Angiogenesis and immune checkpoint dual blockade: Opportunities and challenges for hepatocellular carcinoma therapy
si-Qi Li, Yang Yang,Lin-Sen Ye
Abstract The disease burden related to hepatocellular carcinoma (HCC) is increasing. Most HCC patients are diagnosed at the advanced stage and multikinase inhibitors have been the only treatment choice for them. Recently, the approval of immune checkpoint inhibitors (ICIs) has provided a new therapeutic strategy for HCC. It is noteworthy that the positive outcomes of the phase III clinical trial IMBrave150[atezolizumab (anti-programmed cell death ligand 1 antibody) combined with bevacizumab (anti-vascular endothelial growth factor monoclonal antibody)],showed that overall survival and progression-free survival were significantly better with sorafenib. This combination therapy has become the new standard therapy for advanced HCC and has also attracted more attention in the treatment of HCC with anti-angiogenesis-immune combination therapy. Currently, the synergistic antitumor efficacy of this combination has been shown in many preclinical and clinical studies. In this review, we discuss the mechanism and clinical application of anti-angiogenics and immunotherapy in HCC, outline the relevant mechanism and rationality of the combined application of antiangiogenics and ICIs, and point out the existing challenges of the combination therapy.
Key Words: Anti-angiogenesis; Immunotherapy; Combination therapy; Vascular endothelial growth factor; Immune checkpoint blockade; Hepatocellular carcinoma
INTRODUCTION
According to the statistics from the National Cancer Center of China, the incidence and mortality of hepatocellular carcinoma (HCC) are increasing annually[1]. The World Health Organization (WHO)estimates that, from 2015 to 2030, approximately 10 million people in China will die due to liver cirrhosis and HCC. Although early-stage disease can be cured by surgical removed, transplantation, or interventional therapy, most patients have unresectable disease at the time of diagnosis[2], and current treatments are insufficient to prevent the high metastasis and recurrence rates after HCC treatment.
Currently, immunotherapy is receiving a great deal of attention in the treatment of tumors. Among the immunotherapy options, immune checkpoint blockade (ICB)-based immunotherapy which reactivates dysfunctional or exhausted T cells has shown excellent efficacy in a variety of solid cancers and hematological tumors[3-7]. However, 50%-80% of cancer patients still do not benefit from immunotherapy, and many of them suffer serious adverse events (AEs) during treatment[8]. In fact, there is still no clear mechanism to explain the tolerance of many cancers to immune checkpoint inhibitors (ICIs).HCC is a solid tumor with complex pathophysiological barriers. It is difficult for external lymphocytes to penetrate and infiltrate into tumor tissue. In addition, the rapidly growing tumor cells release immunosuppressive factors, which result in HCC forming an immunosuppressive immune microenvironment, which greatly limits the efficacy of immunotherapy[9]. In addition, the rapidly growing tumor cells release several factors, which result in HCC forming an immunosuppressive immune microenvironment, that greatly limits the efficacy of immunotherapy[10,11]. Therefore, normalizing tumor vasculature and improving the tumor hypoxic microenvironment is expected to reverse the immunosuppressive microenvironment of HCC and promote HCC immunotherapy.
The TME is mainly composed of the vasculature, resident or infiltrating immune cells and various stromal cells. Previous studies have shown that abnormal tumor vasculature promotes the formation of an immunosuppressive TME[11]. Therefore, therapies that promote normalization of the vasculature are of great significance for enhancing immunotherapy of HCC. This review outlines measures to normalize the vasculature of HCC and the common immunotherapy regimens for HCC, and further describes and discusses how to normalize the tumor vasculature to improve the efficacy of immunotherapy in HCC(especially ICB). Additionally, we discuss the challenges associated with emerging combinations of vascular normalization therapy and immunotherapy for HCC.
ABNORMAL ANGIOGENESIS AND VASCULAR NORMALIZATION MEASURES FOR HCC
Abnormal angiogenesis and vascular endothelial growth factor/vascular endothelial growth factor receptor
The excessive growth and abnormal proliferation of tumor cells depend on rapid tumor angiogenesis.Tumor angiogenesis not only provides tumor cells with oxygen, nutrients and removes waste, but also serves as a channel for metastasis of tumor cells and immune cell infiltration[12,13]. However,compared with vessels in normal tissues, tumor neo-vessels have obvious aberrations in both structure and function[11]. Leakage is one of the most notable features of tumor vessels. On the one hand, this property can lead to tumor hypoxia and decreased intra-tumoral pH by impairing perfusion, and on the other hand, leakage will increase interstitial pressure in the TME[10]. Tumor cells overcome these harsh conditions through multiple mechanisms to gain a survival advantage[11]. Abnormal vessels limit the circulation of drugs and immune cells into the tumor, thereby limiting anti-tumor activity[11]. The hypoxia and pH reduction in the tumor caused by abnormal vessels will further lead to abnormal neovascularization, forming a vicious circle.
In the field of cancer research, most studies on angiogenesis has focused on the increased expression of angiogenesis factors [such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), angiopoietin, hepatocyte growth factor, endoglin (CD105,etc)] and decreased expression of anti-angiogenesis factors, such as angiostatin, endostatin, and thrombospondin 1, which is mainly caused by upregulation of the hypoxia-inducible factor protein.Among these, VEGF/VEGF receptor (VEGFR) axis are widely known to play a major role in vascular abnormalities and are crucial for the occurrence and progression of HCC[14-16]. The VEGF family consists of VEGFA, VEGFB, VEGFC, VEGFD and placental growth factor (PlGF) 1-4, which are involved in tumor angiogenesis (VEGFA, PlGF), maintenance of new blood vessels (VEGFB), lymph-angiogenesis and angiogenesis (VEGFC/D), vascular permeability (VEGFA/C), chemotaxis (VEGFB), migration(VEGFA, PlGF), differentiation (VEGFD) and survival (VEGFA/B/C, PlGF)[17,18]. VEGFR mainly includes VEGFR1, VEGFR2, and VEGFR3[15]. Of these, VEGFR2 is the critical receptor of this family,which is expressed on almost all endothelial cells and is activated by binding to VEGFA, VEGFC or VEGFD, and VEGFA is its major ligand[19]. This binding results in the phosphorylation cascade that triggers downstream cellular pathways, ultimately leading to endothelial cell proliferation and migration, and the formation and branching of new tumor blood vessels[19]. These neo-vessels often manifest as abnormal leaky vasculature, resulting in high interstitial pressure and severe hypoxia or necrosis in tissue regions, further promoting the malignant potential of tumor cells[20]. Previous studies have shown that circulating VEGF levels are increased in HCC, and increased VEGFA has been shown to be associated with angiogenesis in HCC[21,22]. In addition, related studies also observed a positive association between increased local and circulating VEGF and high tumor micro-vessel density with rapid disease progression and decreased survival. These findings support the application of therapies that target the VEGF/VEGFR pathway in HCC[21,22].
Vascular normalization measures in HCC and targeting of the VEGF/VEGFR axis
Various molecular and physical mechanisms have been reported to contribute to tumor vascular dysfunction, the most prominent of which is the imbalance of angiogenic signaling mediated by proand anti-angiogenic molecules[11,23]. In normal tissue, this balance is precisely maintained to ensure normal vascular morphology and function[24]. However, during the process of carcinogenesis, this balance usually tends to angiogenesis, and the generated neo-vessels are immature abnormal vessels without complete structure[24]. In view of the key role of the VEGF/VEGFR axis in abnormal angiogenesis of HCC, rational targeting of this axis can promote the normalization of tissue vessels and limit the occurrence and development of HCC.
In the past few decades, the development of anti-angiogenesis therapy has mainly focused on blocking VEGF[17,18]. Several studies have also focused on blocking VEGF signaling by silencing VEGFA expression at the transcriptional and post-transcriptional levels. For example, Zouet al[25]identified emodin that could greatly increase seryl-tRNA synthetase expression in tripe-negative breast cancer (TNBC) cells, consequently reducing VEGFA transcription, and emodin potently inhibited vascular development of zebrafish and blocked tumor angiogenesis in TNBC-bearing mice, greatly improving the survival. Liet al[26] and Dinget al[27] raised that VEGF small interference RNA can precisely and efficiently silence VEGF expression and block VEGF signal pathway, leading to a significant decrease in tumor blood vessels and suppression of tumor growth and metastasis. However,these studies have only been tested in animals. Preclinical evidence suggests that monotherapy which blocks VEGF reduces micro-vessel density, inhibits tumor growth in many cancerous subcutaneous xenografts, and even inhibits tumor cell metastasis[28,29]. Ferraraet al[30] researched and developed the first anti-angiogenesis inhibitor (bevacizumab), a recombinant humanized monoclonal antibody that blocks VEGFA. Bevacizumab binds to VEGF in the bloodstream, thereby inhibiting the interaction between VEGF and VEGFR. In clinical trials of combination therapy for HCC, multiple lines of evidence suggest that bevacizumab has a potential therapeutic effect[31,32].
On the other hand, many anti-angiogenesis therapies in HCC focus on targeting VEGFR. Multikinase inhibitors (MKIs) and monoclonal antibodies (mAbs) were developed to inhibit VEGFR and its downstream targets to inhibit endothelial cell proliferation, thereby reducing the nutrient and oxygen supply required by tumors. Currently, the VEGFR-targeted MKIs and mAbs used in advanced HCC mainly include sorafenib, regorafenib, lenvatinib, cabozantinib, and ramucirumab (Table 1).
Sorafenib is an oral MKI that blocks VEGFR1-3, c-Kit, PDGF receptor (PDGFR)-b, and FMS-like tyrosine kinase-3 (FLT-3)[33]. The phase III clinical trial SHARP showed a 2.8-mo survival advantage for sorafenib over placebo in patients with advanced HCC. This recent study also showed that sorafenib can benefit patients with HCC regardless of etiology, and patients with hepatitis C appeared to experience greater benefit[34]. Treatment-related AEs were more common in the sorafenib group than in the placebo group (80%vs52%), and the incidence of dose reductions and interruptions was high during treatment. The MKI regorafenib also targets VEGFR, RET, c-Kit, B-Raf, FGFR1 and PDGFR[33]. It is the first therapy to demonstrate a survival benefit in advanced HCC patients who have progressed on sorafenib[35]. Fatigue, hypertension, diarrhea and hand-foot skin reactions were common AEs in the regorafenib-treated group. Other analyses showed that the survival benefit of first line sorafenib and second line regorafenib was more than 24 mo[35]. The targets of lenvatinib include VEGFR1-3, FGFR1-4,PDGFRa, RET and c-Kit. Recently, a phase III study of lenvatinibvssorafenib in patients withunresectable HCC showed that overall survival (OS) with lenvatinib was non-inferior to sorafenib[36].The most common AEs in the lenvatinib group were diarrhea, fatigue,etc[36]. It should be noted that patients with tumors with more than 50% hepatic masses or involvement of branches of the main portal vein were excluded from the trial (NCT01761266); thus, further clinical trials should be conducted.Despite this problem, lenvatinib remains the only drug in first-line clinical trials that was positive against the proven active control, sorafenib. In addition to targeting VEGFR2, c-Kit, RET, FLT-3, Tie2,and Axl, cabozantinib has the unique property of inhibiting c-Met, and its potential activity was observed in phase II trials[37]. The subsequent phase III clinical trial, which compared cabozantinib to placebo in advanced HCC, met its primary endpoint of improved OS after up to two prior treatments,one of which included sorafenib[38]. Hypertension, pneumonia were common AEs in the cabozantinib group[38]. Ramucirumab, the mAb that antagonizes VEGFR2, improved OS in a phase III study in patients with sorafenib progression or intolerance with baseline alpha-fetoprotein (AFP) ≥ 400 ng/mL[39]. Hypertension and hyponatremia were the only over grade 3 AEs in patients of the test group[39].Based on the results of the previous phase III study, patients can be selected for treatment based on baseline AFP values. A survival benefit was observed with ramucirumab in a subgroup of patients with higher baseline AFP (400 ng/mL), which is the first positive clinical trial in the biomarker-selected HCC population[39].
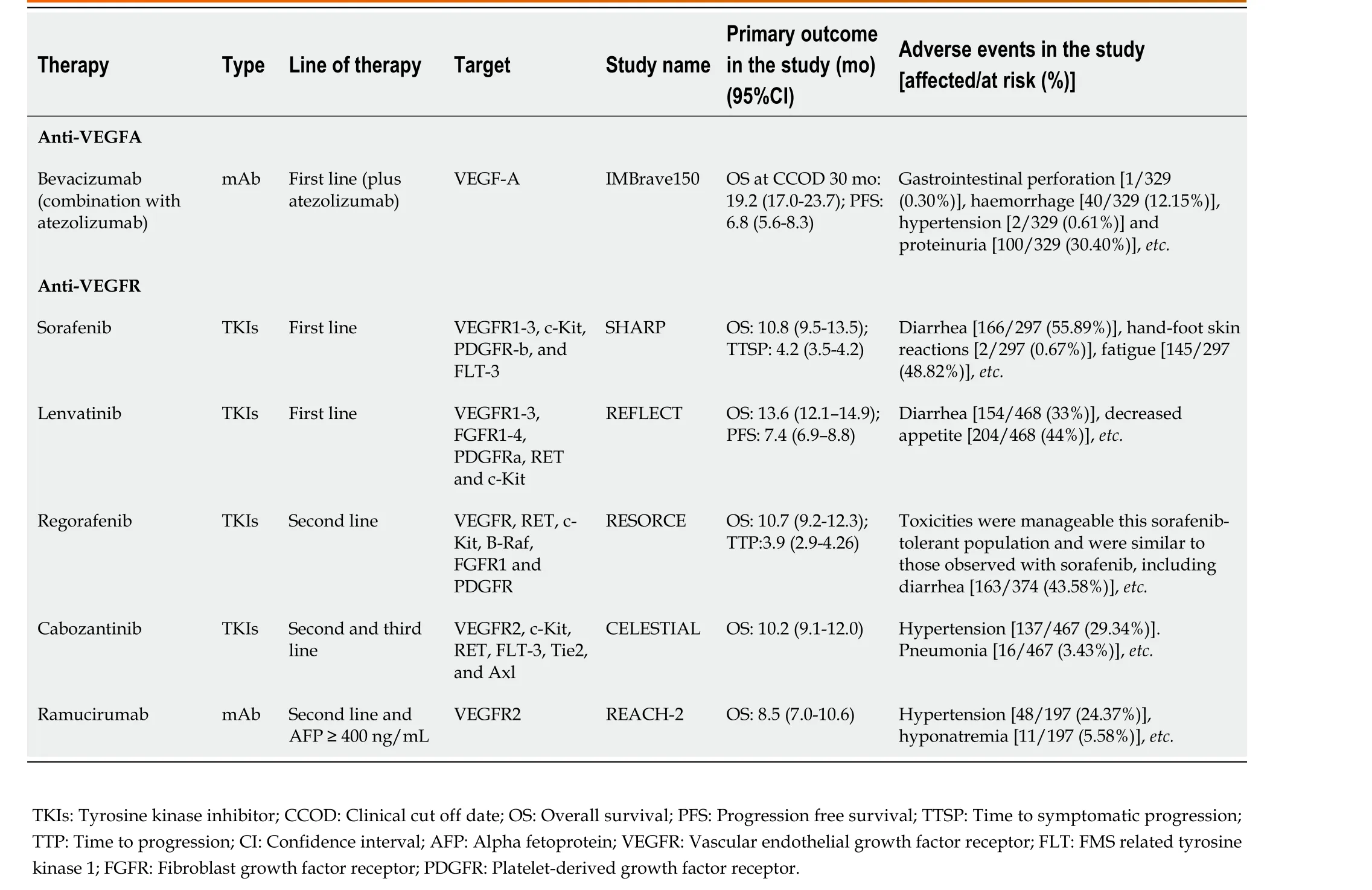
Table 1 Currently approved anti-angiogenic therapy for advanced hepatocellular carcinoma
IMMUNOTHERAPY IN HCC
Driven by the success of ICB therapy in melanoma, ICB has been extensively studied in a variety of malignancies including HCC[40,41]. Long-term liver injury or chronic hepatitis infection often leaves the liver in a state of chronic inflammation[42]. Moderate inflammation can defend against pathogens and repair tissue damage, whereas persistent liver inflammation can disturb the microenvironment,thus favoring carcinogenesis. On the one hand, hepatic endocrine cytokines play a key role in tumorigenesis through regulating regulatory T cells (Tregs) that inhibit CD8+T cell activation[43]. It was previously reported that Tregs derived from hepatitis B virus-positive HCC tumors exhibited higher programmed cell death protein 1 (PD-1) expression and superior inhibitory capacity against CD8+T cells. On the other hand, cytotoxic immune populations frequently express markers of exhaustion such as PD-1, cytotoxic t-lymphocyte associated antigen 4 (CTLA-4) and lymphocyte activating gene 3[44].Under normal conditions, these molecules inhibit T cell activation to maintain inflammatory homeostasis, protect tissue integrity, and prevent unnecessary autoimmunity[45]. However, in tumors,expression of these markers of exhaustion is inversely correlated with their function, making them a prime target for ICBs to revitalize and restore the cytotoxic capacity of CD8+T cells[43]. At the same time, the expression of PD-1 and its ligand (PD-L1) in tumor cells is upregulated, and when it binds to PD-1 expressed by T cells activated by tumor infiltration, it induces T cell exhaustion and suppresses the anti-tumor immune activity of these immune cells, thereby enabling tumor cells to evade immunity[46].ICBs generate robust multitarget immune responses and can even induce long-lasting tumor remissions in some patients. Inhibition of the PD-1/PD-L1 interaction reverses the depleted state of these cytotoxic immune cells and reactivates their antitumor activity[46,47]. In HCC, the mAbs pembrolizumab and nivolumab that target PD-1, and nivolumab combined with ipilimumab (a mAb directed against CTLA-4), has been approved in the United States for sorafenib-treated patients, based on promising results from clinical trials[48-50]. A clinical trial confirmed the efficacy and safety of PD-1-targeting immunotherapy in HCC[51]. However, subsequent phase III trials of nivolumabvssorafenib in first-line therapy failed to meet the primary survival endpoints[50,52]. The combination of phase III nivolumab and ipilimumab is currently under evaluation (NCT04039607).
In the current phase III clinical treatment of HCC, immune combination therapy has attracted considerable attention, especially the combination of ICIs and anti-angiogenic inhibitors (Table 2). Lenvatinib combined with pembrolizumab, bevacizumab combined with atezolizumab (PD-L1-targeting mAb)(T+A combination), and cabozantinib combined with atezolizumab have all obtained encouraging results in clinical studies[53-55]. Of these, the combination of bevacizumab and atezolizumab has been successful in phase III clinical trials of first-line treatment of HCC (IMbrave150). Compared with sorafenib, this combination improved the primary endpoints: OS and progression-free survival, and this combination was shown to be safe and improved quality of life[56]. Hypertension and proteinuria were typical side effects of bevacizumab and were the top two AEs in this test group. Upper gastrointestinal bleeding, another known side effect of bevacizumab, occurred in 7% of patients in this group, and was within the range of previous evaluations of bevacizumab AEs for the treatment of HCC[57,58]. Elevated transaminases and pruritus are common side effects of atezolizumab[32]. As this study applied the usual inclusion criteria in HCC clinical trials and included only Child-Pugh A patients, further clinical trials are pending[32].
Angiogenesis and immune checkpoint combination blockade in HCC
As shown above, the success of the phase III trial (IMbrave150) with the combination of atezolizumab and bevacizumab in advanced HCC is groundbreaking as it is the first treatment with a better survival rate than sorafenib since the approval of sorafenib in 2007 and is also the only successful first-line immunotherapy combination therapy for HCC in the world[32]. Antibodies targeting VEGF not only inhibit tumor growth but also reprogram the TME from immunosuppressive to immune activation[8,59]. Based on these findings, PD-1/PD-L1 inhibitors combined with VEGF/VEGFR inhibitors have attracted extensive attention in the treatment of HCC. Next, we will outline the mechanism and rationality of PD-1/PD-L1 inhibitors combined with VEGF/VEGFR inhibitor therapy, and the biomarkers of response to targeted immune combination therapy.
Mechanism and rationality
In the tumor area, VEGF released by hypoxic cancer cells promotes tumor cell growth and metastasis by angiogenesis[8]. On the other hand, VEGF can also promote the malignant progression of tumors by affecting the TME (Figure 1). The tumor immune cycle mainly includes seven steps, the release of tumor antigens, the uptake and presentation of tumor antigens by dendritic cells (DCs), the initiation and activation of T cells, the migration of T cells to tumors, the invasion of T cells against tumors, the recognition of tumor cells by T cells, and the attack of tumor cells by T cells[60]. VEGF is involved in almost every step of the tumor immune cycle and finally promotes tumor immune escape[61-64]. VEGF enhances the mobilization and proliferation of immunosuppressive cells, including Tregs, tumorassociated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs)etc, enhances the release of immunosuppressive cytokines[8,59], and promotes M1-TAMs to M2 type polarization. In addition, Tregs and TAMs release immunosuppressive factors, such as VEGF and angiopoietin 2, which form positive feedback to further promote tumor progression[65]. Furthermore, VEGF inhibits the maturation and antigen presentation of DCs. Thus, even in the presence of neoantigens, VEGF can reduce the proliferation and activation of naive CD8+ cells by inhibiting DCs[65]. VEGF prevents antigen activated CD8+ T cells from infiltrating tumor tissue by promoting the formation of abnormal tumor vessels. In addition, VEGF forms an immunosuppressive TME that inhibits the function of T cells in tumors during the effector phase of the immune response[65]. Therefore, inhibition of VEGF/VEGFR interaction not only normalizes vessels but also enhances antitumor immunity.
Previous studies have shown that inhibition of VEGF/VEGFR can enhance antitumor immunity.Gabrilovichet al[63] found that the targeted drugs which inhibit VEGF/VEGFR lead to an enhanced antigen-presenting capacity of DCs. In addition, studies also found that these drugs inhibit the production of Tregs, TAMs and MDSCs at tumor sites, and negatively regulate the expression of immunosuppressive cytokines such as transforming growth factor-beta and interleukin-10[66].Therefore, blocking VEGF/VEGFR reprograms the immunosuppressive TME[67]. At the same time, the combination of PD-1/PD-L1 antibody can further enhance the antitumor activity of T cells. First, by reversing the VEGF-mediated suppression of DCs maturation resulting in efficient priming and activation of T cells[67,68]; second, by normalizing tumor vasculature and promoting efficient T cell infiltration into tumors[69]; and third, VEGF/VEGFR inhibitors inhibit the activity of MDSCs, Tregs,and TAMs, leading to the reprogramming of the immunosuppressive microenvironment to immune activation[61]. Finally, PD-1/PD-L1 inhibitors enhance the ability of T cells to attack tumor cells. These four aspects can lead to effective antitumor immunity and tumor growth inhibition. As described above,the use of molecularly targeted drugs against VEGF/VEGFR reactivates the aberrant immunosuppressive TME caused by VEGF, and finally allows the tumor cells to be effectively attacked[60,62]. A recent study of T+A therapy showed that the improved outcome of the combination of bevacizumab and atezolizumab compared with atezolizumab alone was mainly related to higher Tregs expression,suggesting that bevacizumab inhibits VEGF-enhanced antitumor immunity mainly related to inhibiting the function of Tregs[70]. Notably, previous studies have shown that anti-PD-1 therapy can increase tumor blood perfusion by normalizing blood vessels in breast and colorectal cancer models, which is closely related to its antitumor efficacy[71]. These studies form the rationale for the combination of VEGF/VEGFR inhibitors and PD-1/PD-L1 inhibitors.

Table 2 Phase III clinical trials of combinations of anti-angiogenic inhibitors and immune checkpoint inhibitors in hepatocellular carcinoma
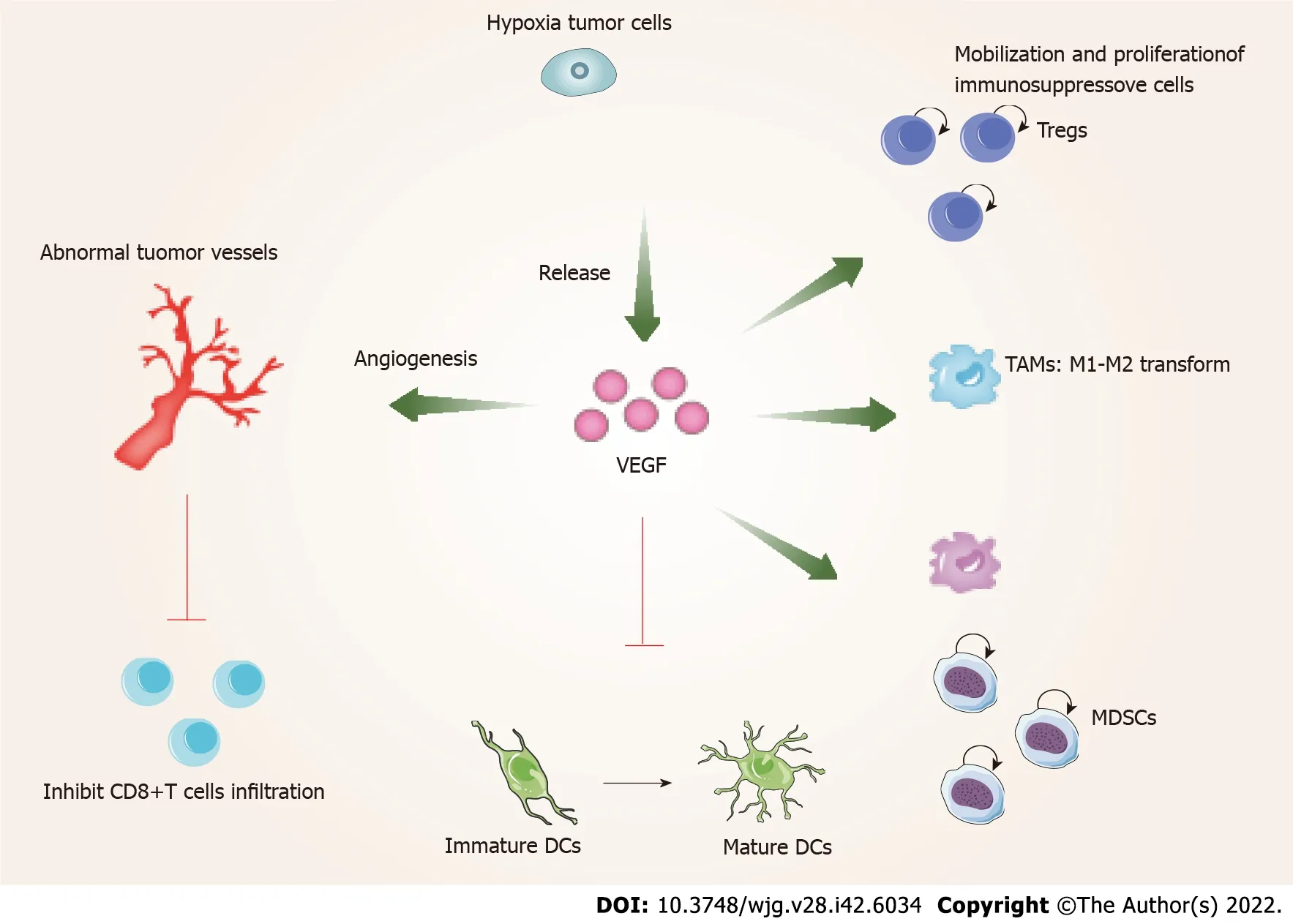
Figure 1 Vascular endothelial growth factor promotes the formation of an immune suppressive microenvironment. VEGF: Vascular endothelial growth factor; DCs: Dendritic cells; MDSCs: Myeloid-derived suppressor cells; TAMs: Tumor-associated macrophages; Tregs: Regulatory T cells.
Biomarkers to assess the response of targeted immunotherapy
A major factor limiting the benefit of angiogenesis and immune checkpoint dual blockade for HCC is the lack of biomarkers to predict patients who can benefit from treatment. PD-L1 expression in tumor specimens was not associated with prognosis in HCC patients treated with nivolumab[48]. This study demonstrated that further comprehensive tumor and stromal immune scores, or tumor gene signatures should be explored. A recent study showed that pre-existing immunity (high expression of CD274, Teffector signature and intra-tumoral CD8+ T cell density) was positively associated with better clinical outcomes with the T+A combination and reduced clinical benefit was associated with a high Treg to effector T cell (Teff) ratio and expression of oncofetal genes (GPC3, AFP)[70]. Hatanakaet al[72] reported that C-reactive protein and AFP could be useful for predicting therapeutic outcomes and treatmentrelated AEs in HCC patients treated with the T+A combination. These results indicate that the clinical studies of bevacizumab combined with atezolizumab, lenvatinib combined with pembrolizumab,cabozantinib combined with atezolizumab and other combination therapies are a valuable platform for the analysis of other potential biomarkers of response to targeted immunotherapy and offer several new possibilities for identifying subpopulations of patients who benefit from these treatments. In several cancers, tumor mutational burden (TMB) and microsatellite instability (MSI) are associated with longer OS after ICB therapy. Considering the low frequency of TMB and MSI in HCC, their predictive applications in HCC are limited. However, it is worth noting that studies have shown that HCC with high TMB and low MSI responded to nivolumab for more than 2 years[73]. Thus, much more research is needed to determine the biomarkers of targeted immunotherapy in HCC.
CONCLUSION
The global disease burden of HCC is increasing year by year. According to statistics, the annual incidence of HCC may exceed 1 million cases in the near future, and most patients are in advanced stages at diagnosis. Currently, only reasonable systemic treatment can effectively delay the progression of HCC. This article describes the characteristics and treatment strategies of abnormal angiogenesis in HCC, and briefly reviews the immunotherapy of HCC. The strategy and rationality of angiogenesis and immune checkpoint dual blockade are further discussed. Among these combinatorial strategies, the success of the IMBrave150 clinical trial demonstrated that bevacizumab altered the tumor immune microenvironment, enabling greater responses to ICB, successfully transforming the immunosuppressive TME to an immune-activated microenvironment. Therefore, the efficacy achieved by the combination of anti-PD-1/PD-L1 antibody and VEGF/VEGFR inhibitor may be due to normalization of the TME. In addition to the combination of atezolizumab and bevacizumab, other combination therapies targeting the same mechanism have also received attention. While the clinical development of VEGF/VEGFR-targeted drugs is due to their anti-angiogenesis inhibitory effects, the potential of this class of drugs is as immunomodulators in combination with immunotherapy.
In the post-sorafenib era of advanced HCC treatment, a great number of combination therapies are being studied. However, one of the biggest challenges with combination therapy is the discovery of predictive biomarkers to accurately identify patients most likely to respond to treatment. In the study of HCC, anti-angiogenesis therapy has been used for more than a decade and ICB has been approved for several years, but these two therapies still lack convincing biomarkers. Therefore, for combination therapy, a better understanding of the mechanism of synergistic therapeutic effect and the discovery of predictive biomarkers will help to design more precise treatment regimens.
FOOTNOTES
Author contributions:Li SQ, Yang Y and Ye LS carried out the research for the manuscript and edited all drafts of the paper.
Supported byGuangdong Basic and Applied Basic Research Foundation, No. 2019A1515110654; the National Natural Science Foundation of China, No. 82103448; and China Organ Transplantation Development Foundation, No. YZLC-2021-003.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Yang Yang 0000-0003-4981-4745; Lin-Sen Ye 0000-0001-9632-1949.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
 World Journal of Gastroenterology2022年42期
World Journal of Gastroenterology2022年42期
- World Journal of Gastroenterology的其它文章
- COVID-19 associated liver injury: A general review with special consideration of pregnancy and obstetric outcomes
- Role of radiomics in the diagnosis and treatment of gastrointestinal cancer
- Computed tomography perfusion in liver and spleen for hepatitis B virus-related portal hypertension: A correlation study with hepatic venous pressure gradient
- Novel management indications for conservative treatment of chylous ascites after gastric cancer surgery
- Clinical value of predictive models based on liver stiffness measurement in predicting liver reserve function of compensated chronic liver disease
