Schwann cells differentiated from skin-derived precursors provide neuroprotection via autophagy inhibition in a cellular model of Parkinson’s disease
Jia-Nan Yan, Hai-Ying Zhang, Jun-Rui Li, Ying Chen, Yong-Cheng Jiang Jia-Bing Shen Kai-Fu Ke, Xiao-Su Gu
Abstract Autophagy has been shown to play an important role in Parkinson’s disease. We hypothesized that skin-derived precursor cells exhibit neuroprotective effects in Parkinson’s disease through affecting autophagy. In this study, 6-hydroxydopamine-damaged SH-SY5Y cells were pretreated with a culture medium containing skin-derived precursors differentiated into Schwann cells (SKP-SCs). The results showed that the SKP-SC culture medium remarkably enhanced the activity of SH-SY5Y cells damaged by 6-hydroxydopamine, reduced excessive autophagy, increased tyrosine hydroxylase expression, reduced α-synuclein expression, reduced the autophagosome number, and activated the PI3K/AKT/mTOR pathway. Autophagy activator rapamycin inhibited the effects of SKP-SCs, and autophagy inhibitor 3-methyladenine had the opposite effect. These findings confirm that SKP-SCs modulate the PI3K/AKT/mTOR pathway to inhibit autophagy, thereby exhibiting a neuroprotective effect in a cellular model of Parkinson’s disease. This study was approved by the Animal Ethics Committee of Laboratory Animal Center of Nantong University (approval No. S20181009-205) on October 9, 2018.
Key Words: alpha-synuclein; autophagosomes; autophagy; neural regeneration; neuroprotection; Parkinson’s disease; PI3K/AKT/mTOR pathway; skin-derived precursor Schwann cells
Introduction
Parkinson’s disease (PD) is the most common motor-related disease worldwide (Pereira and Aziz, 2006) and the second most common neurodegenerative disease after Alzheimer’s disease (Barker et al., 2015). The core pathology of PD is characterized by a progressive loss of dopaminergic neurons and the formation of α-synuclein (α-syn)-containing Lewy bodies, both of which usually occur in the substantia nigra and corpus striatum (Spillantini et al., 1997; Zhao et al., 2021). Increasing evidence has shown that autophagy dysfunction, endoplasmic reticulum stress, oxidative stress, and mitochondrial dysfunction may be involved in the pathogenesis of PD (Janda et al., 2015; Wu et al., 2016; Kwon et al., 2019; Zhu et al., 2019; Rao et al., 2020). Currently available medications fail to recover dopaminergic neuron loss and can produce their own adverse effects, such as dyskinesias and neuropsychiatric complications (Barker et al., 2015; Ray Chaudhuri et al., 2018).
Cell-based therapies, such as therapies using embryonic stem cells, neural stem/precursor cells, and mesenchymal stem/stromal cells, could be good strategies to replace standard levodopa therapy (Pires et al., 2017; Amin et al., 2019; Han and Hu, 2020; Forbes and Andrews, 2021). Assinck et al. (2020) reported that transplantation of skin-derived precursor differentiated into Schwann cells (SKP-SCs) diminished scarring and decreased pathological thickening of the bladder wall in rats with chronic spinal cord injury. Our preceding study reported that an SKP-SC-derived acellular matrix is beneficial for bridging the sciatic nerve gap in rats (Zhu et al., 2018). The results showed that SKP-SCs significantly increased cell proliferation and decreased the apoptotic ratio. Another previous study of ours showed that SKP-SCs had significant neuroprotective effects in a cellular model of PD (Chen et al., 2020). Therefore, cell-based therapies may be potential therapeutic strategies for PD.
SKPs are obtained from the perivascular layer of the dermis around the dermal papilla and the bulge area of hair follicles (Thomas et al., 2020), which are an unlimited source of multipotent cells. These SKPs can be differentiated into peripheral neural cells (Liebmann et al., 2012), and a large number of studies have reported that SKPs have protective effects on nerves (Wagner et al., 2014; Mozafari et al., 2015; Thomas et al., 2020). Zhu et al. (2018) presented SKP-SC-generated acellular matrix-modified chitosan/silk scaffolds to bridge the rat sciatic nerve gap. Our previous study has shown that the neuroprotective effect of SKPSCs is related to inhibiting caspase-3 activity, increasing the mitochondrial membrane potential, and upregulating the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/Bcl-2 signaling pathway (Chen et al., 2020). Moreover, we found that SKP-SC conditioned medium (CM) influenced autophagy induced by 6-hydroxydopamine (6-OHDA) neurotoxicity. However, the specific mechanism is not yet clear.
Autophagy plays an important role in the pathogenesis of PD (Hou et al., 2020; Lu et al., 2020; Zhang et al., 2021). Zhao et al. (2021) reported that microglia protected neurons by clearing neuron-released α-syn via regulating autophagy. Urano et al. (2018) reported that the expression of Parkinson protein 7 was upregulated by 6-OHDA treatment with a significant increase in LC3B-II levels. After adding the autophagy inhibitor bafilomycin A1, LC3B-II levels continued to increase. Moreover, autophagy was dramatically activated, the LC3B-II/LC3B-I ratio significantly increased, and the level of p62 decreased after 6-OHDA treatment (Kang et al., 2019). All of the above findings indicate that autophagy can clear α-syn aggregation and that overactivated autophagy may promote disease progression. In autophagy-related gene 7-deficient mice, the inhibition of autophagy led to phenotypes similar to neurodegenerative diseases, such as progressive dyskinesia, dopaminergic neuron loss, oligomerized α-syn accumulation, and the presence of ubiquitin aggregates, which supports the study of autophagy in clearing aggregation-prone proteins in PD (Ahmed et al., 2012).
The mammalian target of rapamycin (mTOR) signaling pathway, which is a central regulator of autophagy, is involved in regulating cell growth, proliferation, and life span (Chen and Zhou, 2020). The autophagy process, including initiation, progression, and termination, is modulated by the mTOR signaling pathway, which controls the activity of the UNC51-like kinase 1 (ULK1) complex, the vacuolar protein sorting 34 complex (Yang et al., 2018; Valet et al., 2020; Zhang et al., 2020). Recently, it was reported that astragalus polysaccharide could upregulate cell viability and autophagy levels, and inhibit the PI3K/AKT/mTOR pathway in 6-OHDA-injured PC12 cells (Zhang et al., 2020). Collectively, published findings support that autophagy plays a crucial role in PD. However, the underlying molecular mechanism of SKP-SCs through autophagy is still unknown. This study aimed to investigate whether autophagy regulated by SKP-SCs is involved in the neuroprotective effects in PDin vitro.
Materials and Methods
Cell culture
SH-SY5Y cells (RRID: CVCL_0019), a human neuroblastoma line identified by short tandem repeat, were purchased from American Type Culture Collection (Manassas, VA, USA). Dulbecco’s modified Eagle medium (Hyclone, Logan, UT, USA) mixed with 10% fetal bovine serum (Gibco, Billings, MT, USA) and 1% penicillin-streptomycin (Beyotime, Shanghai, China) was used to culture SH-SY5Y cells at 37°C in a 5% CO2. SH-SY5Y cells cultured without any treatment for 12 hours were used as a control group. After pretreatment with concentrated CM for 4 hours, SH-SY5Y cells were cultured with 50 μM 6-OHDA (≥ 98%; MilliporeSigma, St. Louis, MO, USA) for 12 hours.
SKP-SCs were prepared by a previously described method (McKenzie et al., 2006). SKPs were isolated from the back skins of five newborn (P2-3) pups of green fluorescent protein-transgenic Sprague Dawley specific-pathogenfree rats (supplied by the Laboratory Animal Center of Nantong University; license No, SYXK (Su) 2017-0046). This study was approved by the Animal Ethics Committee of Laboratory Animal Center of Nantong University (approval No. S20181009-205) on October 9, 2018. The newborn pups were anesthetized by hypothermia; the pups were put into a glove finger on ice until they no longer responded to a toe pinch. The pups were sacrificed by decapitation according to the lab animal protocol. After successful passaging and culture with SC differentiation medium, SKPs were differentiated into SKPSCs, which have been detected immunocytochemically by marker proteins such as glial fibrillary acid protein, calcium binding protein S100β, and p75 neurotrophin receptor (Biernaskie et al., 2006; Chen et al., 2020). SKP-SCs were maintained in SC differentiation medium, which consisted of Dulbecco’s modified Eagle medium/F12 medium (3:1; Gibco) containing 2% N2supplement (Gibco), 1% fetal bovine serum, 0.1% heregulin-1β (Gibco), and 0.02% forskolin (Gibco).
CM collection
The SC differentiation medium was used to cultivate SKP-SCs. After culturing for 48 hours, SKP-SC-CM was collected. Control medium (control CM) was the same as the SC differentiation medium without the SKP-SC culture. Then, the samples were filtered through a syringe filter (Millipore, Bedford, MA, USA) and centrifuged at 4°C. The two CMs were centrifuged at 4000 ×gfor 10-15 minutes with an Amicon Ultra-15 centrifugal filter from Millipore. The two types of concentrated CMs were collected and stored in a refrigerator (Sanyo Electric, Osaka, Japan) at -80°C for the subsequent experiments.
Cell counting kit-8 assay
The viability of SH-SY5Y cells was evaluated by the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technology, Kumamoto, Japan). SH-SY5Y cells were plated in 96-well plates with 2 × 104cells/well and then pretreated with 6-OHDA for 12 hours with or without SKP-SC-CM. Each well was treated with 10 μL rapamycin (RAPA; MedChem Express, Princeton, NJ, USA) or 3-methyladenine (3-MA; ApexBIO, Houston, TX, USA) and incubated for 10 hours at 37°C. The viability of each CM sample was measured by absorbance at 450 nm using a BioTek plate reader (BioTek, Covina, CA, USA).
TdT-mediated dUTP nick-end labeling assay
A TdT-mediated dUTP nick-end labeling (TUNEL) assay was performed using a TUNEL-488 apoptosis assay kit (Beyotime). After washing with phosphate buffered saline once, the SH-SY5Y cells were fixed for 30 minutes with 4% paraformaldehyde at room temperature. Next, 50 μL TUNEL detection solution was added to the cells, which were then placed in the dark for 1 hour at 37°C. The cells were mounted with an anti-fluorescence quencher and kept at 4°C. Samples were observed under a fluorescence microscope (Olympus, Tokyo, Japan).
5-Ethynyl-2′-deoxyuridine assay
The 5-ethynyl-2′-deoxyuridine (EdU) proliferation assay kit (Beyotime) was used to evaluate cell proliferation. After the different treatments, SH-SY5Y cells were incubated with EdU for 2 hours and fixed with 4% paraformaldehyde. Next, reaction mixture was added and then incubated for 30 minutes in the dark. After treatment, the cells were incubated with Hoechst 33342 for 10 minutes at room temperature. Images were taken using a fluorescence microscope.
Western blot analysis
SH-SY5Y cells were collected in radioimmunoprecipitation buffer (Beyotime) mixed with 50× protease inhibitor cocktail (Beyotime), 50× phosphatase inhibitor cocktail (Beyotime), and 50× ethylene diamine tetraacetic acid (Beyotime). Samples were centrifuged at 15,000 ×gfor 13 minutes at 4°C. The collected supernatants were mixed with 5× sodium dodecyl sulfate sample buffer (Beyotime) and boiled for 10 minutes at 100°C.
The proteins were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes for 1.5 hours. The membranes were blocked with 5% nonfat milk in phosphate buffered saline-Tween 20 for 1 hour, and then incubated with the primary antibodies overnight at 4°C: α-syn (rabbit, 1:800, Cat# ab51253, RRID: AB_869973, Abcam, Cambridge, MA, USA), microtubule-associated protein 1 light chain 3 (LC3B; rabbit, 1:1000, Cat# ab192890, RRID: AB_2827794, Abcam), tyrosine hydroxylase (TH; rabbit, 1:1000, Cat# 25859-1-AP, RRID: AB_2716568, Proteintech, Wuhan, China), p62 (rabbit, 1:1000, Cat# ab109012, RRID: AB_2810880, Abcam), phospho-PI3K (p-PI3K; rabbit, 1:1000, Cat# ab182651, RRID: AB_2756407, Abcam), total PI3K (rabbit, 1:1000, Cat# ab191606, RRID: AB_2891324, Abcam), phospho-AKT (p-AKT; rabbit, 1:1000, Cat# ab38449, RRID: AB_722678, Abcam), total AKT (rabbit, 1:500, Cat# ab8850, RRID: AB_2112117, Abcam), phospho-mTOR (p-mTOR; rabbit, 1:1000, Cat# CST2971S, RRID: AB_330970, Cell Signaling Technology, Danvers, MA, USA), total mTOR (rabbit, 1:500, Cat# 66888-1-Ig, RRID: AB_2882219, Proteintech), and β-actin (mouse, 1:1000, Cat# ab8226, RRID: AB_306371, Abcam). Next, the membranes were washed with phosphate buffered saline-Tween 20 and incubated with the appropriate secondary antibody at room temperature for 1 hour: goat anti-mouse IgG (1:5000, Cat# SA00001-1, RRID: AB_2722565, Proteintech) or goat antirabbit IgG (1:5000, Cat# SA00001-2, RRID: AB_2722564, Proteintech). Finally, the membranes were visualized with an enhanced chemiluminescence system (Beyotime). Images were analyzed using Image J v1.6.0 (National Institutes of Health, Bethesda, MA, USA). The optical densities were normalized to the control group and the autophagy level was defined by the LC3B and p62 levels.
Immunofluorescence analysis
After fixation with 4% paraformaldehyde, SH-SY5Y cells were blocked with 0.5% Triton X-100 (Biobasic Inc., Shanghai, China) supplemented with 10% goat serum (Biobasic Inc.) for 30 minutes and then incubated with primary antibody LC3B (1:500), p62 (1:500), and TH (mouse, 1:250, Cat# 66334-1-Ig, RRID: AB_2881714, Proteintech) overnight at 4°C. Next, the cells were covered with fluorescent antibodies at room temperature for 2 hours: Alexa Fluor 488-conjugated goat anti-rabbit IgG (H+L) (1:1000, Cat# A0423, RRID: AB_10698242, AB_2891323, Beyotime) and Alexa Fluor 647-conjugated goat anti-mouse IgG (H+L) (1:1000, Cat# A0473, RRID: AB_2891322, Beyotime). After washing with phosphate buffered saline-Tween 20, the nuclei were stained with Hoechst 33342. Images were captured using a fluorescence microscope (Olympus).
Transmission electron microscopy
After the different treatments, SH-SY5Y cells were washed several times to remove impurities derived from the culture medium. Cells were collected by scraping them into a centrifuge tube, followed by centrifugation at 1000 ×gfor 5 minutes to pellet the cells. Then, 2.5% electron microscopy-grade glutaraldehyde fixative buffer (Cat# G7651, MilliporeSigma) was added into the tube to fix cells for 2 hours at 4°C. Next, the fixative solution was gently absorbed, and no more than 100 μL of 40°C 1% agar solution was added at the wall closest to the cell mass. After the cell mass solidified with the agar, the cell mass was gently removed and suspended in a fixative solution. Samples were stored at 4°C until electron microscopy (HT7700, Hitachi, Tokyo, Japan) analysis.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). One-way analysis of variance followed by Tukey’spost hoctest was performed using GraphPad Prism 5.00 (GraphPad Software, San Diego, CA, USA). Significance was accepted at the 0.05 level of probability.
Results
SKP-SCs attenuate 6-OHDA-induced cytotoxicity and downregulate autophagy in SH-SY5Y cells
Our previous study demonstrated that SKP-SC-CM showed a cytoprotective effect in SH-SY5Y cells as measured by CCK-8 assay (Chen et al., 2020). Here, immunofluorescence showed that 6-OHDA significantly increased the intensity of LC3B immunoreactivity, where the highest intensity of LC3B was observed when cells were treated with 50 μM 6-OHDA for 12 hours (Figure 1A). This same trend was observed for the intensity of p62 (Figure 1B).
To examine the effects of SKP-SC-CM on 6-OHDA-injured cells, we measured the expression levels of TH and α-syn by western blot assay. TH is the rate-limiting enzyme in catecholamine biosynthesis, and thus is crucial in regulating the biosynthesis of dopamine (Lindenbach et al., 2017). Additionally, abnormal accumulation of α-syn protein has been reported during the progression of PD (Thomsen et al., 2021). In the present study, pretreatment with SKP-SC-CM markedly reduced α-syn expression (P< 0.05,vs. 6-OHDA group) and significantly increased TH expression (P< 0.01,vs. 6-OHDA group,Figure 1C).
We also found that compared with the control group, the 6-OHDA induction group had an increased level of autophagy, and SKP-SC-CM inhibited the increased autophagy. The LC3BII/I ratio and the expression of p62 significantly decreased in the SKP-SC-CM group (P< 0.05,vs. 6-OHDA group,Figure 1D), which indicates that the mechanism underlying SKP-SCCM-mediated neuroprotective effects against 6-OHDA may be associated with autophagy regulation (Figure 1E-H).

Figure 1|Effect of SKP-SCs on cell viability and autophagy in SH-SY5Y cells under 6-OHDA intervention.
Regulation of 6-OHDA-induced autophagy in SH-SY5Y cells by SKP-SC-CM
To further confirm the effects of autophagy regulation by SKPSC-CM, the autophagy activator RAPA or autophagy inhibitor 3-MA was added 2 hours before 6-OHDA in the 6-OHDAtreated cells. As shown inFigure 2A, SKP-SC-CM increased cell viability, which was decreased after treatment with 6-OHDA (P< 0.05,vs. 6-OHDA group). The cell viability of the SKP-SC-CM + RAPA group was lower than that of the SKP-SC-CM group (P< 0.001). Pretreatment with 3-MA increased cell viability in the 6-OHDA + 3-MA group compared with that of the 6-OHDA group (P< 0.01).
SKP-SC-CM significantly increased proliferation and inhibited the 6-OHDA-induced apoptosis in SH-SY5Y cells (Figure 2BandC). Pretreatment with RAPA reduced the increased rate of proliferation (P< 0.001,vs. SKP-SC-CM group), whereas pretreatment with 3-MA increased proliferation after exposure to 6-OHDA (P< 0.05,vs. 6-OHDA group,Figure 2D). We also examined apoptosis by TUNEL assay. As shown inFigure 2E, apoptosis in the SKP-SC-CM treatment group was decreased compared with that in the 6-OHDA group (P< 0.001), which was similar to the changes seen in the 6-OHDA + 3-MA group (P< 0.001,vs. 6-OHDA group). In contrast, apoptosis in the SKPSC-CM + RAPA group was significantly increased compared with apoptosis after SKP-SC-CM treatment alone (P< 0.001).
We also measured the expression levels of TH and α-syn. The TH level increased after pretreatment with SKP-SC-CM or the autophagy inhibitor 3-MA compared with the 6-OHDA group (P< 0.01 for both comparisons,Figure 2FandG). Similarly, SKP-SC-CM or 3-MA decreased the expression of α-syn in comparison with 6-OHDA treatment alone. RAPA reversed the decrease in α-syn expression caused by pretreatment with SKP-SC-CM (P< 0.01,vs. SKP-SC-CM group,Figure 2FandH).

Figure 2|An autophagy agonist diminishes the neuroprotective effects by reversing autophagy inhibition by SKP-SC-CM.
To investigate the relationship between autophagocytosis and the neuroprotective effect of SKP-SC-CM, we examined autophagy levels. The LC3B-II/I ratio and expression of p62 significantly decreased after treatment with SKP-SCCM (P< 0.01,vs. 6-OHDA group) or 3-MA (P< 0.001,vs. 6-OHDA group). Moreover, treatment with RAPA increased autophagy (P< 0.05,vs. SKP-SC-CM group,Figure 3A-C). Immunofluorescence staining of LC3B and p62 were both upregulated in the 6-OHDA group compared with the control group. The addition of SKP-SC-CM or 3-MA to the 6-OHDAtreated cells decreased the intensity of both LC3B and p62 (Figure 3DandE). Autophagosomes, the hallmark structures of autophagy, form characteristic double-membrane vesicles (Kwon et al., 2019). We found an increasing number of autophagosomes after the treatment of 6-OHDA. This result suggests that 6-OHDA injury may be related to the overactivation of autophagy. In addition, transmission electron microscopy analysis demonstrated that SKP-SC-CM or 3-MA in 6-OHDA-injured SH-SY5Y cells decreased the number of autophagosomes compared with 6-OHDA treatment alone (Figure 3F). These results suggested that SKP-SC-CM downregulated excessive autophagy in the 6-OHDA-induced cell model.
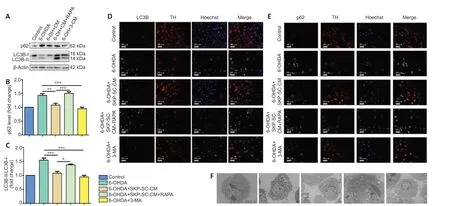
Figure 3|RAPA weakens the protective effects of SKP-SC-CM by activating autophagy in 6-OHDA-induced SH-SY5Y cells.
SKP-SC-CM regulates 6-OHDA-induced autophagy by upregulating the PI3K/AKT/mTOR pathway
The PI3K/AKT pathway exhibits prosurvival and antiapoptotic effects in neurodegenerative disease (Rai et al., 2019). The mTOR complex 1 (mTORC1) is a major negative regulator of autophagy, and the PI3K/AKT pathway is a main upstream modulator of mTORC1 (Heras-Sandoval et al., 2014). In the present study, p-PI3K and p-AKT expression was increased after SKP-SC-CM treatment compared with those in the 6-OHDA group (P< 0.001 andP< 0.01, respectively). Additionally, these increases were diminished by treatment with RAPA (P< 0.01 andP< 0.001, respectively,vs. SKP-SCCM group). Notably, the 3-MA group similarly increased p-PI3K and p-AKT expression (P< 0.001 andP< 0.01, respectively,vs. 6-OHDA group,Figure 4AandB).
Finally, we examined the levels of phosphorylated mTOR (p-mTOR) and total mTOR protein using western blot assay. AsFigure 4Cshows, the ratio of p-mTOR/mTOR in the SKP-SC-CM and 3-MA pretreatment groups was increased compared with that in the 6-OHDA group (P< 0.01 for both comparisons). After the addition of RAPA to SH-SY5Y cells, the p-mTOR/mTOR level decreased (P< 0.05,vs. SKP-SC-CM group). These results suggested that SKP-SC-CM protected against 6-OHDAinduced neurotoxicity by regulating autophagy via the PI3K/AKT/mTOR pathway (Figure 4D-F).
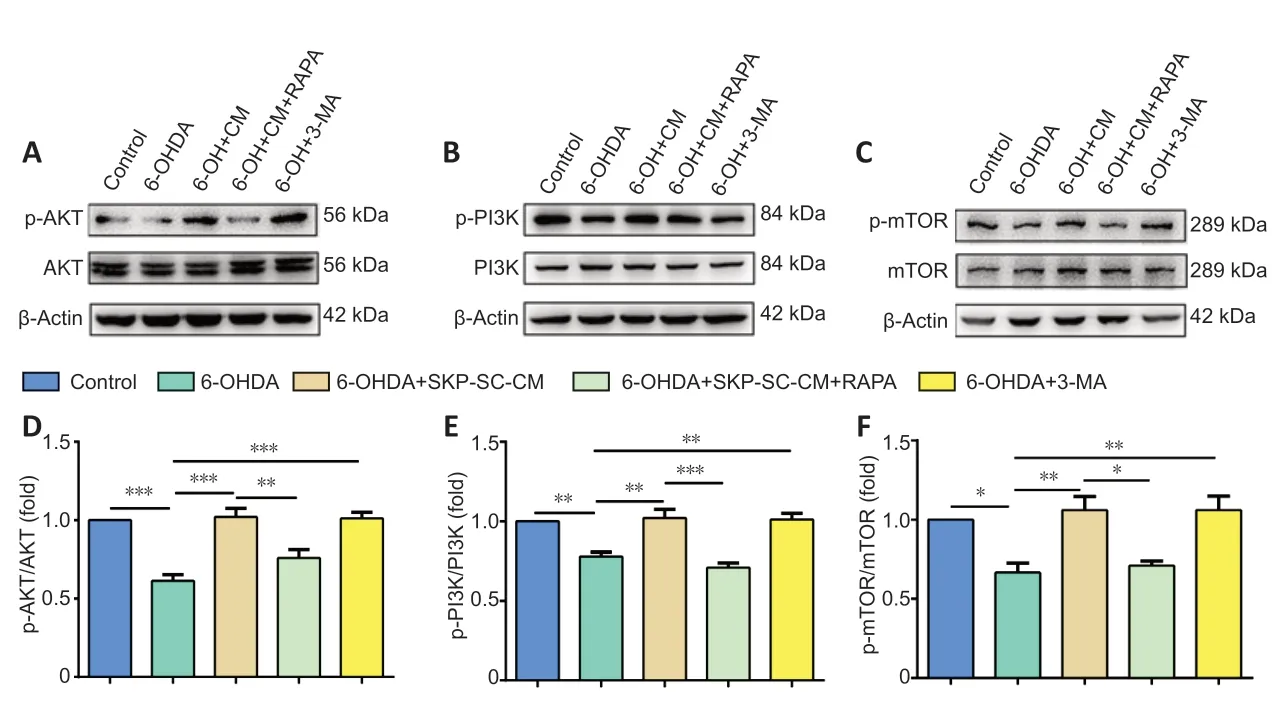
Figure 4|SKP-SC-CM regulates 6-OHDA-induced autophagy by upregulating the PI3K/AKT/mTOR pathway in SH-SY5Y cells.
Discussion
In the present study, we showed that SKP-SC-CM reduced the abnormal accumulation of α-syn and increased the number of viable cells. Furthermore, we found that SKPSC-CM regulated autophagy through the PI3K/AKT/mTOR pathway, which is a significant autophagic pathway that is involved in many diseases. One study reported that adiposederived mesenchymal stem cells played a protective role by reducing autophagy in mice after stroke (Kuang et al., 2020). Another study showed that midbrain astrocyte-derived neurotrophic factor played a cytoprotective role by inhibiting autophagy, downregulating adenosine 5′-monophosphateactivated protein kinase phosphorylation, restoring cell viability, and reducing apoptosis (Zhang et al., 2017a). Stem cells and neurotrophic factors play a key role in protecting against nervous system diseases by regulating autophagy. In our study, we found that SKP-SC-CM regulated excessive autophagy, as indicated by a decreased LC3B-II/I ratio and p62 level compared with those after 6-OHDA treatment. Our results are consistent with the studies above. However, some studies have pointed out that inhibiting autophagy may have adverse effects (Zhang et al., 2017b). Zhu et al. (2019) found that Apelin-36 exerted the cytoprotective effect and enhanced autophagy in 1-methyl-4-phenylpyridinium-treated SH-SY5Y cells.
Tovilovic et al. (2013) reported that AKT activation caused by an arylpiperazine compound contributed to autophagy inhibition in PD. Impaired activity of the PI3K/AKT signaling pathway has been found to be related to tau protein hyperphosphorylation, which may trigger pathogenesis the in central nervous system (Curtis and Bandyopadhyay, 2021). In the present study, we found that SKP-SC-CM significantly activated the AKT pathway, which is consistent with the study by Tovilovic et al. (2013). Normally, mTORC1 maintains ULK1 phosphorylation at the P757 site and inhibits the interaction between AMPK and ULK1, thus inhibiting the initiation of autophagy (Kim et al., 2011). Moreover, the activated ULK1 complex recruits PI3K and is involved in the nucleation of phagophores and installation of incipient phagosome membranes (Brier et al., 2019). A previous study has reported that the PI3K/AKT/mTOR pathway is involved in the conversion of reactive astrocytes toward oligodendrocyte lineage cells to repair spinal cord injuries (Park et al., 2021). Our study also found that SKP-SC-CM significantly increased the ratio of p-mTOR/mTOR, which is consistent with the inhibition of excessive autophagy induction in SKP-SC-CM treatment. Our results are consistent with the studies above. In conclusion, our study found that activation of PI3K/AKT/mTOR regulated excessive autophagy.
It is important to note some limitations that exist in our study. SKP-SC-CM contains multiple complex components, and the substance responsible for the neuroprotective effect remains to be elucidated. Future studies will need to be completed in animals to determine the effect of SKP-SCsin vivo.
To conclude, our present data clearly demonstrate that SKPSC-CM has neuroprotective effects in a 6-OHDA-induced SHSY5Y cell model of PD. This effect appears to be induced by the inhibition of autophagy and activation of the PI3K/AKT/mTOR pathway. Thus, SKP-SCs may be an attractive target for the treatment of PD in the future.
Acknowledgments:Thanks Dr. Mei Liu, Key Laboratory of Neuroregeneration of Jiangsu and Ministry of Education, Co-innovation Center of Neuroregeneration of Nantong University, for assistance with the experiment implementation.
Author contributions:Study design: XSG, KFK; experiment implementation: JNY, HYZ, YC; data analysis: JRL, YCJ, JBS; reagents/materials/analysis tools supporting: JBS, KFK; manuscript writing: JNY, XSG. All authors read and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Financial support:This study was supported by Technology Project ofNantong of China, Nos. JC2020052 (to XSG), JCZ19087 (to XSG); and the National Natural Science Foundation of China, Nos. 81873742 (to KFK), 81901195 (to JBS), 81502867 (to TX), 82073627 (to TX). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:This study was approved by the Lab Animal Ethical Committee of Laboratory Animal Center, Nantong University, China on October 9, 2018 (approval No. S20181009-205).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Sohyun Moon, New York Institute of Technology College of Osteopathic Medicine, USA; Ulises Gomez-Pinedo, Hospital Clinico Universitario San Carlos, Spain.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- The importance of fasciculation and elongation protein zeta-1 in neural circuit establishment and neurological disorders
- Promoting axon regeneration in the central nervous system by increasing PI3-kinase signaling
- Microglial voltage-gated proton channel Hv1 in spinal cord injury
- Liposome based drug delivery as a potential treatment option for Alzheimer’s disease
- Retinal regeneration requires dynamic Notch signaling
- All roads lead to Rome — a review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease

