Enriched environment for offspring improves learning and memory impairments induced by sevoflurane exposure during the second trimester of pregnancy
Shao-Wei Yin, Yi-Lin Meng, Chuang Li, Yuan Wang
Abstract Studies in animals indicate that sevoflurane exposure in the second trimester of pregnancy has harmful effects on the learning and memory of offspring. Whether an enriched environment can reverse the damage of sevoflurane exposure in the second trimester of pregnancy on the learning and memory of rat offspring remains unclear. In this study, rats at 14 days of pregnancy were exposed to 3.5% sevoflurane for 2 hours and their offspring were treated with an enriched environment for 20 successive days. We found that the enriched environment for offspring increased nestin and Ki67 levels in hippocampal tissue, increased hippocampal neurogenesis, inhibited glycogen synthase kinase 3β activity, and increased the expression of cell proliferation-related β-catenin and apoptosis-related Bcl-2, indicating that an enriched environment reduces sevoflurane-induced damage by increasing the proliferation of stem cells in the hippocampus. These findings suggest that an enriched environment can reverse the effects of sevoflurane inhaled by rats during the second trimester of pregnancy on learning and memory of offspring. This study was approved by the Animal Ethics Committee of Shengjing Hospital of China Medical University (approval No. 2018PS07K) on January 2, 2018.
Key Words: apoptosis; fetal; learning; memory; neurogenesis; offspring; proliferation; sevoflurane; signal pathway
Introduction
General anesthetics, local anesthetics and skeletal muscle relaxants are the most commonly used drugs in the perioperative period. Sevoflurane has a low blood gas partition coefficient and high general anesthesia efficiency. It is widely used not only in adult surgery, but also in infants and preschool children who do not tolerate surgical anesthesia. In recent years, many studies including our own have confirmed that sevoflurane exposure during pregnancy can impair the learning and memory function of offspring (Chung et al., 2017; Park et al., 2017; Wang et al., 2018a, b; Wu et al., 2018; Chai et al., 2019a, b). Thus, efforts to find a safe and effective way to intervene or reduce the neurotoxicity of sevoflurane on the offspring of pregnant women who use sevoflurane in the second trimester of pregnancy has become a focus of current research.
In 2009, a prospective epidemiological cohort study of 5320 children was conducted. The results showed that there was no significant difference in the learning ability at the age of 5 years between children delivered by cesarean section under non-general anesthesia or general anesthesia (Sprung et al., 2009). There is no definite conclusion about whether general anesthetics affect the neural development of newborns. Many studies have indicated that general anesthetics have serious neurotoxicity in animals and cause many pathophysiological changes, including neuronal apoptosis, dendritic structure change, nerve development injury, and inhibition of neurotrophic factor production (Wang et al., 2018a, b). However, it is still unclear whether general anesthetics have a similar effect in humans (Devroe et al., 2021).
After birth, brain structure and function undergo a process of continuous development and maturation, and environment plays an important role in neural development (Braun et al., 2019; Ohta et al., 2020). Early postnatal experiencedependent developmental plasticity is an important field of sensory nervous system research. In particular, enriched environment-induced brain plasticity has attracted the attention of researchers (Akhund-Zade et al., 2019; Gelfo, 2019; Gorantla et al., 2019; Singhal et al., 2019), and not only has important theoretical significance, but also has broad potential application prospects. Nestin and Ki67, which are related to nerve proliferation, are closely associated with neural development and learning and memory ability (Wang et al., 2018a, b). In addition to proliferation, neuronal apoptosis also plays an important role in neural development (Wang et al., 2018a, b).
This study focused on the enriched environment intervention for the offspring of pregnant rats who received sevoflurane anesthesia in the second trimester of pregnancy. We investigated whether the enriched environment reverses the sevoflurane-induced neural damage in offspring. The findings will help to develop safe and feasible treatment strategies for anesthetic-related learning and memory functional impairment.
Materials and Methods
Animals
The Animal Breeding Center of Shengjing Hospital of China Medical University (license No. SCXK (Liao) 2015-0001) provided 50 Sprague-Dawley rats (both sexes, 14 days old, 380-420 g, clean-grade condition). The day of pregnancy was the day of a positive smear, after female and male rats were paired. This study was approved by the Animal Ethics Committee of Shengjing Hospital of China Medical University (No. 2018PS07K) on January 2, 2018. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines.
Twenty-four pregnant rats were included in the study and divided into three groups. On the 14thday of gestation, pregnant rats were randomly divided into control (n= 8), sevoflurane (n= 8) and sevoflurane + environmental enrichment groups (n= 8).
Pregnant rats were then placed in the anesthesia box (selfmade box with length of 70 cm, width of 40 cm and height of 50 cm). The top of the box had two holes, 20 cm apart and 15 cm in diameter. Rats in the sevoflurane and sevoflurane + environmental enrichment groups were treated with 3.5% sevoflurane (Abbott Laboratories Ltd., Chicago, IL, USA) by spontaneous breathing in the anesthesia box for 2 hours, and rats in the control group were treated with 30% oxygen for 2 hours.
After the offspring were born, eight litters (24 offspring) from each group were randomly selected with three offspring from each litter serving as replicates. The offspring of the control and sevoflurane groups were fed normally in standard cages (BFSS19909, Nanjing Bianzhen Biotechnology Co., Ltd., Nanjing, China). The offspring of the sevoflurane + enriched environment group received 2 hours (8:00-10:00) of enriched environment intervention every day from days 8-28 after birth. For the environmental enrichment intervention, the offspring (5-6 offspring per cage) and the mother rats were put into the enriched environment cage and provided food and waterad libitum, pipes, bridges, wooden houses and rotating wheels of various shapes and colors in the cage (supplies from Grammy Pet Store, Shenyang, China;Figure 1A). We reset the objects every day to avoid repetition. After the intervention, the offspring rats and their mothers were raised in standard cages. The weight of offspring rats was measured before intervention each day. A water maze test was carried out on day 29 after birth (Figure 2).
Morris water maze
Eight litters from each group (with three offspring per litter as replicates) were randomly selected for the Morris water maze task (image acquisition and analysis system: Shenyang Furui Infrared Technology, Shenyang, China; Shanghai Science and Technology Co., Ltd., Shanghai, China) on day 29. The water maze experiment was carried out as previously described (Wang et al., 2018a, b). Each mouse was randomly placed into the bucket at the midpoint of each quadrant wall. The time, swimming speed and distance from the starting position to finding and staying on the platform were recorded. The underwater platform was removed and the midpoint of the quadrant corresponding to the original platform area was selected as the target square. The number of times the rats crossed the platform in 90 seconds and the activity time in the target quadrant were recorded.
Tissue preparation
After the water maze test, eight litters (three offspring within each litter as replicates) were sacrificed by CO2asphyxiation (10 L/min, carbon dioxide perfusion for about 35 minutes); after providing a normal supply of air or oxygen, the concentration of CO2was continuously increased for 35 minutes until respiratory and cardiac arrest. Hippocampal tissue was placed into 4% formaldehyde solution for 24 hours or kept in liquid nitrogen for the following test.
Immunofluorescence
Immunohistochemistry for nerve proliferation proteins in hippocampal tissue was performed as previously described (Wang et al., 2018a, b). The antibodies used in this experiment were rabbit anti-Ki67 monoclonal antibody (1:50, Cat# sc-15402, RRID: AB_2250495, Santa Cruz Biotechnology, Dallas, TX, USA) and mouse anti-nestin monoclonal antibody (1:50, Cat# sc-33677, RRID: AB_627995, Santa Cruz Biotechnology). The samples were incubated with primary antibodies overnight at 4°C. Fluorescein isothiocyanate-labeled chicken anti-mouse immunoglobulin G (IgG) (1:100, Cat# A-11001, RRID: AB_2534069, Invitrogen [China], Shanghai, China) and tetramethylrhodamine-labeled goat anti-rabbit IgG (1:50, Invitrogen [China], Cat# A16101, RRID: AB_2534775) fluorescent secondary antibodies were added the next day. After incubation with 4,6-diamino-2-phenylindole in the dark for 5 minutes, a fluorescence microscope (Tocris Bioscience, Bristol, UK) was used to obtain photographs. The relative fluorescence expression was analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
Western blot analyses were performed in accordance with methods described by Zhang and Xie (2012). Briefly, tissue proteins were extracted from homogenate, cells were lysed with lysate, and then proteins in cells were extracted. After determining the protein concentration, 50 μg of protein was electrophoresed. The protein was separated by 80-120 V electrophoresis and then transferred to polyvinylidene fluoride membranes using 100 V. To detect cell proliferation and apoptosis in hippocampal tissue, we used the following primary antibodies: mouse anti-nestin (1:1000, Santa Cruz Biotechnology, Cat# sc-33677, 220 kDa, RRID: AB_627995), rabbit anti-Ki67 (1:1000, Santa Cruz Biotechnology, Cat# sc-15402, 345 kDa, RRID: AB_2250495), mouse anti-Bcl-2 (1:1000; Santa Cruz Biotechnology, Cat# sc-8044, 28 kDa, RRID: AB_626717), mouse anti-glycogen synthase kinase-3β (GSK-3β, 1:1000; Santa Cruz Biotechnology, Cat# sc-81462, 43 kDa, RRID: AB_1123754), mouse anti-β-catenin (1:1000; Santa Cruz Biotechnology, Cat# sc-59737, 90 kDa, RRID: AB_781850), and mouse anti-β-actin (1:5000; Santa Cruz Biotechnology, Cat# sc-8432, 42/43 kDa, RRID: AB_626630). The samples were incubated with primary antibodies overnight at 4°C. After incubation with goat anti-mouse IgGhorseradish peroxidase (Santa Cruz Biotechnology, Cat# sc-2031, RRID: AB_631737, 1:5000) or goat anti-rabbit IgGhorseradish peroxidase (Santa Cruz Biotechnology, Cat# sc-2030, RRID: AB_631747, 1:5000) at 37°C for 1 hour, bound proteins were visualized using electrochemiluminescence (Quantity One, Bio-Rad, Beijing, China). The protein levels were calculated relative to β-actin. The quantitative analysis was conducted using ImageJ software.
Real-time polymerase chain reaction
Real-time polymerase chain reaction was performed as previously described (Wang et al., 2018a, b). Total RNA was extracted from hippocampal tissue using TRIzol (Invitrogen, Carlsbad, CA, USA), and then the reverse transcription reaction was performed using the SuperScript II Reverse Transcriptase (Invitrogen). Real-time polymerase chain reaction assay was done using SYBR Green polymerase chain reaction Master Mix (Applied Biosystems, Foster City, CA, USA). Primer sequences are shown inTable 1. Primer design was carried out by primer blast in National Center of Biotechnology Information. Target mRNA levels were based on the CT method (Wang et al., 2018a, b) and normalized byβ-actinmRNA.

Table 1 |Primer sequences
Terminal deoxynucleotidyl transferase dUTP nick end labeling assay
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL; A111-01, Vazyme, Nanjing, China) was performed as previously described (Wang et al., 2018a, b). Three offspring tissues were used for the TUNEL experiment. Hippocampal slices were incubated with 10% bovine serum albumin at 37°C for 30 minutes, and then incubated with TUNEL at 37°C for 2 hours. After incubation with 4,6-diamino-2-phenylindole in the dark for 5 minutes, slides were covered with mounting medium (Vector Laboratories, Burlingame, CA, USA), then observed under a fluorescence microscope. The fluorescence intensity was quantified. Cells positive for TUNEL were considered apoptotic. The quantitative analysis was conducted using ImageJ software.
Statistical analysis
No statistical methods were used to predetermine sample sizes; however, our sample sizes are similar to those reported in previous publications (Wang et al., 2018a, b). Statistical analysis was performed using one-way analysis of variance followed by least significant differencepost hoctest.P< 0.05 was considered a significant difference. Statistical analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL, USA).
Results
Effects of enriched environment on the body weight of offspring rats after sevoflurane exposure in the second trimester of pregnancy
There was no significant differences in body weight between the three groups at birth and before the beginning of the enriched environment (P> 0.05). Over time, the weight of offspring rats in the sevoflurane + environmental enrichment group increased more than that in the sevoflurane group, although the difference was not statistically significant (P> 0.05;Figure 1B).
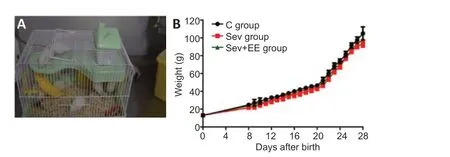
Figure 1|Effects of enriched environment on the body weight of offspring rats exposed to sevoflurane inhalation in the second trimester of pregnancy.
Figure 2|Flow chart of experimental procedure.
Enriched environment ameliorates the learning and memory impairment of offspring exposed to sevoflurane inhalation in the second trimester of pregnancy
The activity track of the Morris water maze spatial exploration test for the three groups of offspring rats is shown inFigure 3A. The activity of the control group demonstrated a clear preference for the target quadrant. The sevoflurane group lacked a clear preference; the activity was evenly distributed throughout the whole region. The activity of the sevoflurane + environmental enrichment group was increased compared with that of the sevoflurane group. The swimming speed of the sevoflurane group was significantly lower than that of the control group (P< 0.05), and the swimming speed of the sevoflurane + environmental enrichment group was significantly higher than that of the sevoflurane group (P< 0.05,Figure 3B).
In the water maze directional navigation test, the escape latency of the sevoflurane group was significantly longer than that of the control group (P< 0.05), and the escape latency of the sevoflurane + environmental enrichment group was significantly shorter than that of the sevoflurane group (P< 0.05,Figure 3C). Additionally, the path length to the platform of the sevoflurane group was significantly longer compared with that of the control group (P< 0.05), and the path length to the platform of the sevoflurane + environmental enrichment group was significantly shorter compared with that of the sevoflurane group (P< 0.05,Figure 3D). In the water maze spatial exploration test, the duration in the target quadrant of the sevoflurane group was significantly shorter than that of the control group (P< 0.05), and duration in the target quadrant of the sevoflurane + environmental enrichment group was significantly increased compared with that of the sevoflurane group (P< 0.05,Figure 3E). The number of platform crossings in the sevoflurane group was significantly decreased compared with that in the control group (P< 0.05), and the number of platform crossings in the sevoflurane + environmental enrichment group was significantly increased compared with that in the sevoflurane group (P< 0.05,Figure 3F). These results suggest that an enriched environment for offspring reduces learning and memory functional impairment induced by sevoflurane inhalation by mothers in the second trimester of pregnancy.
Enriched environment improves the proliferation of hippocampal neural stem cells in offspring exposed to sevoflurane inhalation
The immunoreactivity of Ki67 and nestin in the sevoflurane group were significantly lower than those in the control group, and the immunoreactivity of Ki67 and nestin in the sevoflurane + environmental enrichment group were significantly higher than those in the sevoflurane group (allP< 0.05,Figure 4A). Western blot and real-time polymerase chain reaction were used to detect the protein and mRNA expression, respectively, of nestin and Ki67 in the hippocampus of offspring rats. The protein and mRNA expression of nestin and Ki67 in the sevoflurane group were significantly lower than those in the control group (allP< 0.05), and the protein and mRNA expression of nestin and Ki67 in the sevoflurane + environmental enrichment group were significantly higher than those in the sevoflurane group (allP< 0.05;Figure 4BandC). These results suggest that an enriched environment reduces the inhibition of hippocampal neural stem cell proliferation induced by sevoflurane anesthesia.
Enriched environment affects the proliferation and apoptosis of hippocampal neural stem cells in offspring exposed to sevoflurane inhalation
The number of TUNEL-positive cells in the sevoflurane group was significantly higher than that in the control group, and the apoptotic level in the sevoflurane + environmental enrichment group was lower than that in the sevoflurane group (P< 0.05;Figure 5A). Previous studies have shown that sevoflurane can regulate the proliferation and apoptosis of hippocampal neural stem cells by affecting the GSK-3β pathway (Wang et al., 2018a, b). The results of western blot and real-time polymerase chain reaction showed that in the hippocampus of the sevoflurane group, the protein and mRNA expression of β-catenin and Bcl-2 were significantly lower than those in the control group (P< 0.05), and the protein and mRNA expression of GSK-3β were significantly higher than those in the control group (P< 0.05). Compared with the sevoflurane group, the sevoflurane + environmental enrichment group had significantly increased protein and mRNA expression of β-catenin and Bcl-2 (P< 0.05), and significantly decreased protein and mRNA expression of GSK-3β (P< 0.05;Figure 5BandC). All of these results suggest that an enriched environment for offspring ameliorates the alterations in proliferation and apoptosis of hippocampal neural stem cells induced by sevoflurane inhalation by mothers in the second trimester of pregnancy through the GSK-3β pathway.
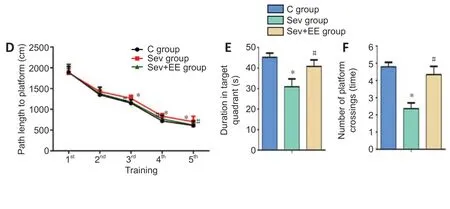
Figure 3|Effect of enriched environment on the learning and memory impairment of offspring exposed to sevoflurane inhalation in the second trimester of pregnancy.
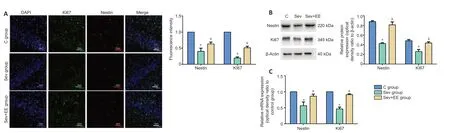
Figure 4|Effect of enriched environment on the proliferation of hippocampal neural stem cells of offspring exposed to sevoflurane inhalation in the second trimester of pregnancy.


Figure 5|Effect of enriched environment on hippocampal neural stem cells through GSK-3β pathway.
Discussion
This study is the first to confirm that an enriched environment after birth can improve learning and memory impairments of the offspring caused by sevoflurane (Consorti et al., 2019; Fàbrega et al., 2019; Kentner et al., 2019). Many studies have found that enriched environmental stimulation can improve synaptic plasticity of the nervous system, promote brain development of neonatal rats and repair brain injury induced by anesthetics (Mkwanazi et al., 2019; Moreno-Jiménez et al., 2019; Zhu et al., 2021), improve hypoxic-ischemic brain injury of neonatal rats (Ohline and Abraham, 2019; Valencia et al., 2019; Yu et al., 2020), and contribute to the repair of injury after stroke in elderly animals (Watanasriyakul et al., 2019). However, the effect of an enriched environment on the neural damage of offspring induced during pregnancy is not clear. A previous study in rats stressed during pregnancy reported that providing the mother with an enriched environment during pregnancy can produce a neuroprotective effect on offspring (Zuena et al., 2016; Pooriamehr et al., 2017). McCreary and Metz (2016) also provided mothers with an enriched environment during pregnancy and reported that the offspring exhibited transient learning and memory enhancements. This indicates that an enriched environment during pregnancy can stimulate the mother to produce a series of changes, such as neuroendocrine changes, which then have a transient impact on the offspring. However, this kind of intervention during pregnancy does not directly affect the offspring, produces effects with short duration, and is difficult to control. At present, there have been no studies to verify whether an enriched environment after birth can improve the neural damage of offspring caused by anesthetics during pregnancy. Therefore, this study chose direct environmental intervention on offspring after birth to observe whether direct intervention after birth produces beneficial effects. The intervention method of enriching the environment after birth has the advantages including low cost, no trauma, no pain, and is easy to implement. The conclusion of this study provides a potential direction for the clinical treatment of brain injury possibly induced by anesthetics during pregnancy. However, the optimal starting time of postnatal intervention and the optimal treatment course need further animal experiments to confirm.
We also found that an enriched environment after birth can reduce the inhibition of proliferation of hippocampal neural stem cells induced by sevoflurane inhalation. This indicates that postnatal enriched environmental stimulation can increase the proliferation of cells in the offspring hippocampus, which is consistent with the conclusion of Zhang et al. (2015). However, Kempermann et al. (1998) found that an enriched environment had no obvious effect on the neural proliferation of the dentate gyrus in adult and aged mice. Döbrössy et al. (2003) studied the effect of spatial learning on nerve proliferation, and found that learning had no effect on nerve proliferation in the dentate gyrus of rats aged 2 months. These contradictory results may be related to the different observation time or the sensitive period of proliferating cells. Shortly after new cells are produced, they begin to extend axons outward and establish relationships with target cells. During this period, cells may be more sensitive to an enriched environment, whereas cells that have not yet extended axons are not sensitive to an enriched environment, and thus alterations in proliferation would not observed. This may be the cause of the contradiction between the experimental data.
This study also found that an enriched environment can weaken the effect of sevoflurane on the apoptosis of hippocampal neurons in rats. The GSK-3β signaling pathway is closely related to hippocampal neurogenesis. GSK-3β and β-catenin play an important role in the process of hippocampal neurogenesis (Wang et al., 2018a, b). GSK-3β phosphorylates β-catenin and mediates its degradation. Activation of the Wnt signaling pathway inhibits GSK-3β activity and causes intracellular β-catenin to accumulate in the cytoplasm, so as to promote the proliferation of neural stem cells and inhibit their apoptosis. The results showed that the protein and mRNA expression levels of β-catenin and Bcl-2 in hippocampus of offspring rats in the sevoflurane + environmental enrichment group were higher than those in sevoflurane group, and the protein and mRNA expression levels of GSK-3β were lower than those in sevoflurane group. These results suggest that an enriched environment may inhibit neuronal apoptosis in the hippocampus of offspring rats.
We have demonstrated that an enriched environment can alleviate some adverse effects of sevoflurane. However, the adverse effects of sevoflurane may involve multiple pathways. We specifically examined cell proliferation and apoptosis and the GSK-3β pathway, but further research is needed to examine other pathways. Future studies should confirm the role of the GSK-3β pathway by inhibiting the pathway and observing the effects. Additionally, we did not investigate how the enriched environment affects the learning and memory ability of offspring by regulating the GSK-3β pathway. Future research could construct gene-deficient mice to investigate this mechanism.
In conclusion, an enriched environment inhibited the apoptosis of hippocampal neurons and the ameliorated learning and memory impairments of offspring rats exposed to a high concentration of sevoflurane during pregnancy.
Author contributions:Study design: YW, SWY; experiment implementation, data analysis and manuscript writing: SWY, CL, YLM; technical/material support and supervision: YW. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.Institutional review board statement:The study was approved by the Animal Ethics Committee of Shengjing Hospital of China Medical University (No. 2018PS07K) on January 2, 2018.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- The importance of fasciculation and elongation protein zeta-1 in neural circuit establishment and neurological disorders
- Promoting axon regeneration in the central nervous system by increasing PI3-kinase signaling
- Microglial voltage-gated proton channel Hv1 in spinal cord injury
- Liposome based drug delivery as a potential treatment option for Alzheimer’s disease
- Retinal regeneration requires dynamic Notch signaling
- All roads lead to Rome — a review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease

