Maternally expressed gene 3 regulates retinal neovascularization in retinopathy of prematurity
Yu Di, Yue Wang, Yue-Xia Wang, Xue Wang, Yuan Ma, Qing-Zhu Nie
Abstract The mouse model of oxygen induced retinopathy is suitable for the study of various retinal neovascularization diseases, including retinopathy of prematurity. The maternally expressed gene 3 (MEG3) has been demonstrated to have an inhibitory effect on diabetic retinopathy. In this study, we investigated the role of MEG3 overexpression in oxygen-induced retinopathy in mice. The results showed that MEG3 overexpression effectively inhibited the production of retinal neovascularization in oxygen-induced retinopathy mice. It acts by down-regulating the expression of phosphoinositide 3-kinase, serine/threonine kinase, and vascular endothelial growth factor and pro-inflammatory factors. MEG3 overexpression lentivirus has a future as a new method for the clinical treatment of retinopathy of prematurity. The animal experiments were approved by the Animal Ethics Committee of Shengjing Hospital of China Medical University, China (approval No. 2016PS074K) on February 25, 2016.
Key Words: long noncoding RNA; maternally expressed gene 3; neurodevelopment; oxygen-induced retinopathy; phosphoinositide 3-kinase; retinal neovascularization; retinopathy of prematurity; serine/threonine kinase; vascular endothelial growth factor
Introduction
The incidence of retinopathy of prematurity (ROP) is increasing worldwide (Rivera et al., 2017; Bancalari and Schade, 2020). In current clinical practice, intravitreal antivascular endothelial growth factor (VEGF) has proven to be effective for the treatment of ROP (Mueller et al., 2017). However, anti-VEGF drugs are expensive and can lead to serious complications (Morin et al., 2016; Wu and Wu, 2018). Therefore, it is important to study other drugs that can inhibit retinal neovascularization (RNV) to aid in the clinical treatment of ROP.
Studies on long noncoding RNAs (lncRNAs) have been shown to participate in several pathophysiological activities (Lekka and Hall, 2018; Wang et al., 2019). Maternally expressed gene 3 (MEG3) is a lncRNA that plays an important role in many biological processes and is widely expressed in eye tissues. MEG3 has a pro-apoptotic role and can inhibit tumor growth, partly by stimulating the accumulation of the tumor suppressor protein, p53 (Zhou et al., 2012, 2007).
The deletion of MEG3 is related to the development of cerebral vessels (You and You, 2019). In MEG3 knockout mice, angiogenesis after cerebral infarction was reduced (Shen et al., 2018). MEG3 can also interact with its targets VEGFA and VEGF receptor 2 (Fang et al., 2013). The deletion of MEG3 can promote the up-regulation of related gene expression in VEGF signaling pathway, thus promoting the increase of micro-vessel density in the cerebral cortex (Shen et al., 2018; Ye et al., 2018; Tong et al., 2019; Xiao et al., 2020). Moreover, MEG3 may be important to the generation of RNV in oxygeninduced retinopathy (OIR) models (Zhan et al., 2017; Ruan et al., 2018). The OIR model in mice is suitable for the study of various retinal neovascularization diseases, including ROP. Pathological angiogenesis is common in most retinal diseases and is detrimental to the visual health of patients. Various reports note that MEG3 can inhibit the formation of neovascularization in diabetic retinopathy (DR), which suggests that MEG3 has a potential inhibitory effect on DR (Zhang et al., 2018; He et al., 2017, 2021). However, the role of MEG3 and its mechanism of action in ROP remain unclear. Our study investigated possible mechanisms of action of MEG3 on RNV in OIR mouse models.
Materials and Methods
Ethics statement
Care and experimental manipulation of animals in this study were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of Shengjing Hospital of China Medical University, China (approval No. 2016PS074K) on February 25, 2016 and was reported compliant with the ARRIVE guidelines (Animal Research: ReportingIn VivoExperiments).
Oxygen-induced retinopathy in mice
Twenty C57BL/6 timed-pregnant dams were purchased from Changsheng Company (Shenyang, China). They were raised in the Animal Experimental Center of Shengjing Hospital Laboratory. Mice were given free access to water and food and were maintained under the conditions of a 12-hour light/dark cycle at ~23°C. One hundred and eighty 7-day-old pups were placed in a hyperoxia tank containing 75 ± 2% oxygen for 5 days. At post natal day 12 (P12), they were placed in normal room air to produce OIR modelling. At P11 (1 day before removal from the oxygen chamber), the right eyes of 90 OIR pups received an intravitreal injection of MEG3 overexpression lentivirus (Genechem, Shanghai, China) (1 μL), forming the OIR-T group, and the right eyes of the other 90 OIR pups received an intravitreal injection of PBS (1 μL), forming the OIR-C group. The intravitreal injection was performed using a micro syringe to insert the needle at 1 mm outside the corneal limbus, after the mouse eyes were dilated with compound tropicamide. A further 90 mouse pups were raised in normoxic conditions over the same time to form the control group.
Retinal vascular morphology
The mice were sacrificed under inhalation anesthesia with isoflurane (2%, Woruide, Shenzhen, China). The eyeballs from 15 mice at P17 in each group were collected. The anterior segment and vitreous of each eyeball were removed under a fluorescence microscope (Nikon, Tokyo, Japan), and the retinal tissue was carefully and completely isolated to ensure the integrity of the retinal morphology. The retinal tissue was incubated overnight at 4°C with Isolectin IB4 Alexa Fluor® dye conjugate (Invitrogen, Carlsbad, CA, USA) dissolved in PBS solution containing 1% TritonX-100 (Solarbio, Beijing, China). After fixation, the retinal tissues were cut into four sections under a stereomicroscope (Shunyu, Nanjing, China), placed on a glass slide and then observed using a fluorescent microscope (Nikon, Tokyo, Japan). Each retina was divided into 12 equal seg ments under the microscope. ImageJ 13.7C (NIH, Bethesda, MD, USA) was used to measure the clock hour scores of neovascularization and the areas of neovascularization and the non-perfusion, as previously described (Chikaraishi et al., 2007).
Hematoxylin and eosin staining
Eyeballs were collected from 15 P17 mice in each group, and then were fixed in 4% paraformaldehyde, passed through gradient dehydration by alcohol and embedded into paraffin sections (Wang et al., 2017). Ten sections of each retinal tissue were selected, deparaffinized, and stained with hematoxylin and eosin (Beyotime, Shanghai, China). The assessor, who counted the preretinal neovascular cell nuclei breaking through the inner limiting membrane (ILM), was blind to the origin of the retinae. Three visual fields were randomly selected from each slice for counting, and the average number of nuclei breaking through the retinal inner limiting membrane was calculated (Said et al., 2017).
Immunohistochemistry
Immunohistochemistry was performed using an Immunohistochemistry Kit (Boster, Wuhan, China). The preparation of paraffin sections of eyeballs was the same as for HE staining. After the paraffin sections were dewaxed, they were heated with 3% citric acid solution for antigen repair, and a goat serum from the kit was used to block the non-specific antigen before the primary antibody incubation (phospho-PI3K: dilution 1:200; phospho-Akt 1/2/3: dilution 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 hours at 37°C, followed by 30 minutes’ incubation with the goat anti-rabbit IgG (dilution 1:200; Zhongshan Jinqiao, Beijing, China) at 37°C. Then DAB reagent was used to develop the color under the microscope for 40 seconds. After re-staining with hematoxylin and gradient dehydration with alcohol, the film was sealed with neutral gum and photographed using an optical microscope (Nikon).
Immunofluorescence
The preparation and main experimental methods of immunofluorescence are similar to those of immunohistochemistry. The primary antibody was incubated (VEGF: dilution 1:200, Santa Cruz Biotechnology Inc.) for 2 hours at 37°C, then the Cy3-labeled (VEGF) goat anti-rabbit IgG (dilution 1:200; Zhongshan Jinqiao, Beijing, China) was incubated for 30 minutes at 37°C. After re-staining with DAPI (Beyotime, Shenzhen, China) the film was sealed with quench resistant tablets (Solarbio) and photographed using a fluorescent microscope (Nikon). The red fluorescence was observed at 620 nm, the blue fluorescence of DAPI was observed at 460 nm. The positive cell counts (150 sections) were averaged across the fifteen mice in each group.
Western blot analysis
The total protein was extracted from the retina of 15 mice at P17 from each group by radio-immunoprecipitation assay (RIPA), then the protein was denatured at 100°C for 5 minutes and separated according to the instructions of 10% rapid gel preparation kit (Yamei, Shanghai, China). A voltage of 80 V was used for the electrophoresis and 200 mA was used for the PVDF membrane transfer, then 5% skim milk was used to block nonspecific protein. The blots were probed overnight at 4°C with the following primary antibodies PI3K, Akt, p-PI3K, p-AKT, VEGF and β-actin (all at dilutions 1:2000, Santa Cruz Biotechnology); IL-1β, IL-6, and TNF-α (all at 1:1000, ImmunoWay Biotechnology Company, Plano, TX, USA) Next, the blots were incubated with horseradish peroxidaseconjugated anti-rabbit secondary antibodies (1:2000, Cat# 65-6120, Molecular Probes) for 2 hours at room temperature, and were visualized using enhanced chemiluminescence (Millipore, Billerica, MA, USA). Finally, the optical density of protein bands was detected using GE AI680 imaging system (GE, Boston, MA, USA). The optical density ratio of target protein to β-actin was regarded as the relative protein expression.
Real-time RT-PCR
We selected fifteen P12 mice retina of each group for PCR (ABI7500 fast, USA) to determine the mRNA level of MEG3 in the retina after intravitreal injection. TRIzol (Takara Bio Inc., Otsu, Japan) was used to extract total RNA from the retinae of mice, and the cDNA was transformed into complementary DNA (cDNA). RT-PCR was performed using SYBR Green RT-PCR main mixture (Takara, Tokyo, Japan). Sequences of primers: MEG3 F: 5′-CTG CCC ATC TAC ACC TCA CG-3′ and R: 5′-CTC TCC GCC GTC TGC GCT AGG GGC T-3′. PI3K, F: 5′-GGC TTG GAC CGA ATG CT-3′ and R: 5′-TTG TTG AAG GCT GTG GC-3′; AKT, F: 5′-AGC AAA CAG GCT CAC AGG TT-3′ and R: 5′-TAA GTC CTC CCC ATC TCC CT-3′; VEGF, F: 5′-CCC GAC AGG GAA GAC AAT-3′ and R: 5′-TCT GGA AGT GAG CCA ACG -3′; β-actin, F: 5′-GTG CTA TGT TGC TCT AGA CTT CG-3′ and R: 5′-ATG CCA CAG GAT TCC ATA CC-3′. The results were normalized with β-actin using the 2-∆∆CTmethod (Livak and Schmittgen, 2011).
Statistical analysis
No statistical methods were used to predetermine sample sizes, but our sample sizes (mouse experiments) are similar to those reported in a previous publication (Di et al., 2021). No animals or data points were excluded from the analysis. HE staining data collection was performed in a blinded manner. All other data analyses were not performed blind to the conditions of the experiments. All experiments were conducted at least in triplicate. Data results were given as the mean ± SD. The Mann-WhitneyUtest was used to analyze the data using SPSS 17.0 (SPSS Inc., Chicago, IL, USA),P< 0.05 indicated statistically significant.
Results
Overexpression of MEG3 in the retina
PCR results show that the expression of MEG3 in the retinas of the OIR-C group was lower than that in the Control group, and the expression in the OIR-T group was significantly higher than that in OIR-C group after intravitreal injection of MEG3 overexpression lentivirus (P< 0.01) (Figure 1).
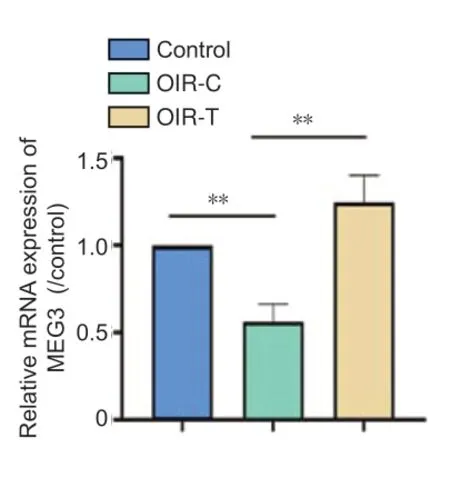
Figure 1|MEG3 mRNA expression in the retina of 12-day-old mice (quantitative real-time PCR).
MEG3 overexpression reduces RNV in OIR mice
The whole retina flat-mounted angiography was used to determine whether MEG3 overexpression can reduce RNV morphologically (Figure 2). The retinal vessels of the OIR-C group were clearly dilated and the non-perfusion area is obvious. The control group and OIR-T group had fewer neovascular clusters and non-perfusion areas (P< 0.05;Figure 2). This result suggests that the overexpression of MEG3 inhibits the neovascularization. Hematoxylin-eosin staining (Figure 3) demonstrated that the number of preretinal neovascular cells in the OIR-T group decreased significantly compared with the OIR-C group (P< 0.01).
How much time did that few more minutes take out of her day? Probably about five. Not so much time out of a busy day. So what if she got home a little later than she had planned?
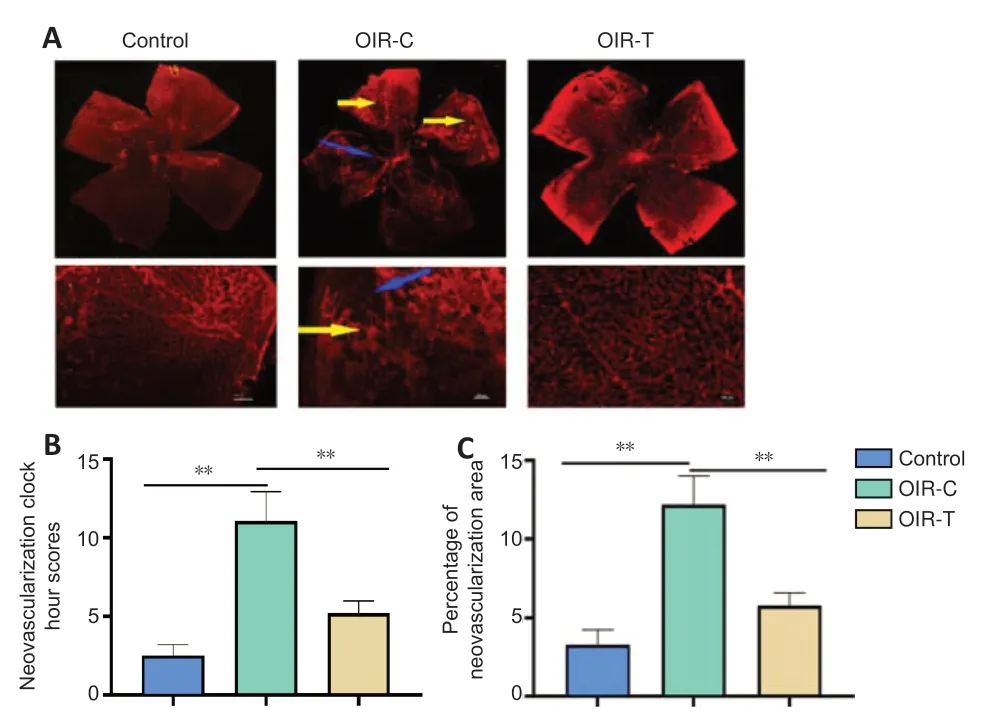
Figure 2|Retinal angiographs in mice at the age of 17 days.
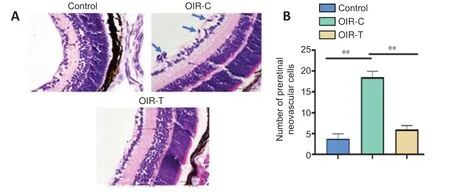
Figure 3|Morphology of retinal tissue in mice at the age of 17 days.
Expression of p-PI3K, p-Akt, and VEGF in retina
Immunohistochemical of retinal sections revealed that p-PI3K and p-Akt were more highly expressed in the OIR-C group than in the control group (P< 0.01) (Figure 4). Immunofluorescence of retinal sections revealed that VEGF was highly expressed in the OIR-C group (Figure 5). However, VEGF expressions were significantly lower in the OIR-T group than in the OIR-C group (P< 0.01).

Figure 4|Immunohistochemical results of p-PI3K and p-Akt in the retina of mice at the age of 17 days.
MEG3 overexpression regulates RNV via VEGF/PI3K/Akt signaling pathway
Western blotting showed that protein expression levels of p-PI3K, p-Akt, and VEGF in the OIR-T group were significantly reduced compared with the OIR-C group (P< 0.01) (Figure 6).
MEG3 overexpression relieves inflammation
We measured the expression of IL-1β, IL-6, and TNF-α to explore the effects of MEG3 on inflammation. The OIR-T group exhibited a significant decrease in inflammatory factors compared with the OIR-C group (P< 0.01) (Figure 7).
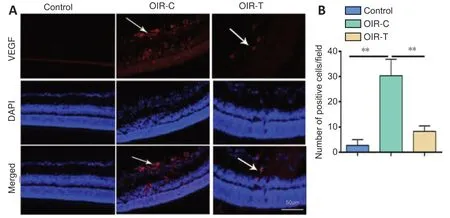
Figure 5|Immunofluorescence results of the retina of mice at the age of 17 days.
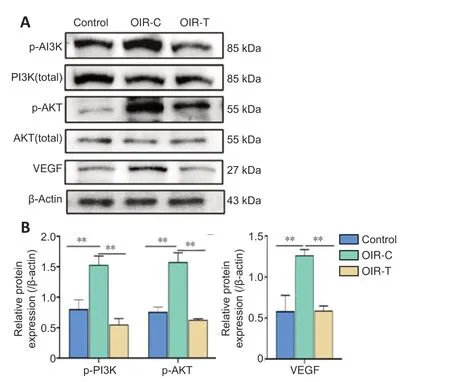
Figure 6|Protein expression levels of p-PI3K, p-AKT and VEGF in the retina of mice at the age of 17 days.
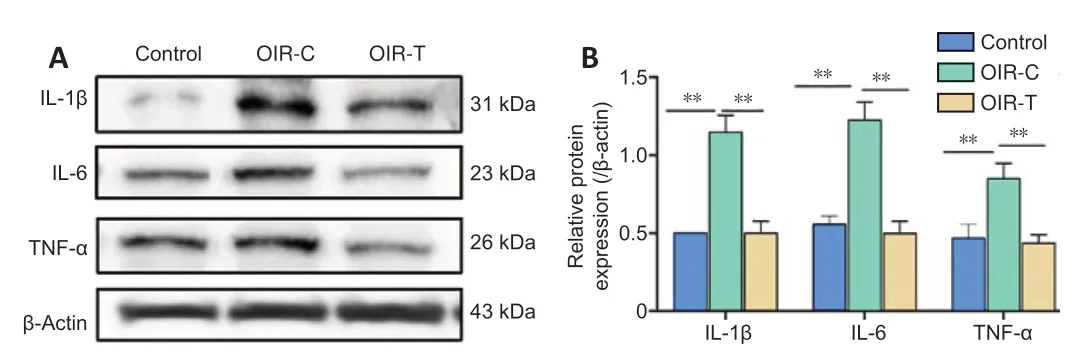
Figure 7|Protein expression level of IL-1β, IL-6 and TNF-α in the retina of mice at the age of 17 days.
Discussion
The formation of pathological angiogenesis is a complex pathophysiological process that is initiated by ischemia and hypoxia and is regulated by multiple factors and pathways including endothelium activation, proliferation, and migration and extracellular matrix remodeling (Hartnett 2015; Cayabyab and Ramanathan, 2016; Darlow and Gilbert, 2019). Angiogenesis first appears in the retinal neuroepithelial layer and then breaks through the ILM and grows along the retinal surface. This results from an imbalance between angiogenesis and inhibitory factors leading to the formation of pathological neovascularization (Kandasamy et al., 2017; Wolf et al., 2020). Some recent results indicate that lncRNA has a coding function and, although this requires further verification, there is sufficient evidence to show that lncRNAs are important for the development of various biological systems (Uchida and Dimmeler, 2015; Wu et al., 2015; Chen and Zhou 2017; Hosseini et al., 2019). Additional evidence shows that lncRNAs can regulate the pathophysiological process (Peng et al., 2016; Chi et al., 2019; Sun and Zhang, 2019).
In some studies, lncRNAs in the OIR group were found to be expressed more than 2-fold compared with that of the control group (Kumar and Goyal, 2017; Yu and Wang, 2018). Our experiments gave similar results. We found that MEG3 is highly expressed in the retina of OIR mice. The OIR model, first proposed by Smith et al. (1994), has been recognized as the standard for research to simulate ROP and other RNV diseases (Kermorvant-Duchemin et al., 2010; Chu et al., 2013). The development of retinal vessels in mice is very similar to that in humans, but they are more easily observed in mice postnatally.
In the present study, MEG3 overexpression lentivirus was injected intravitreally on P11 (1 day before removal from the oxygen chamber). Overexpression of MEG3 was shown to inhibit angiogenesis. Our western blotting results also show that inflammatory factors were lower in retinas of the OIR-T group, which indicates that MEG3 overexpression has an anti-inflammatory effect. PI3K, Akt, and VEGF levels were markedly decreased in the OIR-T group. At the protein and mRNA levels, the results indicate that MEG3 overexpression reduced the expression of VEGF, which then reduced the formation of neovascularization. These results indicate that MEG3 is important in RNV and that MEG3 overexpression inhibits RNV by inhibiting the VEGF/PI3K/Akt signaling pathway. Our results are consistent with those of Ji and Li (2019) and Sun et al. (2019), which indicates that MEG3 participates in angiogenesis through PI3K/Akt pathway.
There are several limitations to this study. Although we have shown that MEG3 overexpression can reduce the RNV in OIR mice and that it affects the PI3K, Akt, and VEGF levels, the mechanisms require further investigation and discussion. In the mouse experimental model, only RNV was simulated, which is very different from the actual pathological changes in human ROP. The OIR mice experienced normal conditions in utero, as the pathological changes occurred only after birth; therefore, the model mice did not suffer the other complications of premature infants.
In conclusion, this study mainly elucidates the important role of MEG3 in RNV but also provides additional insight towards the clinical treatment of ROP.
Author contributions:Study design: QZN; experiment implementation: YD, YW, XW, YM; data collection and analysis: YXW; manuscript drafting: YD. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81600747 (to YD), a grant from Liaoning Department of Education, No. QNZR2020010 (to YD) and a grant from 345 Talent Project of Shengjing Hospital (to YD). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Institutional review board statement:This study was approved by the Animal Ethics Committee of Shengjing Hospital of China Medical University, China (approval No. 2016PS074K) on February 25, 2016.
Copyright license agreement:The Copyright License Agreement hasbeen signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- The importance of fasciculation and elongation protein zeta-1 in neural circuit establishment and neurological disorders
- Promoting axon regeneration in the central nervous system by increasing PI3-kinase signaling
- Microglial voltage-gated proton channel Hv1 in spinal cord injury
- Liposome based drug delivery as a potential treatment option for Alzheimer’s disease
- Retinal regeneration requires dynamic Notch signaling
- All roads lead to Rome — a review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease

