO and lower the reduction overpotential. In addition, the catalytic activity and selectivity of different metals are specific. Combining different metals with Cu to form Cu-based metallic catalysts is an effective strategy to improve the performance of electrochemical CO2RR. Given that*CO intermediate is the key to the production of C2+products, metallic catalysts with high CO selectivity, such as Ag, Au, Zn, etc., are usually combined with Cu to construct Cu-based metallic tandem catalysts103–107. According to the mixing patterns of metals, they can be divided into metallic alloys, metallic heterojunction, and metallic core-shell structures.
3.1.1 Metallic alloys
Alloying is an effective strategy for preparing supported catalysts. Different components of an alloyed catalyst interact and the properties of the original metal are affected due to the formation of an alloy with another metal. There are geometric effects and ligand effects of electronic interaction between different metals108. Meanwhile, the composition, electronic state and element distribution of metallic catalysts affect the binding energy of intermediates109. Another noteworthy point is that alloying can modulate the selectivity and catalytic activity of metallic catalysts. Bell and co-workers46pointed out that the alloy formed on the CuAg surface induced a compressive strain,which inhibited the HER and improved the selectivity of the multi-carbon products. To date, there have been many reports on metallic alloy catalysts, but relatively few with a tandem mechanism.
Among all the hydrocarbons and oxygenates, C2H5OH and C2H4are two C2products of great interest. However, compared with C2H4, the selectivity of C2H5OH is generally lower, which has been explained by density functional theory (DFT)calculations. CH2CHOads, a key intermediate in the formation of C2H5OH and C2H4, was indicated to have a 0.2 eV higher energy barrier when reduced to C2H5OH than that of C2H4110. To significantly improve the selectivity of C2H5OH, Yeo and coworkers111prepared Cu-based alloys by introducing different proportions of the Zn. The yield of C2H5OH at −1.05 V vs. RHE was maximized with the FE of 29.1% over Cu4Zn catalysts. A two-site mechanism was proposed to reveal the reduction process (Fig. 4a). CO2could first be reduced to CO by binding with Cu or Zn (1 → 2). Then, CO will be reduced to CHx(x = 1–3)on Cu sites, but on Zn sites, due to the weak adsorption, CO could desorb and spillover onto Cu sites (2 → 3). Subsequently,CO may intercalate into the bond between Cu and*CH2, forming*COCH2(3 → 4). Finally, after two-step in-depth reduction(4 → 6), the product C2H5OH could be obtained. This work has laid the foundation for the subsequent application of tandem catalysis in the field of electrochemical CO2RR.
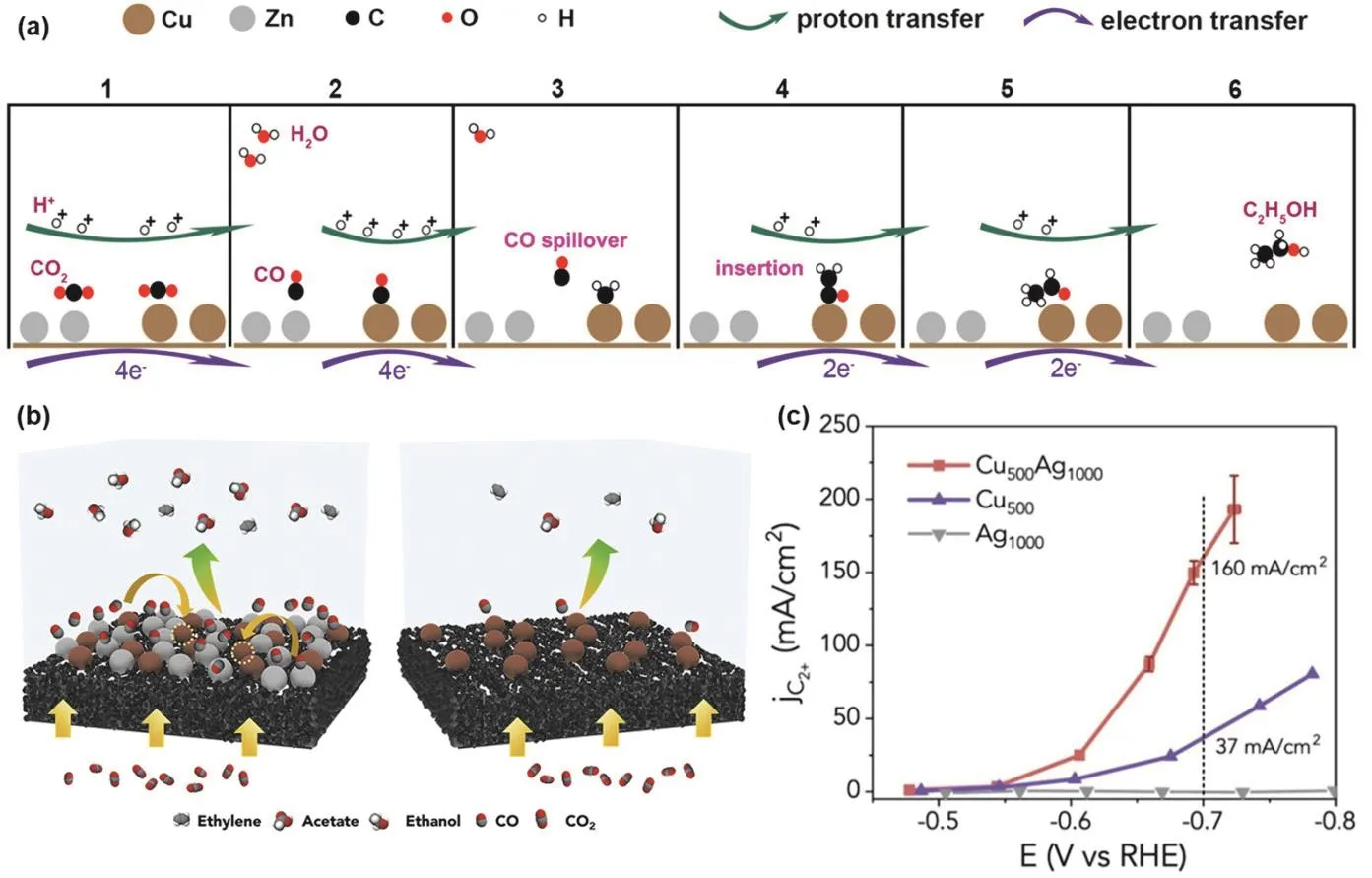
Fig. 4 (a) Mechanism of electrochemical CO2RR to C2H5OH over CuxZn 111; (b) scheme of the Cu-Ag tandem catalysts for high-rate electrochemical CO2RR and C2+ products formation; (c) partial current density towards C2+ products on Cu500Ag1000, Cu500, and Ag1000 catalysts 117.
Very recently, tandem catalysts have been successfully constructed and applied to multi-step reactions for the reduction of CO2. However, their practicality is still questionable due to the mass transfer limitations of CO2. In addition, the microenvironment around the catalyst may change drastically during high speed electrolysis. Gas diffusion electrodes (GDEs)can effectively solve the mass transfer kinetics by constructing a three-phase interface112–116. Therefore, there is a strong need to evaluate the performance of tandem catalysts in the GDE flow cell. Yang and co-workers117reported the high-speed catalytic behavior of Cu-Ag tandem catalysts in GDE (Fig. 4b). The authors explored the influence of local gas on the C2+partial current on the surface of Cu, finding that the normalized intrinsic activity of Cu500Ag1000towards C2H4and C2H5OH in CO2RR was much higher than that of Cu500in CORR. Therefore, the local environment created by tandem catalysts is more favorable for C2+generation than CORR on Cu alone. They proposed that Ag could not only serve as the supply of CO, but also promote the formation of C2+on Cu. The partial current density of Cu-Ag tandem catalysts for C2+products is significantly higher than that of Cu elemental catalyst. In 1 mol·L−1KOH electrolyte at −0.70 V vs. RHE, the partial current density of C2+products increases from 37 to 160 mA·cm−2after adding Ag to Cu to construct a tandem catalyst (Fig. 4c), the conversion rate was increased by a factor of 4, revealing a new catalytic mechanism existing in the three-phase series system.
3.1.2 Metallic heterojunction
In metallic heterojunction, there are clear interfaces and the different arrangement of energy bands on both sides of the interface leads to charge transfer at the interface118. The bonds and interactions of different components enhance the electron transfer rate, which is beneficial to adjust the charge-related properties of the heterojunction119. In addition, the heterojunction can also induce tensile or compressive lattice strain, which affects the adsorption strength of the catalytic sites for intermediates and enhances the catalytic activity120. For heterojunctions as catalysts, the existence of the interface can not only improve the selectivity121, but also enhance the activity of the catalyst and reduce the amount of precious metals, which has good economic benefits122. The unique catalytic sites at the interface are very helpful for the construction of tandem catalysts, which have attracted extensive attention in the field of electrochemical CO2RR123,124.
Morales-Guio et al.125reported for the first time an Au/Cu bimetallic catalyst with a tandem structure, which was synthesized by depositing Au nanoparticles on a flat polycrystalline Cu foil via physical vapor deposition. At low overpotentials, Au nanoparticles acted synergistically with Cu foil to reduce CO2to C2+products at rates more than two orders of magnitude higher than Cu or Au alone. Comparing the reduction rate of CO2and the precipitation rate of CO on the electrode surface, it was found that the desorption behavior of CO occurs on the surface of Au due to unfavorable thermodynamic adsorption. In contrast, CO can be more strongly combined with the surface of Cu to be further reduced. As anticipated, Au/Cu produced a moderate amount of CO, and nearly half of the reduction products escaped the electrode as CO, which also indicates that the CO generated on the Au surface was desorbed and migrated to the Cu surface (Fig. 5a).Benefiting from the strong adsorption behavior of the Cu surface, the CO was further coupled and reduced to multi-carbon products. To further investigate the tandem catalytic mechanism of the system, the C-C coupling selectivity of Cu and Au/Cu was compared, the C2+/C1ratio of Au/Cu catalyst increases exponentially with the decreasing overpotential, indicating that the lower overpotential promotes the C-C coupling rather than the C1pathway. Moreover, the yield of C2+on the Au/Cu catalyst in Fig. 5b is obviously two orders of magnitude higher than that on the single metal Cu catalyst, while the yield of C1is similar,explaining that the enhanced activity and selectivity of tandem catalysts are due to an increase in the rate of C-C coupling rather than inhibition of the C1pathway.
In electrochemical CO2RR, the two components of the heterojunction can catalyze the reaction in an ordered tandem manner. Wang et al.126prepared heterojunction with exposed Ag/Cu interfaces by a method of sequential precipitation followed by electroreduction. Near the interface, components Ag and Cu relay electroreduction of CO2to C2H4. Under the catalysis of Ag, CO2is first reduced to*COOH intermediate, and then reduced to*CO accompanied by the transfer of protons and electrons. Subsequently,*CO is captured by the adjacent Cu component and dimerized, and finally converted into hydrocarbons. The Ag/Cu interface contributes to this tandem catalytic system significantly, resulting in the FE of up to 42% towards C2H4. (Fig. 5c).
The interface of the heterojunction plays a key role in the tandem catalytic reaction, and the area of the interface also affects the catalytic performance. In order to explore the tandem mechanism of the interface at the nanoscale, Buonsanti and coworkers127synthesized Ag1-Cuxnanodimers with tunable size Cu domains by a seed growth method of colloid chemistry,exposing interfaces of different area sizes. The highest FE towards C2H4was achieved on the Ag1-Cu1.1nanodimers with similar sizes of Cu and Ag (Fig. 5d). In addition, the Ag 3d binding energy of the Ag/Cu nanodimers with different Cu domain sizes are all blue-shifted (Fig. 5e), indicating that there is an electron transfer phenomenon during the reaction process.Since Cu has a higher electron chemical potential than Ag, Ag domains act as electron acceptors for Cu domains, which is also thermodynamically favorable128–130. The electron-deficient Cu enhances the binding ability of the surface to CO and promotes the coupling of CO to generate C2H4. Furthermore, Cu has been shown to have an optimal value for electron density which maximizes the production of C2H447, which also explains the interface-dependent FE observed on three different nanodimers.
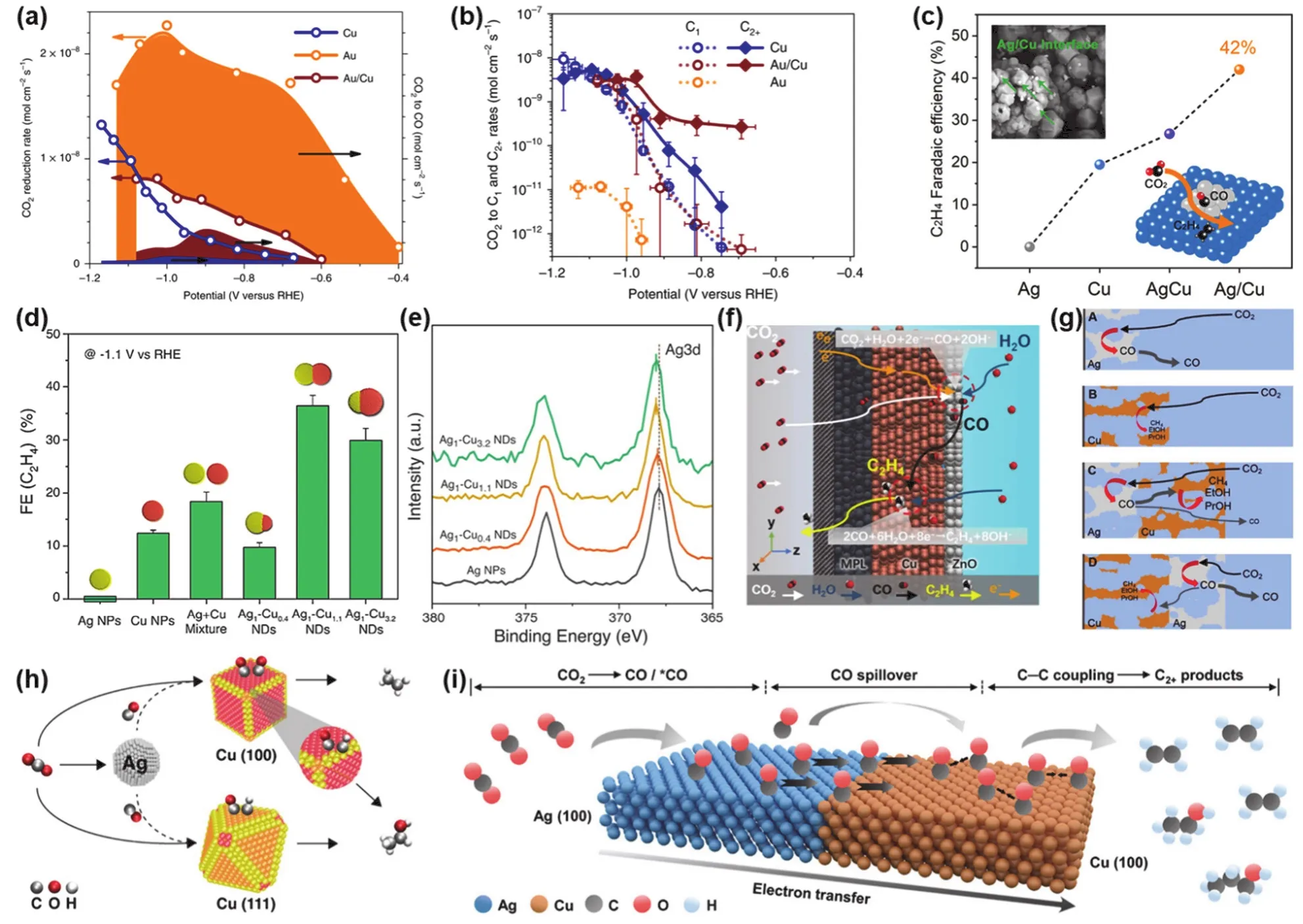
Fig. 5 (a) CO2 reduction and CO production rates as a function of the potential; (b) reduction rates of CO2 to C1 and C2+ products on Cu, Au, and Au/Cu electrodes 125; (c) Ag/Cu interface for relay electrochemical CO2RR to C2H4 126; (d) FE of C2H4 over different Ag/Cu catalysts at −1.1 V vs. RHE; (e) XPS spectra of different Ag/Cu catalysts 127; (f) schematic diagram of the cascade process of CO2 → CO → C2+ on the tandem electrode of the layered structure 133; (g) eelectrochemical CO2RR on oxide-derived Ag, Cu, Cu on Ag, and Ag on Cu foam catalysts. Black arrows indicate mass transport of CO2 from the solution bulk; grey arrows indicate mass transport CO;red arrows indicate electrochemical reaction 134; (h) schematic illustration of electrochemical CO2RR over Cu-Ag tandem catalysts 139;(i) schematic illustration of a plausible CO2RR mechanism on Ag65-Cu35 JNS-100 140.
The C-C coupling kinetics over tandem catalysts could be enhanced by increasing the local concentration of CO intermediates, where the spatial transport of the supplied*CO directly affects the catalytic performance132. Inspired by the concentration distribution of reactants in a plug flow reactor, Wu and co-workers133designed a two-layer tandem GDEs by adding a ZnO catalyst layer to the top space of the Cu catalyst layer,aiming to improve the efficiency of CO utilization (Fig. 5f). The selectivity of the bilayer tandem catalyst for the C2+product was increased by a factor of 1.2 and 1.3 compared to pure Cu and the physically mixed electrode (Cu & ZnO), respectively. In addition, the orientation of the bimetallic arrangement also affects the spatial transport of*CO. Compared with bimetallic nanowire electrodes, bimetallic foam electrodes are kinetically favorable due to their open porous structure, larger surface area,and less resistance to mass transfer during CO2RR. There is an obvious interface separation between the two metal layers of the bimetallic foam electrodes, and the order of the catalysts can be artificially regulated during the preparation process to support the tandem catalysis reaction sequence. Proper sequence of bimetallic foam catalysts can result in higher selectivity for C2+products over C1products. Experimental studies have found that if Cu foam is placed on the top of Ag foam, the formation rate of multi-carbon products, especially C2H5OH and propanol,increases by 64%. Conversely, the reverse order significantly reduces the rate of oxygenate production (Fig. 5g). The origin of this difference lies in the different crystal orientation of the catalyst material and the influence of the proton-electron transfer in each unit step of the CO2RR134. This research opens up a new era in the establishment of tandem catalysis using foam electrodes at the mesoscale range.
In electrochemical CO2RR, different crystal facets of the catalyst also affect the selectivity of the products. The facetdependent selectivity of CO2RR was first reported by Hori and co-workers135in 1995. It has been proven that Cu(100) facet is more conducive to the production of C2+products due to its lower C-C coupling energy barrier, while Cu(111) facet tends to generate C1products136–138. Therefore, when designing an advanced tandem catalyst, the effect of facets should also be considered. Recently, Buonsanti and co-workers139studied the effect of facets on tandem catalysis. They introduced Ag nanoparticles into Cu catalysts exposing different facets to form a tandem catalyst. This effect is further enhanced by tandem catalysis based on the selectivity of different facets to products.Particularly, the Cu nano-octahedrons with exposed (111) facets exhibit remarkable selectivity for CH4, containing a large amount of CHx*intermediates, and Ag in the tandem structure can provide high concentrations of*CO. Thus C2H5OH is further generated by CHx*-*CO coupling (Fig. 5h). In addition, the selectivity of the product C2H4can likewise be enhanced by the combination of the facet effect and tandem catalysis. Ma et al.140synthesized three kinds of Ag-Cu nanostructures with (100)facets. By controlling the surfactant and reduction kinetics of the Cu precursor, the authors achieved restricted growth of Cu with(100) facets on Ag. The schematic illustration for the synthesis is shown in Fig. 5i. Compared to bare Cu, Ag65-Cu35Janus nanocrystals (JNS)-100 could exhibit higher selectivity for C2H4and multi-carbon products at more negative potentials. DFT calculations reveal that the compensating electronic structure and CO spillover in Ag65-Cu35JNS-100 contribute to the CO2RR performance. This study would provide an effective strategy for the design of advanced tandem catalysts for a wide range of CO2RR applications.
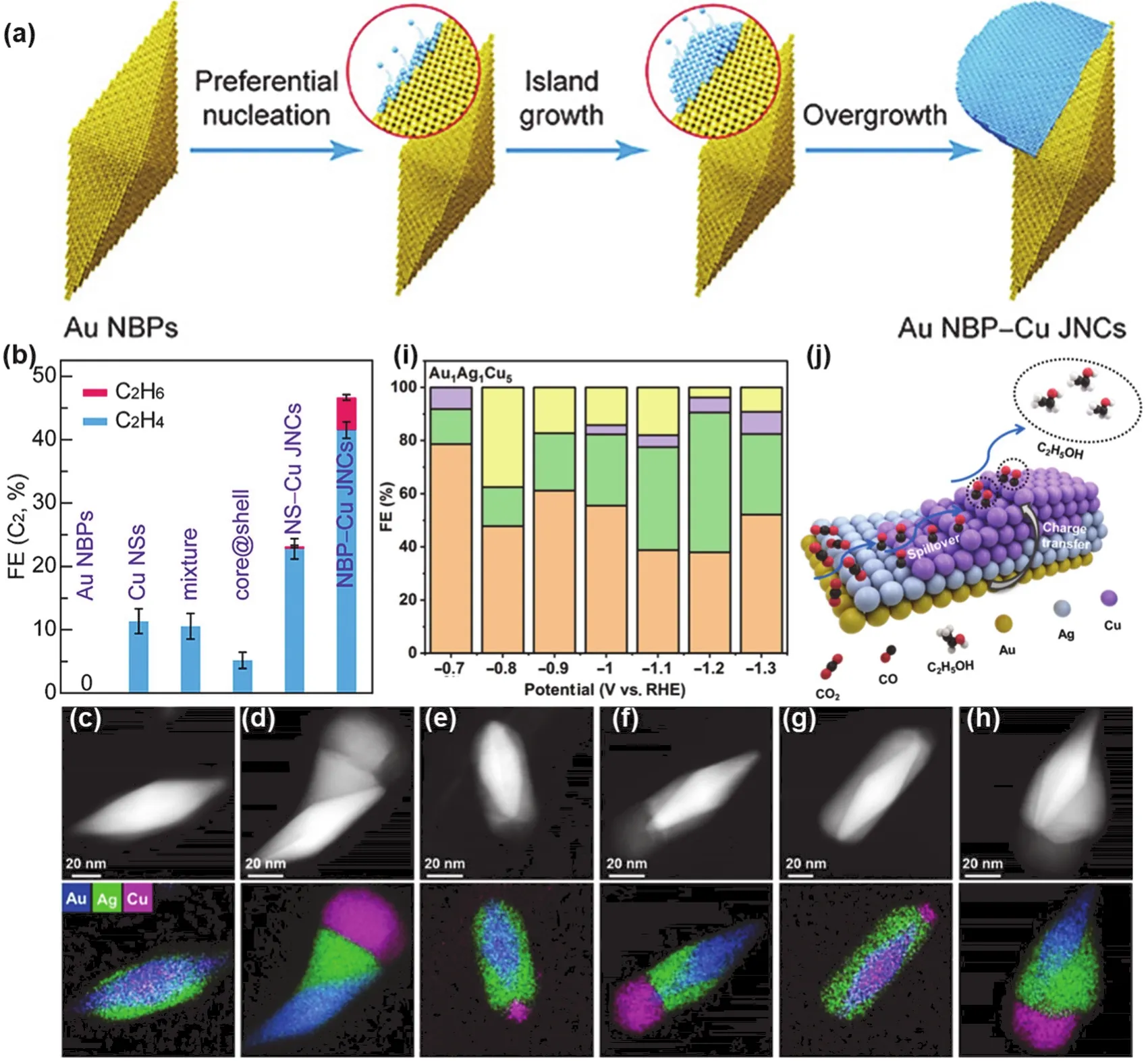
Fig. 6 (a) Schematic diagram of selective growth process of Au NBP-Cu heterojunction; (b) optimal FE of C2 over different catalysts 142;(c–h) HAADF-STEM images of (c) Au1Ag1Cu1 NSs, (d) Au1Ag1Cu5 NSs, (e) Au1Ag2Cu1 NSs, (f) Au1Ag2Cu5 NSs, (g) Au1Ag3Cu1 NSs,(h) Au1Ag3Cu5 NSs, and corresponding EDX elemental maps of Au (blue), Ag (green), and Cu (pink); (i) FE of the CO2RR products over Au1Ag1Cu5 NSs at a potential range from −0.7 to −1.3 V vs. RHE, Orange, H2; Green, CO; Purple, HCOOH; Yellow, C2H5OH; Blue, CH3COOH;(j) schematic illustration of the tandem mechanism for electrochemical CO2RR on asymmetric AuAgCu NSs 143.
Metallic heterojunction is not always a symmetrical system,and asymmetrical structures often occur due to the loss of symmetry elements, with a spontaneous reduction in the degree of symmetry. Any symmetry must have a symmetry break, which includes both “spontaneous symmetry break” and “kinetic symmetry break” scenarios141. Based on the large lattice mismatch between Au and Cu, Jia et al.142synthesized Au/Cu JNS via a seed-mediated growth method by using Au nanobipyramids (Au NBPs) as the seeds (Fig. 6a). During the overgrowth of Cu on Au NBPs, the concentration of surfactant plays a key role and determines the morphology of the Au-Cu nanostructures. Appropriately low concentrations of CTAB and HDA contribute to the site-selective growth of Cu on Au NBPs.This asymmetric space-separated structure significantly promotes the generation of C2hydrocarbons in electrochemical CO2RR with the FE 4.1 times higher than that of single metal Cu(Fig. 6b). The Au NBPs expose ten high-index facets (116) with abundant atomic steps as active centers for COOH*intermediates. A direct contact interface is formed between Au and Cu, which is also a key factor to improve the selectivity of C2products. The excellent CO2adsorption and activation ability of Au promotes the generation of CO, the electron-poor Cu surface has the high adsorption energy for CO, and the CO-CO coupling promotes the generation of C2products. In addition to the above-mentioned binary asymmetric heterojunction, very recently, Zhu et al.143reported the ternary asymmetric metallic nanostructures (NSs). By a multi-step seed-mediated growth method, the authors synthesized six AuAgCu tandem catalysts with different morphology and compositions, which were characterized by high-angle annular dark-field scanning TEM(HAADF-STEM) images and corresponding energy dispersive X-ray (EDX) elemental maps in Fig. 6c–h. It can be seen that with the increase of Cu domains, Cu can be selectively deposited on one end of the pre-synthesized Au@Ag core-shell nanorods,and Ag also undergoes surface reconstruction, resulting in asymmetric trimetallic AuAgCu NSs (Au1Au1Cu5, Au1Ag2Cu5,Au1Ag3Cu5). The unique heterogeneous structure can effectively modulate the selectivity of C2products in CO2RR, especially at a potential of −0.8 V vs. RHE, where the FE of Au1Ag1Cu5NSs for C2H5OH was up to 37.5% (Fig. 6i). The authors attribute this increased selectivity to electronic effects and tandem catalysis,with the tandem mechanism shown in Fig. 6j.
Bi-/tri-metallic heterojunctions have shared interfaces and distinct surfaces that participate in catalytic reactions and play different roles. There are unique active sites at the interface,where the tandem catalysis in CO2RR can be realized and the reaction efficiency can be improved.
3.1.3 Metallic core-shell structures
输电铁塔属于格构式塔架结构,在风载荷或地震载荷作用下,塔架结构中的主材主要受剪力、弯矩和扭矩作用,斜材承受轴力、剪力、弯矩和扭矩,辅材主要受轴向的拉压力.因此,建立铁塔有限元模型时,将主材和斜材简化成梁结构,辅材简化成桁架结构最为合适[16].图2所示为耐张塔的局部结构.为了与实际相吻合,塔架模型的主材和斜材使用梁单元T3D2,辅材使用杆单元B31.
The metallic core-shell structures can be divided into solid core-shell structures and heterogeneous hollow structures,wherein the heterogeneous hollow structures includes yolk-shell structures and interlayer heterogeneous hollow-shell structures144.The bimetallic core-shell structures formed by two metals with different activity and selectivity have an important influence in the field of electrochemical CO2RR due to the short diffusion path, high active surface, low internal resistance and excellent stability. In addition, the core material and shell material can synergistically improve the catalytic performance, which is very suitable for tandem catalysis. In particular, the interlayer heterogeneous hollow multi-shell structures are considered to have great application potential in heterogeneous tandem catalysis145.
Rodriguez and co-workers146synthesized Au@Cu core-shell nanoparticles with a well-defined surface area structure in 2015 for electrochemical CO2RR, pointing out that the thickness of the shell layer affects the activity and selectivity of the catalyst.Later, Zhang et al.147prepared Cu@Ag core-shell tandem catalysts with different Ag shell thicknesses (10.7, 11.2, 11.8,12.2 nm) by a two-step reduction method. The core-shell structure can be clearly seen in the energy dispersive spectroscopy (EDS) elemental mapping image of Cu@Ag-2 (Ag shell thickness of 11.2 nm) as an example (Fig. 7a). In the electrochemical CO2RR performance test, the Cu@Ag coreshell structure with different Ag shell thicknesses has different selectivity of C2H4. Cu@Ag-2 catalyst has the highest FE for C2H4, as shown in Fig. 7b. There is a synergistic effect between the Ag shell and the Cu core. As shown in the catalytic schematic diagram (Fig. 7c), CO2is initially reduced to CO in the Ag shell,and further C-C coupling is carried out on the Cu core. The Ag shell that is too thin will not provide enough active sites to produce CO intermediates, and too thick will prevent the CO intermediates from migrating to the Cu core surface. Therefore,Cu@Ag-2 with appropriate Ag shell thickness exhibits the best catalytic performance. The synergistic effect of the core and shell enhances the bond strength of the*CO intermediate at the interface, promotes electron transfer and increases the electrochemical specific surface area, thereby improving the activity and selectivity of the catalyst.
In the bimetallic core-shell structures, in addition to the above study that the thickness of the shell layer affects the performance of the catalyst, the hollow size between the core and shell will also impact the activity and selectivity of the catalyst. Zhang et al.148developed Au@Cu2O yolk-shell bimetallic catalysts with different hollow size by hydrazine hydration reduction method(Fig. 7d). The active sites are located in the cavity, and the spatial confinement and a tandem catalysis mechanism result in the selectivity shifting from C1to C2. It is worth mentioning that the hollow size of the Cu2O nanocavity could affect the CO concentration in the cavity. Au@Cu2O with a mid-cavity size exhibits the highest current density while achieving the FE as high as 52.3% for C2H5OH at the potential of −0.3 V vs. RHE(Fig. 7e).
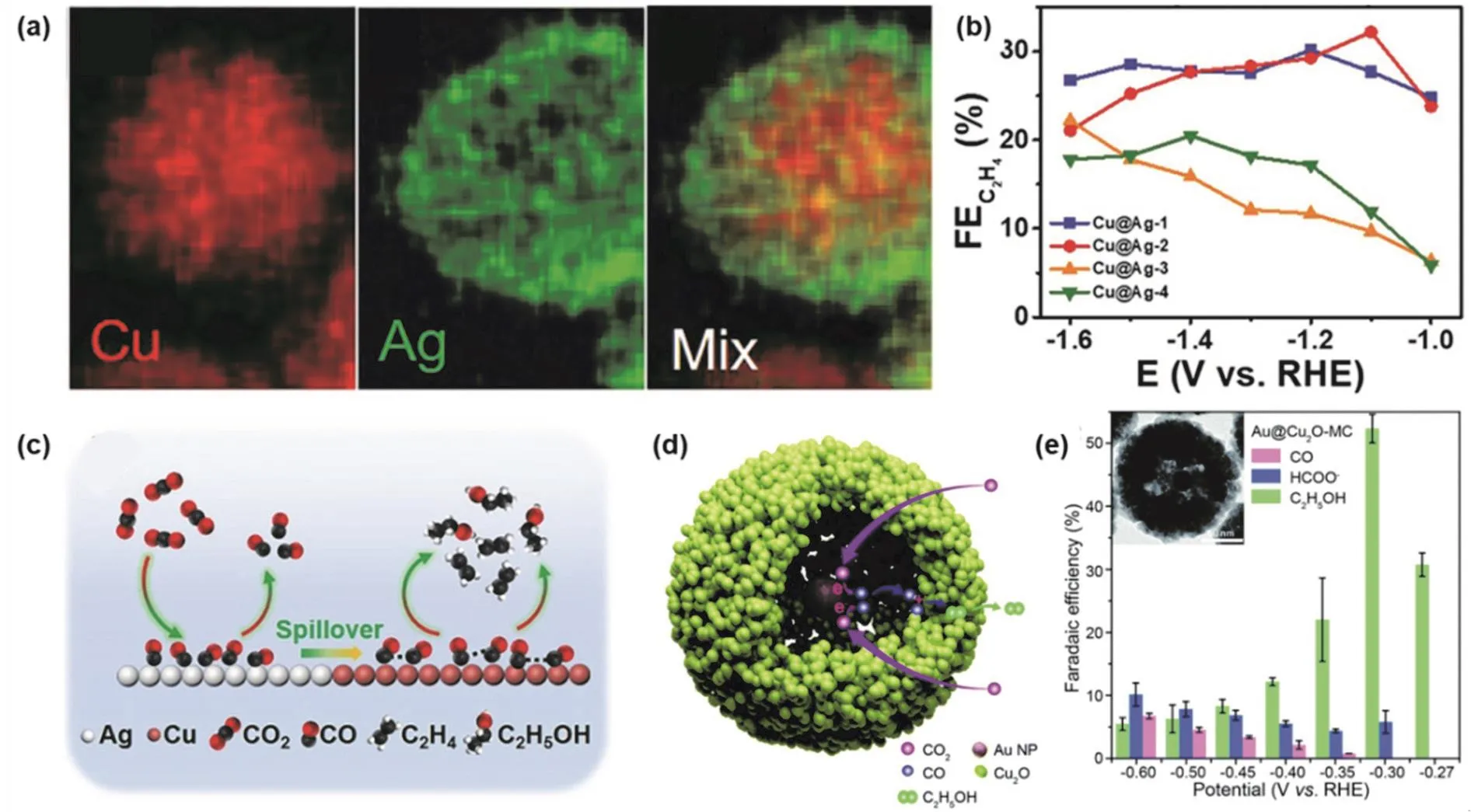
Fig. 7 (a) EDS elemental mappings images for Cu@Ag-2; (b) FE of C2H4 over different Cu@Ag structures; (c) schematic illustration of tandem catalysis for electrochemical CO2RR on Cu/Ag core-shell structure 147; (d) schematic illustration of tandem catalysis for electrochemical CO2RR in the cavity of Au@Cu2O; (e) FE of different products over different catalysts under a range of potentials 148.
Cu-Ag nanocrystals with diameters less than 10 nm have promising applications in the field of CO2RR, especially in tandem catalysis and selective generation of multi-carbon products. The Ag/Cu atomic ratio in core-shell catalysts greatly affects the product selectivity. Although the CO2RR product distribution is still a complex function of overpotential,electrolyte properties, etc., it is still found that a moderate atomic ratio of Ag/Cu composition can greatly improve the selectivity of multi-carbon products and promote the production of hydrocarbons149.
In CO2RR, the short diffusion path between the core and shell can facilitate tandem catalysis process. The core and shell in the core-shell structures cooperate with each other to enhance the bond strength of the*CO intermediate at the interface, promoting electron transfer, and improving the catalytic activity. When designing metallic core-shell catalysts, the influence factors such as shell thickness and hollow size should be considered to achieve further reduction of CO2with the optimal structure.
The above metallic tandem catalysts have contributed significantly to electrochemical CO2RR. Of course, the metallic structures are not limited to alloys, heterojunction, and core-shell structures. Zhang and co-workers150reported a nanoporous hollow-structured bimetallic tandem catalyst Au/CuO-CuO,prepared by annealing Au/CuC2O4/C, which exhibited remarkable selectivity for multi-carbon products, especially C2products with the FE of 52.8% at −1.0 V vs. RHE, the partial current density of 78.77 mA·cm–2at −1.5 V vs. RHE.Furthermore, in a wide potential window range (−1.0 – −1.5 V vs. RHE), the FEC2was stably kept at above 40%. The authors focused on not only the preparation of tandem catalysts, but also the conditions under which tandem catalysis occurs. According to the electrode potential and proton electron transfer resistance,the CO/*CO generation site (Au/CuO) and the CO/*CO reduction site (CuO) were well matched to ensure that there is sufficient CO intermediate at the Au/CuO site, further reduction was obtained at the CuO site. Similar works in the past have contributed to the electrochemical CO2RR. The research on bimetallic tandem catalysts is just at the initial stage, and more novel structures still need to be developed.
3.2 Cu-based framework materials
Organic framework materials are a new class of crystalline porous materials that exhibit excellent performance and broad application prospects in the field of electrocatalysis due to their large specific surface area, tunable pore size and structure151,152.
For example, metal-organic frameworks (MOFs) are organicinorganic hybrid materials composed of organic ligands and inorganic metals, which usually have a tunable topology and a large number of dispersed unsaturated metal centers inside the framework153–158. The porous structure of organic framework materials has a high ability to capture CO2, and they themselves can be used as catalysts to reduce CO2159. In order to improve the selectivity of C2+products, the rational design and controllable synthesis of Cu-based framework material to construct tandem catalysts are highly desired.
Most of the reported MOFs and COFs are insulators, and there are relatively few applications of conductive framework materials in CO2RR. Conductive Cu-based metal-organic frameworks (Cu-THQ) have a high density of square planar CuO4nodes. Zhao et al.160prepared Cu(111)@Cu-THQ tandem catalysts by immobilizing Cu(111) nanoparticles on Cu-THQ by electrochemical synthesis (Fig. 8a). This Cu-based MOF composite material has high electrical conductivity. The FE of C2H4catalyzed by Cu(111)@Cu-THQ is as high as 44.2 ± 3.4% at the potential of −1.2 V vs. RHE, which is much higher than many reported MOF-derived materials. In order to explore the mechanism, the author also carried out DFT calculations. As can be seen in Fig. 8b, the CO2→*COOH,*COOH →*CO pathways are thermodynamically robust at the CuO4site compared to the Cu(111) facet, and subsequently, the barrier for*CO →*CHO is 0.761 eV, while the potential barrier for*CO desorption is only 0.236 eV. Therefore,*CO is more easily desorbed rather than further reduced on the CuO4site of Cu-THQ. In addition,*CO dimerization on Cu(111) facet is thermodynamically favorable (Fig. 8c).
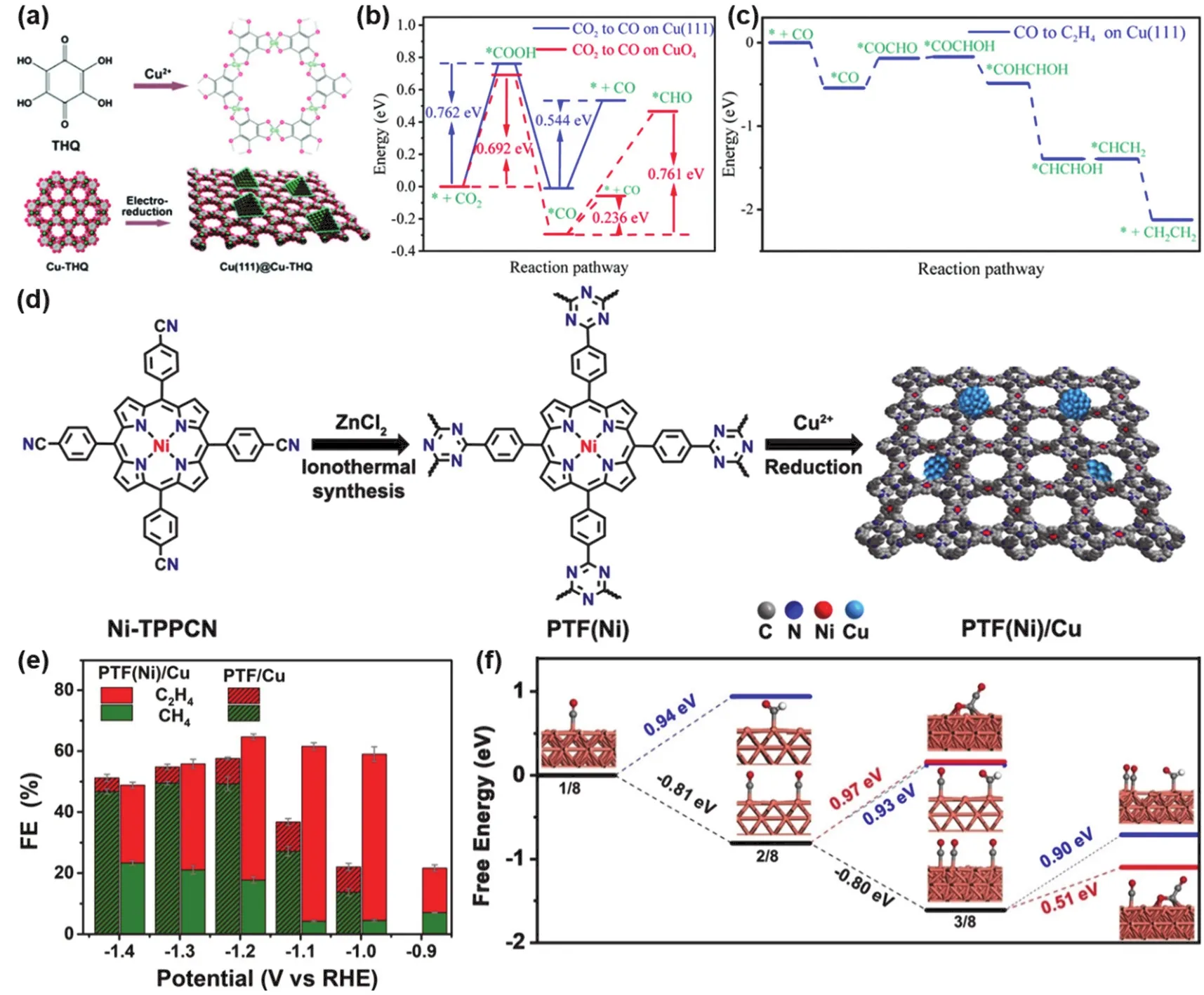
Fig. 8 (a) Illustration of the preparation of Cu(111)@Cu-THQ via electro-reduction; (b) the energy barriers for CO2RR to *CO and*CHO intermediates. Red line indicates on CuO4 sites and blue line indicates on Cu(111) sites; (c) corresponding energy barriers for CORR to C2H4 over pure Cu(111) lattice facets on Cu(111)@Cu-THQ 160; (d) schematic illustration of the preparation of PTF (Ni)/Cu with well-dispersed Cu sites from Ni-TPPCN; (e) FE of C2H4 and CH4 over PTF (Ni)/Cu and PTF/Cu catalysts at a range of potentials;(f) free energies for the formation of CH4 and C2H4 of the rate-determined step at U = 0 V 161.
Meng et al.161prepared PTF(Ni)/Cu tandem catalysts by uniformly dispersing Cu nanoparticles (NPs) on a porphyrin triazine framework anchoring isolated atomic Ni-N sites (Fig.8d). The highly porous structure of the porphyrin triazine framework is suitable for embedding Cu nanoparticles.PTF(Ni)/Cu exhibited high C2H4selectivity with an optimal FE of 57.3% (Fig. 8e). As can be seen from the free energy comparison (Fig. 8f), CO can be easily desorbed from PTF(Ni)and migrate to the nearby Cu(200) surface to form the*CO intermediate. In addition, the tandem catalyst effectively reduces the free energy of C2H4formation.
3.3 Cu-based carbon materials
Due to the good conductivity and electrochemical stability,carbon material has been a beneficial candidate in electrocatalysis162–168. The introduction of heteroatoms (e.g., B,N, P, S, etc.) into carbon materials can change the electronic state and thus affect the coordination structure of catalysts169.Therein, N-doped carbon materials have long been proven to efficiently reduce CO2to C1or even C2+products170–173. N-doped carbon material is a good support material. In CO2RR,defective N sites (e.g., pyridinic N and pyrrolic N) act as active sites that can reduce CO2to CO. The Cu-based N-doped carbon material tandem catalyst contains single-atom dispersed Cu-N-C active sites, which improves the catalytic activity of electrochemical CO2RR174.
In 2016, Rondinone and co-workers175found that the tandem catalyst of Cu nanoparticles supported on N-doped carbon nanospike films showed high selectivity for C2H5OH. It was found that there is a strong covalent bond between the OCCO intermediate and the Cu surface, which makes OCCO highly reduced to -CH3, while the oxygen atom on the other end of OCCO can bind to the less reactive CNS with an appropriate binding energy to prevent complete reduction and thus promote the formation of -CH2OH. This tandem effect promotes the formation of C2H5OH. In Cu-based carbon material tandem catalysts, Cu nanoparticle surfaces often act as active sites for*CO dimerization. Both the mass and size of Cu nanoparticles affect the catalyst selectivity. Sun and co-workers176found that the selectivity of the product C2H4was highest when the mass ratio of Cu nanoparticles to pyridine N-rich graphene (p-NG)support was 1 : 1 and the size of Cu nanoparticles was 7 nm.
In view of the fact that another component in the Cu-based tandem catalyst can provide sufficient CO intermediates to the Cu surface, Wang et al.177introduced CO molecules from the outside of the catalyst and reported the catalytic activity of Cubased catalysts for CO2/CO co-feed. As can be seen in Fig. 9a,the formation rate of C2H4is significantly increased when CO2/CO is co-fed. The co-feed is a designed internal self-feeding mixed tandem catalyst. To simulate this mechanism, the authors constructed a tandem catalyst by combining non-metallic NiNCs for CO production with CuOx, as shown in Fig. 9b.The equivalence of external and internal CO co-feeding using CuOx-NiNC tandem catalysts was demonstrated.
There is an interaction between metallic Cu and carbon materials in Cu-based carbon material tandem catalysts. Under the catalytic oxidation of metal Cu, C will oxidize and burn to form pores. The diffusion of Cu into the pores activates N to pyridinic N or pyrrolic N, which catalyzes the reaction. Lee et al.178reported a tandem catalyst consisting of N-doped C nanofibers surrounded by Cu nanoparticles. Fig. 9c shows the formation mechanism of Cu/N-CNF, a novel tandem catalyst with self-forming structure. CO is generated from the pyridine N active sites and escapes to Cu. The increased CO content on the Cu surface reduces the CO*dimerization barrier, FE of C2H4reaches 62% at the potential of −0.57 V vs. RHE (Fig. 9d). In this tandem catalyst, Cu promoted the combustion of C and the generation of pyridine N near the Cu surface, which promoted the generation of C2H4on the Cu surface in the electrochemical CO2RR.
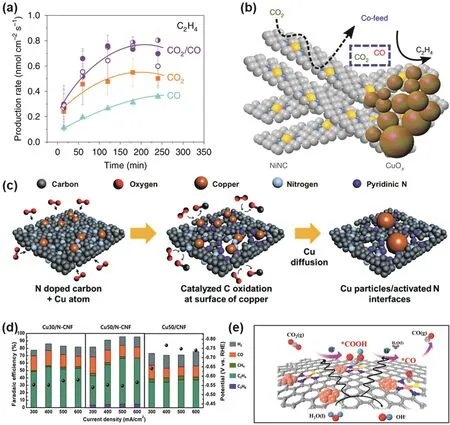
Fig. 9 (a) The formation rate for C2H4 as a function of time under different feeding modes; (b) schematic illustration of tandem catalysis for electrochemical CO2RR on CuOx-NiNC catalyst. Grey, C; Blue, N; Yellow, Ni 177; (c) schematic illustration of Cu particles/N rich C formation during calcination; (d) FE of different products and current density over Cu30/N-CNF,Cu50/N-CNF, and Cu50/CNF catalysts under a range of potentials 178; (e) the mechanism for the production of CO on Cu-S1N3/Cux via electrochemical CO2RR, Orange, Cu; Yellow, S; Blue, N; Red, O; Gray, C; light blue, H 180.
The carbon material can also serve as the substrate of the catalyst to assist the catalyzed reaction of the Cu-based tandem catalyst. Liu et al.179synthesized a Cu1.96S/Cu-N doped carbon nanofiber (Cu1.96S/Cu-NCNF) tandem catalyst by a combined electrospinning and calcination method, which exhibited high selectivity for CO in CO2reduction (FECO> 80%). Theoretical simulations show that Cu acts as a*CO production site in the tandem catalyst, and the adjacent Cu1.96S promotes the desorption of*CO to generate CO molecules, which is due to the covalent hybridization of S 3p and Cu 3d, 4s orbitals. As for why there is no further coupled reduction of CO, it may be due to the steric hindrance in the microporous structure. In addition, Chen et al.180synthesized a Cu-S1N3/Cuxtandem catalyst by a template method, which is a nanoflake-structured carbon-based material composed of N, S co-coordinated asymmetric singleatom Cu sites (Cu-S1N3) and atomically dispersed Cuxclusters.The catalyst exhibited extremely high selectivity to CO products in electrochemical CO2RR, with a FE up to 100%, which is because the Cu-S1N3atomic interface on the carbon-based plane is conducive to the adsorption of*COOH intermediates, and the adjacent Cuxclusters can accelerate the dissociation of H2O to provide abundant*H to the Cu-S1N3site (Fig. 9e). Such a tandem catalytic system improves the activity and selectivity of CO2RR.
3.4 Cu-based polymer-modified materials
Polymer modified Cu electrode is another effective method to improve the activity and selectivity of CO2RR. For instance,when poly (aniline) was coated on Cu nanoparticles, the FE of C2+hydrocarbons can even reach 80% and the FE of C2H4exceeds 40%181. Besides, the Nafion modification of Cu foil achieved a high FECH4of 88%182. Furthermore, the tricomponent copolymer modification of electrodes would not only increase the local concentration of CO2but also facilitate the diffusion of CO2, leading to a high FEC2+of 77%183.
Polymer-modified Cu can improve catalytic activity and selectivity, so that CO2can be reduced to obtain more C2+products. However, the corresponding current density of this strategy is too small to be suitable for industrial production. To promote the CO2RR performance of commercial GDEs at high current density, Duan et al.184recently reported a Cu-based organic-inorganic hybrid materials tandem catalyst Cu0@PIL@CuI(PIL: poly(ionic liquid)). Experiments combined with theoretical studies show that the interface of Cu0-PIL reduces CO2molecules, releasing a large amount of CO into the PIL layer. Thereafter, C-C coupling of CO intermediates occurs at the PIL-CuIinterface. The hierarchical architecture of such hybrids was confirmed by high-resolution transmission electron microscopy images (Fig. 10a). This tandem mechanism enhances the selectivity of C2+products. In addition, there is a weak intermolecular interaction between the PIL layer and the key intermediate*CO, which decreases the C-C coupling barrier and is beneficial to the production of C2+products. At−0.85 V vs. RHE, the FE of C2+is 76.1%, and the partial current density reaches 304.2 mA·cm−2(Fig. 10b).
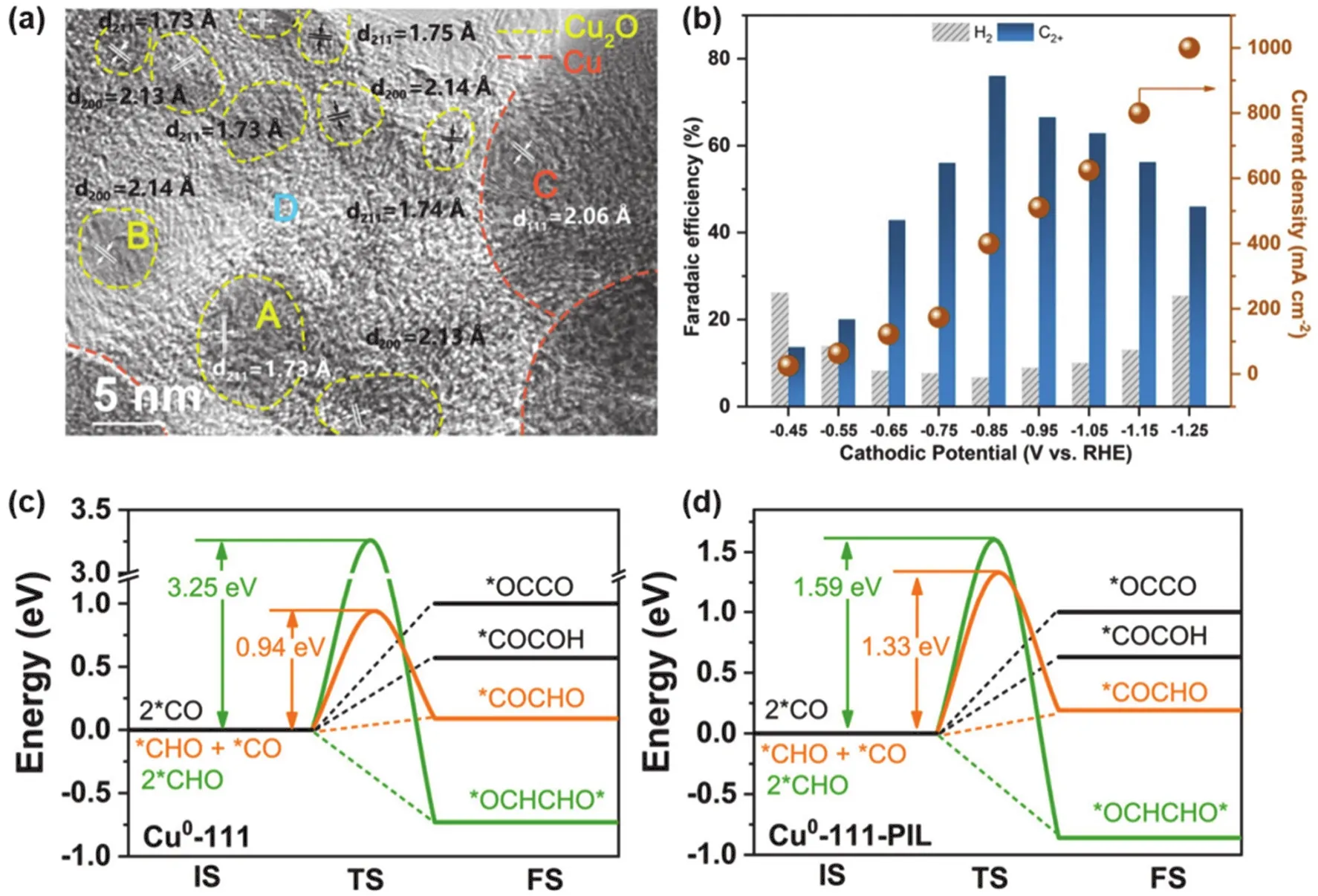
Fig. 10 (a) HR-TEM images of Cu0@PIL@CuI-5. 5 refers to the molar percentage of CuCl to Cu NPs for the synthesis. Yellow, Cu2O;Orange, Cu; (b) CO2RR performance of Cu0@PIL@CuI-5 under different cathodic potentials;(c, d) energy diagrams of C-C coupling on Cu0-111 and Cu0-111-PIL, respectively 184.
To elucidate the effect of PIL itself on electrochemicalCO2RR, the authors carried out a DFT study for Cu0-111 and Cu0-111-PIL models, respectively. On both catalysts, the reduction of CO2to*COOH is endothermic and the reduction of*COOH to*CO is exothermic. C-C coupling generally cannot be directly dimerized by*CO due to its high energy barrier, while the C2+product obtained from the*CHO coupling via the hydrogenation of*CO has a downhill energy, which is thermodynamically and kinetically favorable. In contrast to the coupling of*CO and*CHO to*COCHO, the homo-coupling of*CHO to*OCHCHO*is energetically favorable. As shown in Fig. 10c and Fig. 10d, in the presence of PIL, the activation energy of*OCHCHO*is significantly reduced from 3.25 to 1.59 eV. In conclusion, both theoretical calculations and experimental results indicate that in this tandem structure, PIL contributes significantly to the improved C2+product selectivity, which may be attributed to the interactions between PIL and intermediates(e.g., hydrogen bonding), the induced interfacial electric field,and compatible activity in the formation of*H.
4 Conclusion and perspective
Tandem catalysts, with multiple active sites, can sequentially convert CO2molecules into high value-added chemicals and fuels. Tandem catalysis has been developed as an effective strategy to improve CO2RR performance. In this review, the reaction routes and tandem mechanism of electrochemical CO2RR are firstly introduced. Then, the recent progress of Cubased tandem catalysts for electrochemical CO2RR is systematically summarized, which involves Cu-based metallic materials (including alloys, heterojunction, and core-shell structures) as well as Cu-based framework materials, carbon materials, and polymer-modified materials. The synthesis strategies and electrochemical CO2RR performance of some recently reported Cu-based tandem catalysts are summarized in Table 1. In particular, the preparation methods of various Cubased tandem catalysts and their structure-activity relationship towards CO2RR are discussed and analyzed in detail.Significantly, the performance of electrochemical CO2RR could be enhanced by tandem catalysis via tuning the adsorption energies and local concentration of intermediates, thereby achieving specific multi-carbon products.
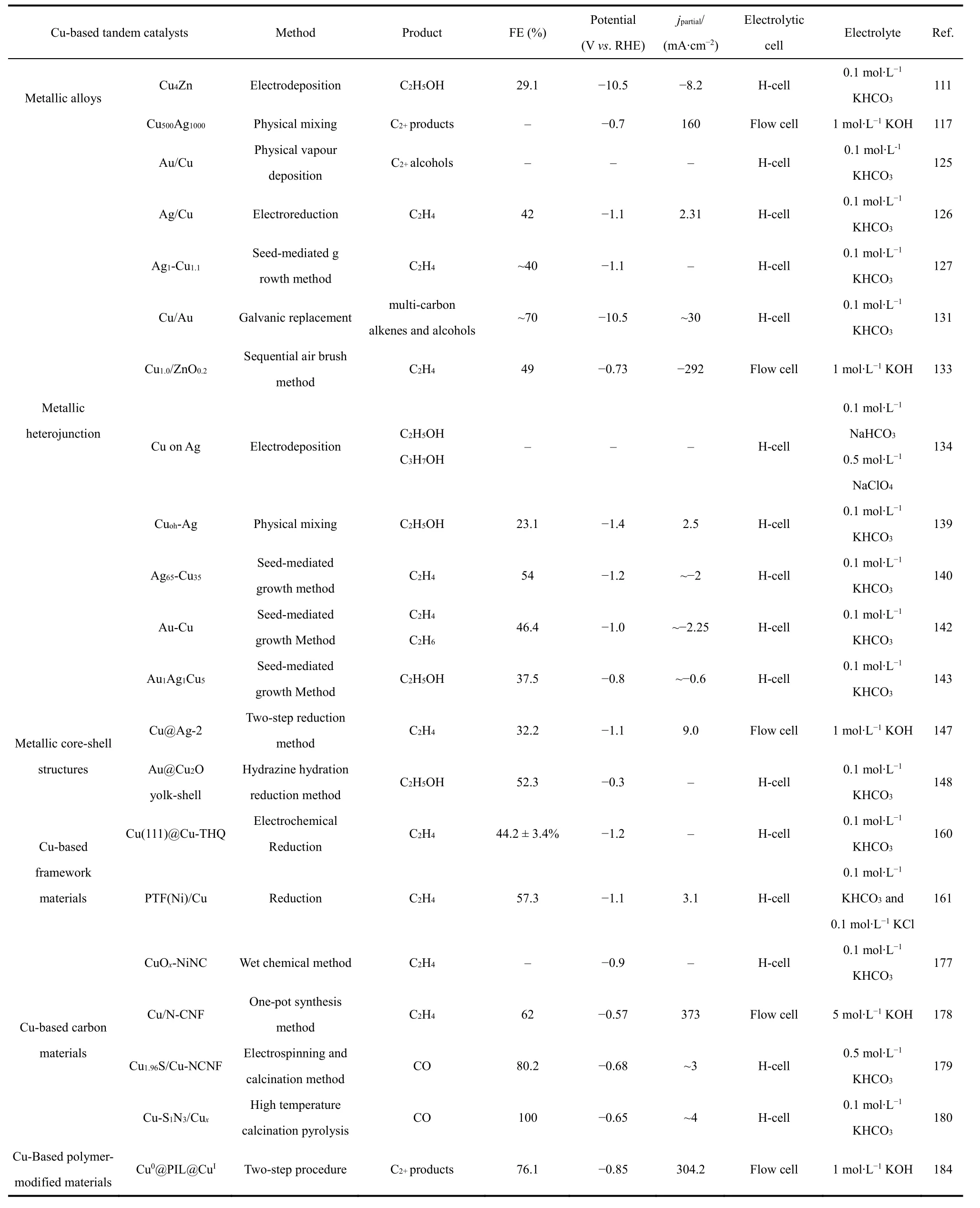
Table 1 Summary of some recently reported Cu-based tandem catalysts for electrochemical CO2RR.
Although the researchers have devoted great efforts to rational design and controllable synthesis of Cu-based tandem catalysts,the development of this field still remains a great challenge. As for the tandem mechanism for electrochemical CO2RR, it is superficial and requires a thorough understanding both experimentally and theoretically. Generally, CO2RR itself involves multiple electron-proton transfer steps, and the intermediates and reaction pathways of different products are intersected. In this regard, identifying and capturing the key intermediate*CO is of great significance for revealing the intrinsic mechanism of tandem catalysis. Thus the use of in situ/operando spectroscopic or electronic imaging techniques is highly desired, such as in situ Raman spectroscopy, Operando attenuated total reflectance surface enhanced infrared absorption spectroscopy, in situ Fourier transform infrared spectroscopy, X-ray absorption fine structure spectroscopy, positron annihilation spectroscopy, aberration-corrected electron microscopes, and scanning tunneling microscopy, etc. As for the catalytic performance, it is difficult to achieve industrial application due to the low solubility of CO2in the electrolyte and the mass transfer limitation at the gas-liquid interface. Therefore, the research on pH and potential-dependent reaction barriers,electrolyte effects and the binding-energy scaling relations of intermediates is hopefully needed. Not only does it need to explore more features of the real interface in CO2RR, it is also important to make more reasonable hypothetical models in material screening studies. Therefore, it is necessary to combine machine learning and in situ spectro-electrochemical techniques to develop multi-scale computational and modeling methods and formulate more rigorous experimental and standardized procedures to gain a deeper understanding of electrocatalytic process on catalyst surfaces. In addition to activity and selectivity, stability is also a key performance benchmark for Cubased catalysts. In order to mitigate the disintegration of the structure and the loss of active sites, strategies such as wrapping graphene oxide and thick CuOxouter layer, and specifically adsorbing halogen ions on the surface of the catalyst have been proposed. However, the stability of Cu-based catalysts, which is especially significant for the industrialization of this technology,remains less investigated across the researches on CO2RR.
Although the research on Cu-based tandem catalysts for electrochemical CO2RR is still at an early stage with many challenges, numerous advanced technologies have been developed that are expected to provide great support for the rational design of more efficient tandem catalysts. It is believed that Cu-based tandem catalysts will boost the application in industrial electrochemical CO2RR with an excellent performance.

