Computed tomography perfusion in liver and spleen for hepatitis B virus-related portal hypertension: A correlation study with hepatic venous pressure gradient
Lei Wang, Yu Zhang, Yi-Fan Wu, Zhen-Dong Yue, Zhen-Hua Fan, Chun-Yan Zhang,Fu-Quan Liu, Jian Dong
Abstract
Key Words: Hepatic venous pressure gradient; Portal hypertension; Computed tomography perfusion;Hepatitis B; Liver cirrhosis
INTRODUCTION
Gastroesophageal variceal bleeding is a common complication of portal hypertension (PH) in decompensated liver cirrhosis. There is a 60% recurrence rate and 20% mortality rate in the 1styear, and it is the leading cause of liver transplantation and mortality[1-4]. The diagnostic criteria for PH include hepatic venous pressure gradient (HVPG) ≥ 5 mmHg. Notably, when HVPG is higher than 12 mmHg,patients have a significantly increased risk of gastroesophageal bleeding. It was reported that HVPG was positively associated with individual risk of gastroesophageal variceal bleeding, and the incidence of variceal bleeding increased proportionally with an increase in HVPG[1,5-8]. In addition, HVPG can be applied clinically for risk stratification, therapeutic adoption, drug efficacy and adverse events for PH[4,9-12]. However, HVPG is an invasive procedure, which has limited its wide application for the evaluation of therapeutic effects or long-term follow-up. Therefore, studies continue to focus on noninvasive evaluation of HVPG, including anatomy (e.g., liver volume, maximal diameter of spleen), lab results (e.g., platelet level, coagulation function), liver function (e.g., Child-Pugh score, model for endstage liver disease [commonly known as MELD] score), liver and spleen stiffness (e.g., FibroScan,FibroTouch, magnetic resonance elastography), and even computation simulation modeling. However,none of these methods has demonstrated satisfactory accuracy and reproducibility.
Computed tomography (CT) perfusion of the liver is traditionally utilized to evaluate liver cancer,metastatic tumors, and liver cirrhosis. Decreased blood flow perfusion from the portal vein system and increased blood flow perfusion from the hepatic artery system can be identified with CT perfusion of the liver[13-16]. Furthermore, liver blood perfusion after transjugular intrahepatic portosystemic stentshunt (TIPS) can be quantitatively assessed with CT perfusion[17]. However, very few reports have focused on the correlation between HVPG and CT perfusion in gastroesophageal bleeding. Talakićet al[13] reported that HVPG had no correlation with CT perfusion in end-stage cirrhosis. Therefore, we aimed to explore the relationship between quantitative indices of CT perfusion with HVPG and the Child-Pugh score and to investigate the feasibility of CT perfusion as a non-invasive imaging tool for HVPG in gastroesophageal variceal bleeding induced by hepatitis B virus (HBV)-related PH.
MATERIALS AND METHODS
Patients
This prospective study was approved by the Institutional Ethics Committee at our hospital, and all written informed consents were obtained from each participant. Patients with recurrent gastroesophageal variceal bleeding resulting from HBV-related PH were randomly recruited from January 1, 2019 to June 30, 2019. All patients previously underwent drug and/or endoscopic therapy and were prepared for the TIPS procedure. The inclusion criteria were as follows: (1) Gastroesophageal bleeding as a consequence of HBV-related PH; (2) CT perfusion and Child-Pugh score available 1 wk before TIPS surgery; and (3) HVPG measured during TIPS and HVPG ≥ 5 mmHg. The exclusion criteria were as follows: (1) Gastroesophageal bleeding caused by any other etiology; (2) liver tumors, including primary and metastatic; (3) any other conditions leading to hemodynamic changes in the liver, such as partial hepatectomy, splenectomy, hepatic tumor surgery, TIPS,etc; (4) any factors affecting liver blood perfusion, such as portal vein thrombosis, cavernous transformation, Budd-Chiari syndrome,etc; (5)dysfunction in vital organs, such as cardiac, renal or respiratory damage/failure; and (6) any factors that reduced the quality of CT images, such as motion and metal artifacts.
CT perfusion and post-processing
CT perfusion was performed by a Revolution CT scanner (GE Healthcare, Chicago, IL, United States)with 16 cm Z-axis coverage axial scanning mode to cover most parts of the liver. Scanning parameters were set as tube voltage 100 kVp, automatic tube current from 50 mA to 200 mA with noise index as 14,slice thickness of 5 mm, rotation speed of 1.0 sec, helical pitch of 0.992:1.000 and 80% adaptive statistical iterative reconstruction (commonly known as ASIR). Initially, 50 mL nonionic contrast media(Omnipaque 350; GE Healthcare) followed by a 50-mL saline chaser were injected through the antecubital vein at a rate of 5 mL/sec, using a dual-head pump injector (Stellant; Medtron, Saarbrucken,Germany). The scanning was fixed with a 9-sec time delay after injection. Then, CT perfusion was performed. The CT perfusion was compromised of 26 pass acquisitions and 25 interscan gap without table movement, including 10 early acquisitions with an interscan gap of 1 sec, 12 acquisitions with an interscan gap of 2 sec, and 4 acquisitions with an interscan gap of 4 sec. Thus, total scanning time was 80 sec. All patients were instructed to avoid deep and irregular breathing during the procedure. A band compressing the upper abdomen was used to reduce respiratory motion artifacts.
Raw data generated by CT perfusion were reconstructed with a thickness of 2.5 mm. Post-processing was performed separately by two radiologists with 11 years and 7 years respectively of experience in the CT perfusion procedure. First, iterative registration reconstruction was performed to correct respiratory motion between each dynamic acquisition. Second, corrected data were post-processed with a commercial software (CT Perfusion 4D AW 4.7; GE Healthcare). Third, regions of interest were placed in the abdominal aorta and portal vein separately for liver perfusion (Figure 1). The region of interest was placed in the abdominal aorta only for splenic perfusion (Figure 2). Then, the perfusion map would be generated automatically for the liver and spleen (Figures 1 and 2). Finally, three volumes-of-interest would be selected in the left and right liver parenchyma without any hepatic vessels. By contrast, three volumes-of-interest were also selected in the superior, medial and inferior splenic parenchyma. Then,average values of perfusion parameters, including liver blood volume (LBV) (mL/100 mL), liver blood flow (LBF) (mL/100 mL/min), hepatic arterial fraction (HAF) (%), splenic blood volume (SBV) (mL/100 mL/min) and splenic blood flow (SBF) (mL/100 mL/min) were calculated and recorded.
HVPG measurement
HVPG was measured according to established standards[18,19] during the TIPS procedure. After fasting for more than 8 h, all patients underwent local anesthesia. The right internal jugular vein was cannulated using the Seldinger technique, and a 5-French balloon catheter (Edwards Lifesciences LLC,Irvine, CA, United States) was placed into the right hepatic vein, and the wedged and free hepatic venous pressure was measured three times in each patient. Then, HVPG was calculated as the difference between average wedged and free hepatic venous pressure.
Statistical analysis
Statistical analysis was performed with SPSS 24.0 software (IBM Corp., Armonk, NY, United States). All data were described as mean ± SD or range [95% confidence interval (CI)]. Kolmogorov-Smirnov was performed for the normal distribution test. Pearson or Spearman was used to evaluate the relationship among quantitative indices. Kappa was applied to analyze the agreement between observers. Patients were classified into two groups according to the HVPG value [> 12 mmHg (moderate)vs≤ 12 mmHg(severe)]. Quantitative indices, including LBV, HAF, LBF, and SBV, were compared between the two groups. Receiver operating characteristic (ROC) was performed to calculate a cutoff value for differentiation between moderate and severe PH. APvalue of less than 0.05 was considered significant.
RESULTS
General data analysis
Initially, 35 patients had portal vein thrombosis. Then, 13 patients with splenectomy, 3 patients with liver tumors and 2 patients with motion artifacts (leading to unavailable CT perfusion) were excluded.Finally, 28 patients (4 female and 24 male) were included in our study, with an age range of 28-years-old to 68-years-old and an average age of 53.7 years ± 10.4 years. Patient characteristics are summarized in Table 1, including demographics, medical history, Child-Pugh class, and HVPG.
Comparisons of Child-Pugh scores in different types of PH
Ten patients had moderate PH (HVPG < 12 mmHg), and the remaining eighteen patients had severe PH(HVPG ≥ 12 mmHg). The median HVPG was 10 mmHg (interquartile range: 9.0 mmHg; range: 8.0-11.0 mmHg) in the moderate PH group and 21 mmHg (interquartile range: 17.5 mmHg; range: 14.0-31.0 mmHg) in the severe PH group. In the moderate PH group, 6 patients were Child-Pugh A and 4 patients were Child-Pugh B. In the severe PH group, 5 patients were Child-Pugh A, 12 patients were Child-Pugh B, and 1 patient was Child-Pugh C. For the moderate PH group, HVPG and Child-Pugh scores were lower than those in the severe PH group (9.6 mmHg ± 1.3 mmHgvs18.9 mmHg ± 4.4 mmHg,P< 0.001) (Table 2).
Correlation of CT perfusion parameters with HVPG
The two radiologists demonstrated good agreement (Kappa = 0.821,P< 0.01) in the evaluation of the CT perfusion parameters. Quantitative parameters of CT perfusion of the liver are summarized in Table 2.Both LBF and LBV in moderate PH were higher than in severe PH (114.6 ± 36.0vs87.9 ± 24.8 and 19.7 ±3.0vs15.5 ± 2.2, respectively, allP< 0.05). No significant difference was observed between the two groups for the other indices (Table 2).
LBF was negatively associated with HVPG (r= -0.398,P< 0.05). LBV was negatively related to HVPG(r= -0.504,P< 0.01) and Child-Pugh (r= -0.563,P< 0.01). SBF was positively related to HAF (r= 0.498,P< 0.01). No association was observed among HAF, SBV, SBF, Child-Pugh score and HVPG. The ROC of LBV for differentiation between moderate and severe PH resulted in an area under the curve of 0.864 with a standard error of 0.075 (95%CI: 0.72-1.00) (Figure 3). Using a cutoff value of 17.85 mL/min/100 mL for LBV, the sensitivity and specificity for detection of severe PH was 80% and 89%, respectively.ROC of LBF resulted in an area under the curve of 0.797 with a standard error of 0.100 (95%CI: 0.60-1.00)(Figure 3). Using a cutoff value of 111.3 mL/min/100 mL for LBF, the sensitivity and specificity for detection of severe PH was 60% and 94%, respectively.
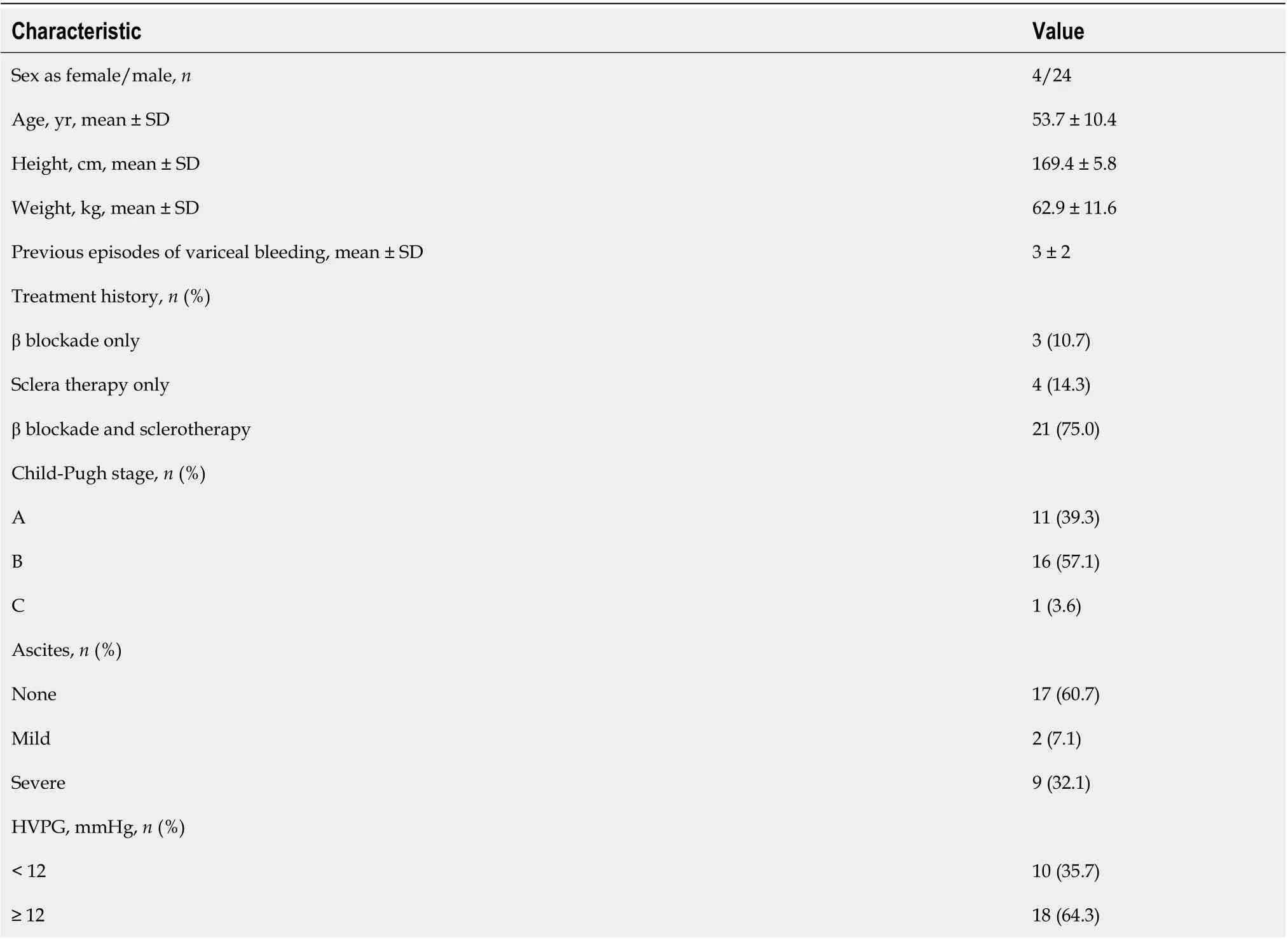
Table 1 Characteristics of the patients
DISCUSSION
HVPG is the gold standard for diagnosis of liver cirrhosis-induced PH and is an independent risk factor for evaluating the prognosis of decompensated liver cirrhosis[5,19,20]. However, as an invasive measurement requiring a complex operation, wide clinical application of HVPG has been limited. It was reported that quantitative parameters (e.g., LBF, LBV) from CT perfusion of the liver can be used to evaluate the blood supply changes in the liver and spleen with good sensitivity and specificity[13,21,22]. Therefore, our study investigated the correlation of CT perfusion for quantitative assessment of PH in HBV-related PH. Our results suggested that LBV and LBF were negatively correlated with HVPG and Child-Pugh scores, and CT perfusion imaging is a potential non-invasive quantitative predictor for PH in HBV-related liver cirrhosis.
In our study, LBV and LBF were negatively correlated with HVPG. This was explained by a significant decrease in hepatic flow[20-22] after hepatitis B infection when patients were suffering from cirrhosis-induced PH. A decrease in hepatic flow results from hepatocyte damage caused by HBV,deconstruction in normal liver structure, deposition of collagen fibers in the perisinusoidal space and formation of pseudo-lobules and fibroses, which together remarkably increases the resistance of the portal vein blood flow into the liver[1,4]. In this study, LBV and LBF were negatively related to HVPG.It is possible that the decrease of LBV and LBF is the consequence of the increase of HVPG, suggesting significantly reduced blood perfusion in the liver as PH increases. Therefore, CT perfusion is potentiallyfeasible for the non-invasive evaluation of HVPG using LBV and LBF in patients with HBV-related PH.

Table 2 Comparison of the moderate and severe portal hypertension groups

Figure 2 Computed tomography perfusion of the spleen post-processing data. A: Regions of interest were placed in the abdominal aorta as the input blood vessel; B: The time-density curve was generated automatically for calculation of splenic perfusion; C and D: The parameters of computed tomography perfusion of the spleen were calculated automatically, including splenic blood flow (C) and splenic blood volume (D).
In this study, liver blood perfusion parameters (e.g., LBV and LBF) in the moderate PH group were significantly higher than those in the severe PH group. For distinguishing moderate PH from severe PH,LBV had a ROC curve with a sensitivity and specificity of 80% and 89%, respectively. LBF had a sensitivity and specificity of 60% and 94%, respectively. Therefore, CT perfusion parameters (LBV and LBF) can be used to distinguish moderate PH and severe PH in PH-induced gastroesophageal variceal bleeding in patients with HBV-related PH.

Figure 3 Receiver operating characteristic curves to differentiate moderate and severe portal hypertension. For discriminating severe portal hypertension, liver blood volume had an area under the curve of 0.864 with a standard error of 0.075 [95% confidence interval (CI): 0.72-1.00], while liver blood flow had an area under the curve of 0.797 with a standard error of 0.100 (95%CI: 0.60-1.00). LBF: Liver blood flow; LBV: Liver blood volume; ROC: Receiver operating characteristic.
LBV was negatively correlated with Child-Pugh score, suggesting that liver reserve function decreases with reduced LBV. Moreover, the Child-Pugh score in the moderate PH group was significantly lower than that in the severe PH group. Similarly, liver reserve function was better in the moderate PH group than the severe PH group. This was related to pathophysiological mechanisms underlying hepatitis B cirrhosis and PH. In addition, HVPG in the severe PH group was significantly higher than the moderate PH group. The intrahepatic portal venous system pressure in severe PH may increase, leading to progressively decreased blood flow and gradually weakening the reserve capacity of liver function. However, in this study, the Child-Pugh score was not associated with HVPG, which was consistent with previous studies[3,7,10,23]. The Child-Pugh score is mainly used to evaluate liver reserve function, which can only provide a crude evaluation of PH.
HAF was not related to HVPG, suggesting no correlation between the hepatic artery perfusion ratio and PH in liver cirrhosis. HAF mainly indicates the proportion of hepatic artery blood supply to the total liver blood supply in cirrhosis. When cirrhosis occurs due to damage in the liver sinusoid and liver lobule structure, the blood in the portal vein meets increasing resistance against its return to the liver.When portal vein pressure increases, the blood supply flowing to the liver is reduced. Likewise,compensatory hepatic artery blood perfusion can increase. However, the portal vein blood supply accounts for about three-quarters of the total liver blood supply[24]. The compensatory increase in hepatic artery blood supply could not compensate for a substantial decrease in blood flow in the liver caused by reduced portal vein blood supply. This buffering effect is not enough to maintain the hepatic blood supply[22-24]. In addition, HAF is affected by various factors, such as blood pressure, blood volume and cardiac function. This might explain why HAF was not correlated with HVPG.
The perfusion parameters of the spleen (e.g., SBF, SBV) were not related to HVPG and Child-Pugh classification. This was consistent with a previous study. However, in that cohort, blood flow and blood volume of the liver were not associated with HVPG[13]. This may be related to different samples included in our study, where patients suffering from liver cirrhosis caused by hepatitis B were classified as relatively moderate cases. Among them, according to the Child-Pugh classification, 11 cases were defined as grade A, 16 cases as grade B, and 1 case as grade C. By contrast, patients included in the previous study were primarily suffering from alcoholic cirrhosis with Child-Pugh grade B and C.Furthermore, in the previous study, all patients were suffering from more severe diseases and were planning for liver transplantation as treatment. Moreover, our study excluded factors that may affect portal vein hemodynamics (such as splenic resection, portal vein thrombosis), which may explain the differences between the two studies.
Limitations existed in our study. First, our study only included cases of HBV-related PH, with a remarkable disproportion in patient sex. The majority of patients were Child-Pugh A and Child-Pugh B.A larger sample size is required to identify the clinical application of CT perfusion in patients with different causes of cirrhosis and higher Child-Pugh scores, including alcoholic cirrhosis, drug-induced metabolic liver disease and autoimmune liver disease. Second, our study primarily targeted patients who were suffering from gastric fundus esophageal variceal bleeding as a consequence of PH and excluded other factors like thrombosis, cavernous transformation and splenectomy that could affect liver hemodynamics. Nonetheless, further research is required to determine its application in PH with multiple complications. Finally, our study did not focus on pathology, laboratory and comparative imaging evaluation (such as volume and elasticity of the liver and spleen). Thus, further research is required.
CONCLUSION
Quantitative parameters of CT perfusion imaging, in particular LBV and LBF, were negatively correlated with HVPG and Child-Pugh scores. Therefore, CT perfusion imaging is a potential application for non-invasive quantitative evaluation of HVPG in patients with HBV-related PH.
ARTICLE HIGHLIGHTS
FOOTNOTES
Author contributions:Dong J, Liu FQ, and Wang L designed the report; Zhang Y, Wu YF, Yue ZD, Fan ZH, and Zhang CY collected the clinical data; Wang L and Zhang Y analyzed and wrote the paper; Dong J and Liu FQ performed quality control; Liu FQ contributed to administrative and financial support; all authors have read and approved the final version of the manuscript.
Supported bythe National Natural Science Foundation of China General Program, No. 81871461.
Institutional review board statement:This study was reviewed and approved by the Ethics Committee of the Beijing Shijitan Hospital, Capital Medical University (Approval No. 201801).
Clinical trial registration statement:This study is registered at ClinicalTrials.gov:http://www.chictr.org.cn/edit.aspx?pid=26048&htm=4. The registration identification number is ChiCTR1800015268.
Informed consent statement:Written informed consent was obtained from each patient.
Conflict-of-interest statement:There are no conflicts of interest to report.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Lei Wang 0000-0002-4374-059X; Jian Dong 0000-0002-2643-0370.
S-Editor:Chen YL
L-Editor:A
P-Editor:Chen YL
 World Journal of Gastroenterology2022年42期
World Journal of Gastroenterology2022年42期
- World Journal of Gastroenterology的其它文章
- Angiogenesis and immune checkpoint dual blockade: Opportunities and challenges for hepatocellular carcinoma therapy
- Clinical value of predictive models based on liver stiffness measurement in predicting liver reserve function of compensated chronic liver disease
- Novel management indications for conservative treatment of chylous ascites after gastric cancer surgery
- Role of radiomics in the diagnosis and treatment of gastrointestinal cancer
- COVID-19 associated liver injury: A general review with special consideration of pregnancy and obstetric outcomes
