Liposome based drug delivery as a potential treatment option for Alzheimer’s disease
Carely Hernandez, Surabhi Shukla
Abstract Alzheimer’s disease is a neurodegenerative condition leading to atrophy of the brain and robbing nearly 5.8 million individuals in the United States age 65 and older of their cognitive functions. Alzheimer’s disease is associated with dementia and a progressive decline in memory, thinking, and social skills, eventually leading to a point that the individual can no longer perform daily activities independently. Currently available drugs on the market temporarily alleviate the symptoms, however, they are not successful in slowing down the progression of Alzheimer’s disease. Treatment and cures have been constricted due to the difficulty of drug delivery to the blood-brain barrier. Several studies have led to identification of vesicles to transport the necessary drugs through the blood-brain barrier that would typically not achieve the targeted area through systemic delivered medications. Recently, liposomes have emerged as a viable drug delivery agent to transport drugs that are not able to cross the blood-brain barrier. Liposomes are being used as a component of nanoparticle drug delivery; due to their biocompatible nature; and possessing the capability to carry both lipophilic and hydrophilic therapeutic agents across the blood brain barrier into the brain cells. Studies indicate the importance of liposomal based drug delivery in treatment of neurodegenerative disorders. The idea is to encapsulate the drugs inside the properly engineered liposome to generate a response of treatment. Liposomes are engineered to target specific diseased moieties and also several surface modifications of liposomes are under research to create a clinical path to the management of Alzheimer’s disease. This review deals with Alzheimer’s disease and emphasize on challenges associated with drug delivery to the brain, and how liposomal drug delivery can play an important role as a drug delivery method for the treatment of Alzheimer’s disease. This review also sheds some light on variation of liposomes. Additionally, it emphasizes on the liposomal formulations which are currently researched or used for treatment of Alzheimer’s disease and also discusses the future prospect of liposomal based drug delivery in Alzheimer’s disease.
Key Words: Alzheimer’s disease; beta-amyloid; blood-brain barrier; brain delivery; drug delivery systems; encapsulated drugs; liposomes; nanoparticles; neurodegenerative diseases; PEGylation; targeted delivery; tau
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder causing problems with memory, reasoning, and judgement. Currently, there is no treatment or cure available for AD. The available drugs only aid in symptom management. Diagnosis of AD entails positron emission tomography to identify betaamyloid protein accumulation one of the culprit proteins that can cause the brain atrophy associated with AD (Varghese, 2005; Kassem et al., 2020). AD patients make up more that 5 million Americans as of 2019, estimating that number to be 16 million Americans by 2050. Currently, AD is the 6thprominent cause of death in the United States. Alzheimer’s care cost in United States for 2018 was upwards of 277 billion dollars (Kassem et al., 2020; No author listed, 2020). Patient living with AD in the late stages suffer with complications such as difficulty altering physical position, requiring support moving around, considerable weight gain or loss, gradual loss of speech, and substantial difficulties with short and long term retention (No author listed, 2020). Physiologically a healthy adult brain encompasses about 100 billion neurons. Neurons are made up of long branching extensions allowing connections between other neurons; these connections are known as synapses. Synapses release surges of chemicals shifting from one neuron and identified by another neuron (Vermunt et al., 2019; No author listed, 2020; Zameer et al., 2020). Around 100 trillion synapses are responsible for the brain’s neuronal circuits; these circuits are what create the cellular foundation of recollections, thoughts, perceptions, feelings, movement, and cognitive skills (Vermunt et al., 2019). The backfire of these synapse connections that are identified as a neurodegenerative disease; AD. The physiological backfire is caused by two proteins beta-amyloid and tau (Li et al., 2020; No author listed, 2020). AD occurs through the buildup of protein fragments known as beta-amyloid plaques or tangles of a protein known as tau (Vermunt et al., 2019). These buildups inhibit neurons from being able to conduct the necessary connections that would lead to a memory recall. Beta-amyloid plaques surround healthy neurons impeding them from connecting to one another. Beta-amyloid plaques preceded by oligomers lead to the destruction and death of neurons; rendering them incapable to process a synapse (Ostro and Cullis, 1989; Vermunt et al., 2019). Oligomers are repeated copies of similar or identical small molecules. Oligomers tend to develop during the early onset of AD, prior to the development of beta-amyloid plaques. Oligomers of beta-amyloid are identified as the highest toxic beta-amyloid group (Harilal et al., 2019; No author listed, 2020). There are several studies and clinical trials for better understanding of disease progression however, no cure has been found yet for AD. Research targeting development of method to eliminate beta-amyloid peptide build-up as well as regulating its production are the potential strategy for AD treatment (Ostro and Cullis, 1989). Researchers have a theory that balance of beta-amyloid peptide in the brain and blood stream can help clear out excess beta-amyloid from the brain (Mourtas et al., 2019; Vermunt et al., 2019). The problem has been targeting of beta-amyloid. The idea is that nanotechnology treatment can now be specified to target the beta-amyloid; introducing a small portion of the beta-amyloid protein into the blood (Vermunt et al., 2019). Tau proteins are the second hallmark of AD and are present in the axons of the nerve cells. The preliminary function of tau proteins is to form microtubules for the transport of necessary nutrients throughout the nerve cells. Nutrients within the nerve cells allow the cells to remain straight and strong. Tau tangles occur inside the neurons rendering them ineffective (Ostro and Cullis, 1989; Villemagne et al., 2013). Tau tangles found inside neurons deprive the neurons of vital molecules or nutrients as a result neuron die off. These physiological occurrence leads to inflammation and atrophy of the brain. These changes inhibit the brain from processing glucose; the main source of energy for brain (Ostro and Cullis, 1989; Vermunt et al., 2019). Patients with AD show evidence of the tau proteins disintegrating within the neurons.
Currently available AD treatment plans are entailed primarily of cholinesterase inhibitors (donepezil, galantamine, rivastigmine), N-methyl-D-aspartate (NMDA) receptor antagonists (memantine), and acetylcholinesterase inhibitor (tacrine) (Spuch and Navarro, 2011). Cholinesterase or acetylcholinesterase inhibitors improve memory, thinking, language, and thought processing (Vermunt et al., 2019). Patients of AD are identified as having lower levels of Acetylcholine (Ach). Cholinesterase inhibitors increase the levels of Ach, by inhibiting the breakdown of acetylcholine in the synaptic cleft and increasing neuron excitation (Kuo and Lee, 2016; Binda et al., 2018). ACh is a chemical courier, vital in learning and recall (Binda et al., 2018). NMDA receptor antagonists improve memory, focus, reasoning, language, and gross task recall. NMDA mechanism of action enables the transmission of electrical indicators among neurons in the brain and spinal plexus. NMDA receptors must remain open, and bind with glycine and glutamate; opening the ion channels. Glutamate is a neurotransmitter that permits cognitive function to take place. Through this regulation of NMDA receptors with such NMDA receptor antagonists such as memantine, neuronal excitability can be decreased. AD patients have an increased amount of glutamate. The disproportionate neuronal excitation causes neuronal impairment. Clinical reviews of the treatment have expressed a relief in care giver burden, and slight improvement on overall quality of life. The treatments have expressed the best result mainly in patients showing mild to moderate symptoms (Johnson and Kotermanski, 2006).
Drugs used for treatment of Neurodegenerative disease are typically administered orally; while they help manage the symptoms of the disease, however they do not accomplish much in delaying the progression The oral medication once ingested does not reach the brain in its full effect as it is metabolized by the liver rendering its availability inefficient to manage the actual disease (Spuch and Navarro, 2011). Treatments using liposomes to transport through the bloodbrain barrier (BBB) could show a potential impact on the actual disease progression by reaching the brain region requiring treatment. The objective of this review is to compile current information on how liposomes as a drug delivery system may play important role as a potential treatment option for AD. This review also briefly touches the challenges associated with drug delivery to the brain. The review describes some of the variation and modification of liposomes and also deals with current research on liposomes addressing their role as a potential treatment option for AD.
Search Strategy and Selection Criteria
Articles cited in this review published from 1997 to 2020 were searched on databases including NCBI PubMed, Web of Science, Scopus, and Google Scholar. The following keywords were used: Alzheimer’s disease; beta-amyloid; blood-brain barrier; brain delivery; drug delivery systems; encapsulated drugs; liposomes; nanoparticles; neurodegenerative diseases; PEGylation; targeted delivery and tau.
Challenges of Drug Delivery into the Blood-Brain Barrier
AD affects the brain, a part of the body associated with challenges in drug delivery and treatment due to presence of protective barriers. The central nervous system has preset barriers to protect the brain and spinal cord; unfortunately, these protective layers pose a barrier therapeutically (Vermunt et al., 2019). The central nervous system is composed of the blood-cerebrospinal fluid barrier and the BBB. These two compartments separate the circulating blood from neurons (Forlenza et al., 2010; Vieira and Gamarra, 2016). The existence of endogenous transporters in the BBB allows for selective admission of nutrients and restricting entrance to foreign substances (Mourtas et al., 2019). Numerous endogenous transporters selectively allow necessary nutrients and minerals through the blood brain barrier to the brain. The same selective entry restricts the entrance of external materials including drugs (Spuch and Navarro, 2011). This system of checks that protects the brain also renders traditional- treatment methods useless to treat areas of the central nervous system. Systemic drugs have to overcome blood brain barrier to reach the brain (Misra et al., 2003; Mourtas et al., 2019). These barriers not only impede the treatment and diagnosis of AD, but also dementia, meningitis viral and bacterial infections, Parkinson’s disease, psychiatric disorders, and acquired immune deficiency syndrome. These disease states offer a need for development of treatment model on targeting the brain. The failure of variousin vitrotrials has led to the search for a vesical that is able to transport the drug to necessary areas to target the brain (Spuch and Navarro, 2011; Davtyan et al., 2014; Vermunt et al., 2019; No author listed, 2020). The boundaries set by the BBB have set new limits for nanotherapeutics to exceed in the areas of affinity, absorption, and function of the delivery system. Modifications to increase affinity and binding of lipophilic nanoparticles onto endothelial cells heightens the transport of drugs by way of endocytosis or lipophilic route (Ostro and Cullis, 1989). Absorption of the drug is necessary to achieve a safe therapeutic index. Nanoparticles provide better release of the drug into capillaries enhancing the chances of the drug to be transported through the BBB. Targeted site delivery allows for the bypass of oral and intestinal absorption barriers and increases the bioavailability of drugs at the target site (Ostro and Cullis, 1989). Further modifications are still needed to bypass the BBB. Surface variations on nanoparticles have allowed the nanoparticles to enter the BBB; avoiding phagocytic opsonization expressing progress in drug concentration delivered to the brain (Karthivashan et al., 2018). Finally, function of the nano transporter prompt receptor mediated transcytosis allowing the drug to pass across the BBB and reach to the brain. Liposomes have been widely studied as the potential nanocarrier to enable the transport of drugs via the BBB (Karthivashan et al., 2018; Naqvi et al., 2020).
Liposomes and drug delivery strategies
Liposomes are spherical, organic nanoparticle formations, composed of lipid bilayer encompassing an aqueous core along with an impermeable exterior lipophilic phospholipid layer (Figure 1) (Ross et al., 2018; Harilal et al., 2019). The aqueous center is enclosed to envelope water-soluble agents for transport ensuring the arrival to the targeted areas (Sokolik and Maltsev, 2015). The aqueous intermediate allows for the polar segment of the molecule continues to stay in link with the polar environment, while still shielding the nonpolar segment. The outer coating surrounding the aqueous center is made up of fat referred to as a phospholipid bilayer (Harilal et al., 2019; Mourtas et al., 2019). The bilayer phospholipid layer helps transports lipid soluble agents in lipid soluble layer of cell membrane (Figure 1). Conventional liposomes are typically containing biological phospholipids and lipids such as monosialoganglioside, 1,2-distearoryl-snglycero-3-phosphatidyl choline, egg phosphatidylcholines, and sphingomyelin (Yu et al., 2011; Bender et al., 2019). The method of liposomes formulation is one way to classify distinct forms of liposome vesicles (Harilal et al., 2019; Mourtas et al., 2019). The liposomes can be classified into three groups; multilamellar vesicles (MLVs), small unilamellar vesicles (SUVs), and large unilamellar vesicles (Corace et al., 2014; Dreier et al., 2016; Kong et al., 2020). The MLVs are made up of numerous lipid layers divided by an aqueous solution; the preparation is spontaneous. MLV are formed by gentle shaking. Small unilamellar vesicles or large unilamellar vesicles vary in size and are created from homogenization of MLV with a single lipid layer (Varghese, 2005; Kassem et al., 2020). Liposomes are entailed of cholesterol plus phospholipid and active drug molecules (Ostro and Cullis, 1989; Vermunt et al., 2019). The location of drug encapsulation is determined by the drugs optimal environment. Understanding the liposomes phospholipid make up allows for a better understanding of the drug’s necessary location for transport. The phospholipid is made up of a hydrophobic tail (2 fatty acids containing 10-20 carbon atoms), and a hydrophilic head (phosphoric acid bound to a water-soluble molecule) (Harilal et al., 2019; Fonseca-Gomes et al., 2020). The lipid can range in size from 25 nm to 5000 nm microscopic fatty material. Therapeutically liposomes can increase a drug’s efficacy, stability, control release, administration via multiple routes, targeted tissue action, and assist in the reduction of unnecessary drug toxicity (Corace et al., 2014; Dreier et al., 2016; Fonseca-Gomes et al., 2020). Liposomes have been long studied as vesicles that can be engineered to offer appeal to endogenous barriers prone to turn away foreign objects (Yadav et al., 2017).
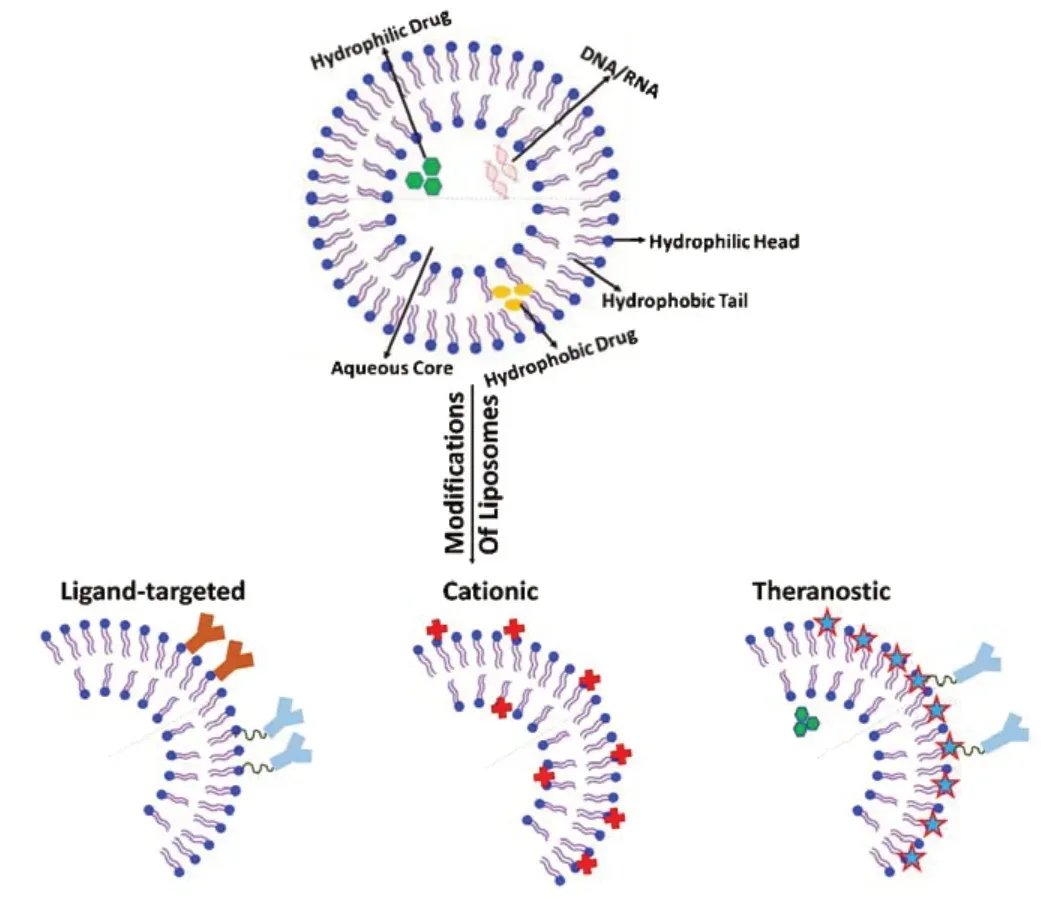
Figure 1|Basic structure of liposome and structural modification of liposomes for infiltration to brain.
Liposome’s traits that allow them to be such excellent vesicles are their ability to transport amphipathic, hydrophilic and hydrophobic drugs, minimal toxicity, excellent compatibility to its exterior environment, no immune system triggering, and targeting site of action for effective drug delivery (Harilal et al., 2019; Mourtas et al., 2019). Liposomes encapsulated agents have enhancing protection from immediate degradation from the external environment (Yu et al., 2011). Benefits of liposome use are a reduction in drug toxicity from exposure to nontarget sites, and an increase of therapeutic effectiveness (Yu et al., 2011; Bender et al., 2019).
Although liposomal formulation have many advantages and are successful in drug development process but they are not free from limitation that result from poor stability of liposomesin vivoand shelf condition The poor stability is due to potential lipid oxidation, hydrolysis, leakage, fission and fusion of the particles and reduction in hydrophilic carriers (Akbarzadeh et al., 2013; Randles and Bergethon, 2013; Pattni et al., 2015). Other limitation of liposomal drug delivery system is drug loading or encapsulation efficiency of drugs. Liposomal formulation of a drug could be created if the encapsulation efficiency of drug is such that the drug can be delivered in therapeutic doses in a reasonable amount of lipid. As lipids in higher amounts may produce toxicity and lead to saturable pharmacokinetics of drugs. The limitation of liposomal drug delivery is drug loading. Drug loading in liposomes can be done through two ways either passively (the drug is loaded or encapsulated during liposomes formation or actively (after liposome formation). Passive encapsulation of hydrophilic drugs which depends on capacity of liposome to trap aqueous buffer that has dissolved drug during the vesicle formation (Sharma and Sharma, 1997; Sharma et al., 2018). The trapping efficiency is less than 30% and is limited by the trapped volume restricted in the liposomes and solubility of drugs. Advances in techniques have provided ways to improve the encapsulation efficiency of hydrophilic drugs. For example, hydrophilic drugs that have amine groups can be actively entrapped by use of pH gradient leading to 100% efficiency. Also, active loading of amphipathic weak acidic or basic drugs can increase the encapsulation efficiency (Sharma and Sharma, 1997; Sharma et al., 2018).
Some other disadvantages associated with liposomes along are concerns of plasma instability, rapid drug release, and uptake to the reticuloendothelial system. These problems associated with liposomal based drug delivery can be addressed by some modification to liposomes. Liposomes can also undergo modifications to correct the short coming by changes in size and lipid components (Kuo and Lin, 2014; Hubin et al., 2019). Modifications are made to a liposome’s structure and properties; depending on the drug it encapsulates, and desired treatment environment needed. An example of structural additive is addition of cholesterol (Corace et al., 2014; Dreier et al., 2016). The addition of cholesterol or 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine to a primarily phospholipid liposome helps address the issue of a liposomes instability in plasma and reduction in rapid release. Also, addition of polyethylene glycol (PEG) to the surface of the liposome have shown to increase the circulation time of the drugs up to 55 hours thereby increasing the half-life of the encapsulated agents (Ostro and Cullis, 1989; Morgan, 2011; Harilal et al., 2019). The presence of PEG on surface of liposomes also reduces the mononuclear phagocyte system uptake (stealth liposomes). The stealth liposomes have been successful in avoiding mononuclear phagocyte uptake however stealth liposomes have been unsuccessful in avoiding the reticuloendothelial uptake; hence the need for a modification and the introduction of ligand targeted liposomes evolved (Kuo and Lin, 2014; Balklava et al., 2015) (Figure 1). Other modifications made to liposome include target ligand mediated liposomes, and antibodies conjugated by PEG to ensure liposome stabilization and successful treatment (Figure 1). Ligand targets offer a customized liposome that can enhance the rate of drug buildup in targeted tissue. Ligands can be comprised of antibodies, peptides, and proteins attached directly on agent loaded liposome’s phospholipid head or to a PEG chain (Figure 1). The amino acid composed ligand offers a high affinity to targeted cells and offer a less toxic treatment to the patient reducing systemic exposure (Kuo and Lin, 2014; Balklava et al., 2015; Kuo and Tsao, 2017). Negatively charged agents present a challenge in drug delivery. Liposomes with negative charges are thought to be removed swiftly from circulation decreasing therapeutic availability (Spuch and Navarro, 2011; Kuo et al., 2017). Neutral or positively charged liposomes decrease the rate of reticuloendothelial uptake allowing for an effective drug potency to reach the targeted area (Harilal et al., 2019; Mourtas et al., 2019). This manipulation of the liposome does diminish the liposomes’ ability to cross the BBB (Kuo et al., 2017). The drug delivery by liposomes occurs using passive diffusion, vesicle retention, and vesicle erosion. Liposomes are affiliated with three specific models of cell interaction for transport of agents adsorption, fusion, and endocytosis. The liposome allows improved control over the amount of time the therapeutic agent spends in the circulatory system thereby reducing toxic side effects, and prolonging the therapeutic action (Kuo and Tsao, 2017; Kong et al., 2020).
Liposomes offer the ability to transport large amounts of drug enveloped into one vesicle. Though liposomes may seem very promising however it does not come without its limitations (Kuo and Lin, 2014; Balklava et al., 2015). Administered orally the liposome would not be feasible as the stomach low pH, and possible interaction with bile salt would render the liposome unstable. Engineering liposomes to be functional in the optimal treatment environment makes them vulnerable to temperature, ultrasonic waves, magnetism, light, and pH (Kong et al., 2020). Liposomes have been observed in all its possible threats, including macrophages (Kuo and Lin, 2014). Ensuring the liposome is stable has entailed securing the bilayers are not degraded at the time of administration such as expected when taken orally, and controlling the drug release to the targeted area (Pillot et al., 1997). To ensure that the drug system does not fail, several variations of this nanocarrier are under study. The earlier mention example of how some alterations to the liposome surface would render it unable to penetrate the BBB and how the addition of targeted ligands on the other hand would enhance its ability to enter the BBB (Figure 1) shows some modified liposomes that can infiltrate the brain. The objective of a liposome is to deliver a therapeutic level of drug to the targeted area, and avoid immunological attack (Harilal et al., 2019; Mourtas et al., 2019). Some other types of liposomes currently being evaluated in research are virosomes, immunoliposomes, and gene-bases liposomes which are described below (Table 1).
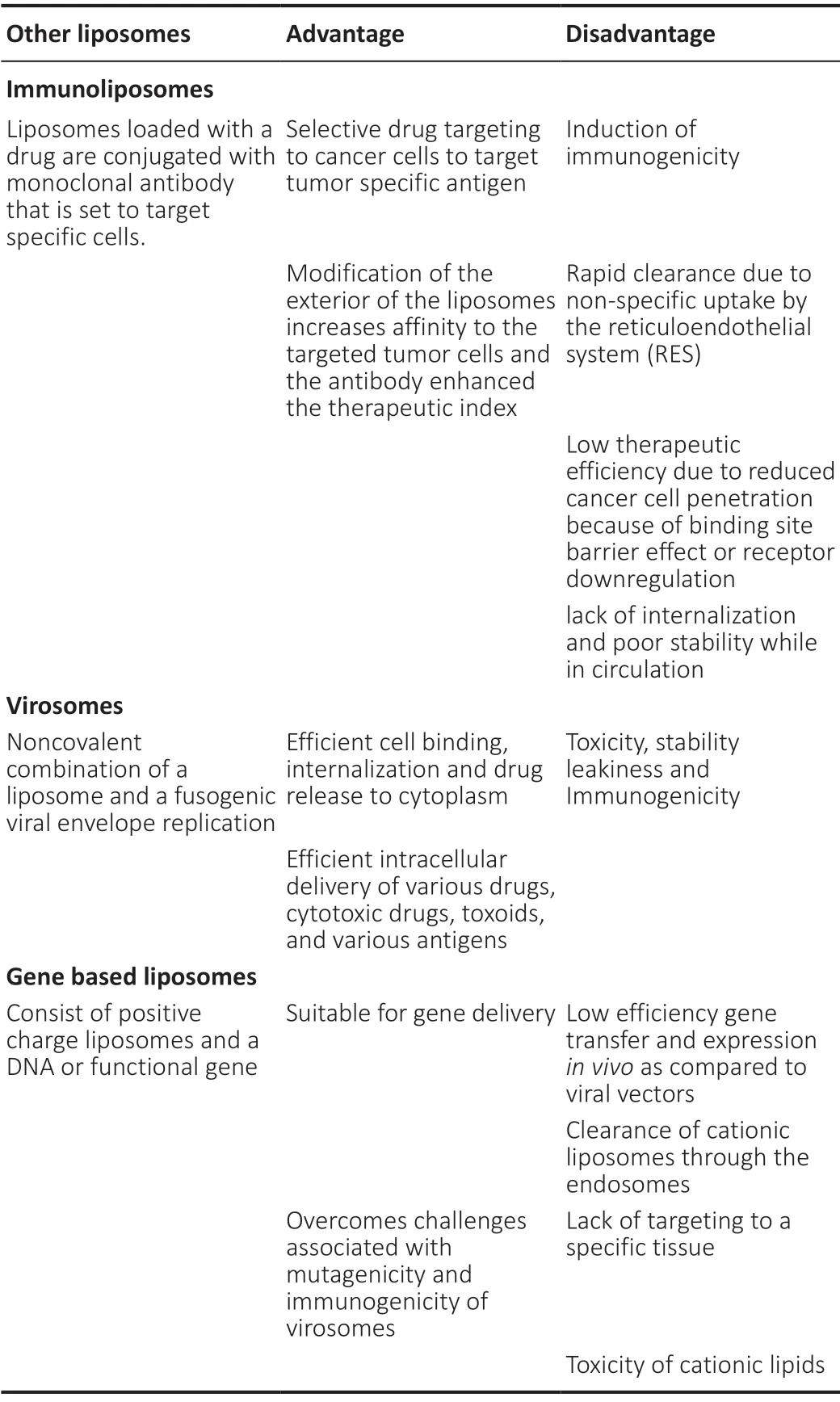
Table 1 |Comparison of modified liposomes
Fundamentals of Virosomes
Virosome is a type of liposome formulated from the bounding of noncovalent linking of a fusogenic viral envelope and a liposome (Spuch and Navarro, 2011; Carrera et al., 2013; Onodera et al., 2014). The construction of a virosome is done so by using viral fragments along with a nonviral transporter. Pseudo virions can also be constructed to enable treatment. Hemagglutinating virus of Japan or hepatitis B virus have been studied in the use of virosomes (Spuch and Navarro, 2011; Onodera et al., 2014; Kuo and Tsao, 2017). Hemagglutinating virus of Japan derivatives packaged in a liposome are being used to effectively transport DNA, siRNA, and anticancer agents (Carrera et al., 2013). Hemagglutinating virus of Japan triggered anti-tumor activity selective for cancer. Virosomes have even been researched recently for the development of a preventative and therapeutic inoculation for AD (Kuo and Chou, 2014). The inoculation containing amyloid peptides has been reported to have had positive results in many AD mice models. The effectiveness of the inoculation conveyed inhibition and removal of amyloid plaques in infected mouse brain tissue. The future research in further development of the vaccination will provide more information on the promises virosomes have to offer to neurodegenerative therapy (Carrera et al., 2013; Kuo and Chou, 2014).
Fundamentals of Immunoliposomes
Immunoliposomes are liposomes loaded with a drug and are conjugated with monoclonal antibody that are set to target specific cells. Modification of the exterior of the liposomes Increases affinity to the targeted tumor cells and the antibody enhanced the therapeutic index (Harilal et al., 2019; Mourtas et al., 2019). Immunoliposomes have shown successin vivotumor suppression therapy. Liposomes protected the encapsulated agent from macrophage through modifications, but immunoliposomes trigger immune response. Reduction of immune response by the use of immunoliposomes occurs by using Fc portion of the IgG antibody, and using humanized antibody (Spuch and Navarro, 2011; Carrera et al., 2013; Onodera et al., 2014). The Fc fragment is modified to target the human transferrin receptor. A transferrin receptor is a membrane glycoprotein and is expressed in different tissues and cells. Transferrin is present on brain endothelial cells, red blood cells and certain tumor cells. Transferrin receptors play important role as iron binding blood plasma glycoprotein that control transfer of iron into cells thereby regulating level of free iron in the cells and brain. Recent studies indicate that transferrin can cross BBB via endocytosis. RecentIn vitrostudies indicate liposomes conjugated with transferrin could penetrate BBB. Two techniques of modifications to boost affinity is the binding of the antibody directly to the head of a liposome’s phospholipid; the second technique is to attach the antibody to a PEG chain. The use of the PEG chain also enhanced the antibody retention (Carrera et al., 2013; Mourtas et al., 2019). Liver metabolism of drug is another issue that is addressed by Fc fragment; the active model used in immunoliposome is Fab-PEG-liposome. Fab-PEG-liposome has a longer retention in the blood as it avoids uptake from phagocytosis (Carrera et al., 2013; Onodera et al., 2014).
Fundamentals of Gene-Based Liposomes
DNA being a sizeable anionic molecule is difficult to transport across cells (Carrera et al., 2013; Onodera et al., 2014). Circulating deoxyribonuclease enzyme breaks down DNA, rendering it impossible to reach the target location intact. In mouse models, liposomes have demonstrated the ability to transport DNA effectively intracellularly. The components of gene-based liposomes consist of an amine hydrophilic head and a phospholipid (Carrera et al., 2013). The addition of the amine head would categorize this liposome as a cationic liposome (Figure 1) shows cationic liposome and explains the interaction between positive and negative charged molecules. The cationic liposomes have a positive exterior charge within a conventional biological pH (Kuo and Tsao, 2017). The focus of correction in gene-based liposome is to effectively reach a therapeutic threshold without a reduction of the active agent through rapid clearance. The idea is to add ligands that can target affected tissue within the cellular level without sacrificing the treatment benefit of a cationic liposome (Kuo and Tsao, 2017).
Liposome based drug delivery in treatment of AD
Various liposome-based drug delivery systems are studied as a potential treatment for AD (Table 2). Research on multi-functional and modified liposomes that aid in transport of drug across the BBB along with targeting the AD pathology is burgeoning. Some of the liposome formulation and multifunctional liposomes for potential treatment of AD are described below.
Oxidative damage is hallmark of neurogenerative diseases including Alzheimer’s disease, evidence suggest antioxidant can protect from beta amyloid toxicity. Curcumin is an extract from rhizome of the turmeric root and is used as a nutritional supplement. Pharmacologically curcumin has shown antibacterial, anti-cancer, anti-fungal, anti-inflammatory, and anti-Alzheimer properties due to its anti-oxidative properties. (Morgan, 2011; Davtyan et al., 2014; Qi et al., 2017; Chen et al., 2020). However, due to the low solubility, instability in the circulatory system, and rapid metabolism, curcumin has limited therapeutic availability taken directly in systemic administration (Morgan, 2011; Davtyan et al., 2014). The solubility was thought to be improved by having it nano transported. Studies suggest loading curcumin into a liposome increases the stability and improvise the solubility of the curcumin (Davtyan et al., 2014; Qi et al., 2017). Curcumin targets Aβ and reduces amyloid plaque formation thereby reducing amyloid associated toxicity. Mourtas et al. (2011) developed liposomes decorated with curcumin. Curcumin derivative showed high affinity for Aβ42fibrils and thereby reducing Aβ aggregation and β amyloid plaque formation. Other study done by the same group to make a multifunctional nanosized liposomes to target β amyloid. PEG -curcumin liposomes additionally decorated with antitransferrin antibody to mediate transport of curcumin through blood brain barrier demonstrated reduction in Aβ deposit and formation in hippocampus of APP/PS1 mice (Mourtas et al., 2011, 2014)
A study of multifunctional liposomes involved Liposomes loaded with mAPO and phosphatidic acid (PA). PA shows increased affinity towards binding of Aβ while mAPO enhances the crossing of blood brain barrier. This dual function mAPOPA Loaded Liposome led to disaggregation of Aβ fibrilsin vitro. The synergistic effect of both mAPO and PA was more than either of them alone (Balducci et al., 2014). Apolipoprotein E2loaded liposome has shown to have therapeutic benefits in AD through gene adaptation. ApoE2loaded to target brain tissue showed treatment benefit in AD. The model used wasin vitrousing mice AD brains administered systemic liposomes enveloping therapeutic genes (Szoka and Papahadjopoulos, 1980; dos Santos Rodrigues et al., 2019). The results of the intravenous injection with surface modified (transferrinpenetratin) liposome were effective in delivering of the therapeutic agent past the BBB (Davtyan et al., 2014; Qi et al., 2017). The mice brains had increased levels of ApoE showing a potential treatment for AD (dos Santos Rodrigues et al., 2019).
Intranasal H102 peptide loaded liposomes are studied as a potential treatment option for AD. A study including nanoparticle drug delivery system with β-sheet breaker peptide H102 demonstrated that the conjugation of H102 to liposomes has dual function of targeting the blood brain barrier and also Aβ42targeting. The study indicated neuroprotective effect of H102 loaded liposomes. The spatial learning and memory in AD mouse model were improved in H102 loaded liposomes as compared to control group. The H102 loaded liposomes seem to be a promising candidate for targeted drug delivery to brain to the AD lesions (Davtyan et al., 2014; Zheng et al., 2015).
Rivastigmine is the only FDA approved treatment for AD. However, the problem associated with oral administration of rivastigmine has poor stability and poor permeability to bypass the BBB which limits the level of efficacy of drug. To overcome this problem, alternate nasal delivery methods for rivastigmine loaded liposome are studied. To maintain stability, a positive charged inducer DDAB (Didecyldimethyl ammonium bromide) is added to liposomes, and this aids in electrostatic repulsion and decrease in interaction between liposomes. Further addition of PEG leads to formation of highly stable ESS. This modification increased the plasma and brain level of drugs compared to rivastigmine itself (electrosteric stealth liposome) (Malekpour-Galogahi et al., 2017; Kong et al., 2020). Studies are being conducted to check if rivastigmine loaded liposomes were able to reach the brain without metabolization which may lead to slowing of progression of disease (Mutlu et al., 2011; Malekpour-Galogahi et al., 2017; Nageeb El-Helaly et al., 2017).
Nano sweepers are small particles composed of chitosan core with Pegylated Aβ targeting sequences and Beclin-1 (inducer of autophagy). These nano sweepers capture extracellular Aβ and prompt their uptake into cells and also increase digestion and autophagy of Aβ. A study investigating this nano sweeper in APPswe/PS1dE9 transgenic mice shows degradation of both insoluble and soluble Aβ decreased the Aβ toxicity and also increased neuronal cell survival. The process was referred to as the mice’s brain swept; these mice were found to have fewer memory challenges (Luo et al., 2018).
In a recent study, a liposome with a wheat germ bond was used to improve neuronal survival in AD. The liposome is embedded with curcumin and a nerve growth factor the surface is than modified with wheat germ agglutin. The multifunctional liposomes down regulate the phosphorylation of two important kinases in the MAPK (Mitogen activated protein kinase) pathway which are important for neuronal survival and apoptosis. In these multifunctional liposomes, curcumin inhibits aggregation of Aβ and also alters the activity of kinases involved in apoptosis pathway. NGF activates Tyrosine Kinase A receptor which plays important role in neuronal survival. Wheat germ agglutin and PEGylation helps in improving the delivery of drugs across BBB (Kuo and Lin, 2015; Kuo et al., 2017).
A study on the quercetin loaded liposome via nasal delivery in animal model of AD showed that quercetin loaded liposome diminished the degeneration of cholinergic neurons. The possible mechanism of neuroprotection of quercetin loaded liposomes were to some extent via reduction of oxidative stress as demonstrated by increase superoxide dismutase (Phachonpai et al., 2010).
Coarce et al. (2014) developed multifunctional liposomes for delivery of an anti-Alzheimer drug tacrine hydrochloride for delivery via nasal route. These liposomes formulation consisted of phosphatidyl choline and cholesterol which were supplemented with α-tocopherol and/or Omega3 fatty acids. This methodology resulted in increase in permeation of tacrine through nasal mucosa along with enhancement in neuroprotective effect because of presence of with α-tocopherol and Omega3 fatty acids (Corace et al., 2014).
Many liposomal formulations are in clinical trials in USA for cancer treatment, fungal infection, Kaposi’s sarcoma in acquired immune deficiency syndrome. Unfortunately, liposomal formulation for brain specific drug delivery in clinical practice is limited (Pattni et al., 2015; Bulbake et al., 2017). Few liposomal drugs for brain drug delivery are listed inTable 3.

Table 3 |Status of liposomal formulations in clinical trial for brain drug delivery
Future and Prospect of Liposomes Based Drug Delivery in Alzheimer’s Disease
AD is a major culprit in diagnosed dementia worldwide. Neurodegenerative therapy comes with a variation of caveats due its difficulty in both diagnosis and treatment. The physiological barrier, BBB has restricted the use of viable oral treatments due to its impermeable structure. AD patients are typically diagnosed based on the loss of cognitive function, impaired language, the inability to rationalize, and behavioral impairment. AD also affects a patient’s quality of life. There is decline in quality of life not only for the patient, but also for the care giver leading to fatigue associated with taking care of the patient. Therapeutically few FDA approved options are available in the symptomatic management of AD. Rivastigmine, cholinesterase inhibitor is the gold standard for treatment of AD. Current available drugs aid in symptom management, however they do not delay the progression of disease. Advances in nanotechnology has led to research that allows targeted drug delivery to specific region. Liposomal drug delivery as a diagnostic and therapeutic vesicle has potential for the treatment of neurodegenerative disorders. The evolution of the liposomal drug loaded technology improves the biological distribution of the agents, reduction in reticuloendothelial uptake by macrophages, and decrease in free drug, hence reducing systemic toxicity. The variations in liposomes through modifications allows for specific targeting to reach the brain by overcoming the BBB. Multifunctional liposomes have potential to target more than one pathology of neurodegenerative diseases and thereby slowing down the disease progression.
Liposomes come with many advantages such as high loading capacity, facilitated fusion to cells (fusogenicity), hydrophilic and hydrophobic drug loading capability, and intracellular drug release via fusion. Loaded liposomes can increase the efficacy, stability, controlled release, targeted action, and therapeutic index of a drug. However, there are some disadvantages of liposomes such as high cost, leakage and fusion of enveloped agent, low solubility, and short halflife. Liposomes have a very strategic storage standard. The liposomes stability is put into question if improperly stored due to temperature, light, and even susceptibility to agitative motion. Liposomes based drug delivery nonetheless require further trials to better understand the most effective and safe route of administration. Dosage concentration is still to be determined in many of the uses of liposomes as drug delivery system. Liposomes promote drug accumulation, it is necessary to ensure the clearance rate is therapeutically relevant, as well as not toxic.
Future endeavors of research are expected to implementing the magnification of the advantages that liposomes have to offer; variation in sizes, large load transport capability, ability to offer sustained agent delivery, increase in efficacy, enhancement in therapeutic index, improved in stability of agent, selective passive targeting, and reduction in toxicity of transported agent. A variation in composition of liposome, uniformity must be observed from batch to batch. The concern with the lack of uniformity in manufacturing process of liposomes in sizable quantities leads to increased cost of production of liposome. Future research in this direction will lead to affordable cost of liposomal based drug delivery system. Reticuloendothelial system uptake of liposomes still needs further research development. The route liposomes administration is varying; though more of the recent studies are focused on liposomes as inoculations or intranasal route of drug delivery, research in the safe route of drug administration is warranted.
Although liposomes have many challenges, still liposomesbased drug delivery for the treatment of AD shows promising and potential treatment option for slowing down the progression of AD pathogenesis. The variation in liposomes and modification allows for specific targeting to cross the blood brain barrier and reach the target area in the brain. Future research is expected to be in direction of development of multifunctional liposomes that can target more than one aspect of brain pathology. Also, further strategies and research are required for safe administration of liposomal drugs delivery system.
Author contributions:Conceptualization, review, editing, and supervision: SS; writing original draft preparation: CH. Both authors have read and agreed to the published version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriatecredit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Georg Petroianu, Khalifa University, United Arab Emirates; Lionel F, Gamarra, Hospital Israelita Albert Einstein, Brazil.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- The importance of fasciculation and elongation protein zeta-1 in neural circuit establishment and neurological disorders
- Promoting axon regeneration in the central nervous system by increasing PI3-kinase signaling
- Microglial voltage-gated proton channel Hv1 in spinal cord injury
- Retinal regeneration requires dynamic Notch signaling
- All roads lead to Rome — a review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease
- SARS-CoV-2 involvement in central nervous system tissue damage

