Progress in perisynaptic Schwann cell and neuromuscular junction research
Chandler L. Walker
The neuromuscular junction (NMJ) is widely studied for its utility in investigating synaptic properties and processes and neuromuscular changes in response to injury, aging, and disease. The NMJ consists of three essential anatomic components, the pre-synaptic motor axon terminal, the post-synaptic nicotinic acetylcholine receptors (AchRs) on the muscle, and the perisynaptic Schwann cell (PSC), also known as the terminal Schwann cell, that caps the synapse (Figure 1A). In addition to this tri-partite construction, another cell called the kranocyte is known to be involved in the structural makeup of the NMJ though even less is known about this cell type. The PSC is a protective cellular covering for the NMJ and serves various dynamic functions under normal and pathological conditions. The NMJ is a complex multi-component site of communication between motor axons and target musculature. The PSC is a specialized non-myelinating Schwann cell that protects, nourishes, and regulates synaptic function at the NMJ (Alvarez-Suarez et al., 2020). A dynamic reciprocal communication network exists between the PSC and muscle to adapt to and help modulate alterations to NMJ activity in healthy adults. The PSC produces and secretes trophic factors that influence the axon’s health, post-synaptic muscle, and overall integrity of the NMJ. Likewise, muscle also secretes trophic factors and other chemical mediators that influence the PSC and associated localized structures at the NMJ.
These interactions also directly affect PSCs to aid in neuromuscular reinnervation following nerve injury. Retraction of the motor axon terminals and Wallerian degeneration of the axonal segments distal to the injury site occurs acutely and rapidly post-injury. As part of this early response, localized acetylcholine decreases, and the PSC becomes activated and modifies its biochemistry and morphology. Some changes include an upregulation of glial fibrillary acidic protein and neuregulin-1/Erb receptor signaling as the extended cytoplasmic processes of the PSC interact with and guide the regenerating axon toward its muscular target. Trophic factors and guidance cues produced by activated PSCs can also directly influence neuromuscular reinnervation (Figure 1B). The result is PSC-facilitated reinnervation and reestablishment of the NMJ. Expression of G-coupled protein receptor, GPR126, by PSCs improves the ability of axons to successfully reinnervate NMJs after injury, with evidence suggesting it affects trophic factor production and influences target skeletal muscle chemokine and inflammatory response (Jablonka-Shariff et al., 2020). However, the extent of GPR126 expression fluctuations in PSCs over time post-injury is unclear. The correlation between its expression and changes in other components of the NMJ that influence regenerating motor axon innervation requires further investigation (Figure 1B).
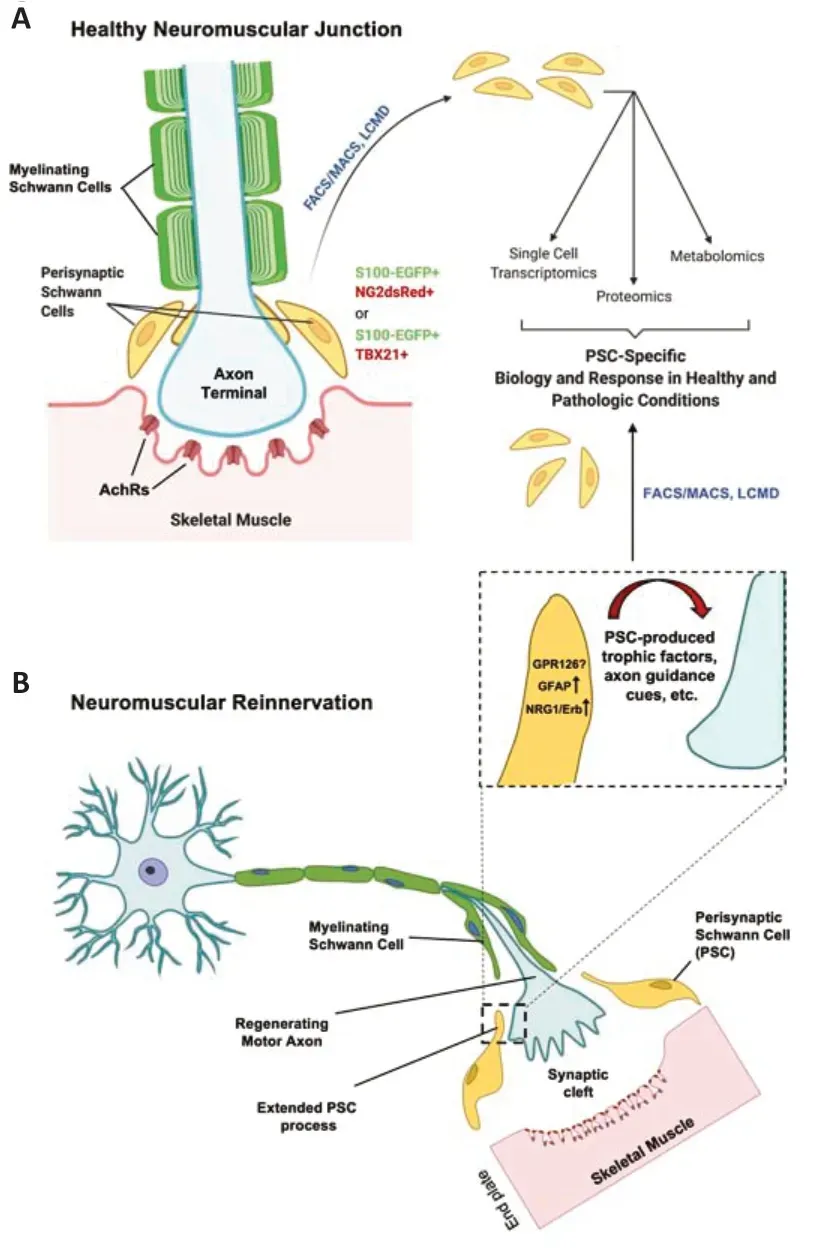
Figure 1|NG2 and TBX21 expression improves the ability to study normal and pathological responses of PSCs at the NMJ.
In neuromuscular diseases such as amyotrophic lateral sclerosis (ALS), pre-symptomatic denervation of skeletal muscle occurs, and early PSC changes are also observed. As demonstrated by Carrasco et al. (2016) a mouse model of ALS exhibits fewer PSCs at the NMJ than their wild-type counterparts. The PSCs that are present are misaligned with the post-synaptic AchRs under normal conditions (Carrasco et al., 2016). This disorganization of PSCs varies in different muscles. Those exhibiting early pre-symptomatic PSC abnormalities are more likely to be affected by the onset of denervation of motor axons from the NMJ in later stages of disease progression. Evidence to date has clearly shown relevance for PSC presence, reactivity, and morphologic alteration in innervated, denervated, and reinnervated states of the NMJ.
Despite the increasingly clear importance of the PSC in NMJ integrity and function, it has remained surprisingly challenging to study the PSC as an individual cell type. This difficulty has been due in no small part to the fact that PSCs are closely associated with other structures at the NMJ, making them hard to separate, and they express many of the same markers as other SCs, including S100 (Jablonka-Shariff et al., 2021). As such, specific markers for PSCs have not been easily identifiable. Though some markers and labeling methods for PSCs have been previously identified, such as peanut agglutinin and monoclonal antibody 2A12 labeling (Astrow et al., 1998), it is necessary to understand better the development, normal and pathological functions of PSCs. To do this, clearer and more consistently evident markers of PSCs are needed. A few have been identified, with two recent advancements highlighting specific proteins that could aid in efforts to progress PSCs research in health and disease.
Neuron-glial antigen-2 (NG2):Long studied as a marker of glial progenitor cells such as those of the oligodendrocyte lineage (McTigue et al., 2006), NG2 proteoglycan, also known as chondroitin sulfate proteoglycan 4, has recently been shown to be expressed by PSCs at the NMJ (Castro et al., 2020). By crossing mice with enhanced green fluorescent protein expressed in S100+cells (S100-EGFP) with the NG2dsRed mouse, Castro et al. (2020) were able to identify dual-labeled S100-NG2 positive cells at the motor endplate on the extensor digitorum longus muscle under normal physiologic conditions. Importantly, co-labeling these fluorescently labeled proteins did not present any adverse outcomes for PSC morphology or behavior. The group further determined retention of this marker combination under conditions of stress. A nerve crush injury was performed, and the co-expression of S100-EGFP and NG2dsRed was maintained at the NMJ, despite clear denervation observed as early as one day post-injury.
This approach affords the ability to track and better understand the PSCs changes that occur following denervation. In addition, correlations of other morphological and protein expression changes at the NMJ can be determined, such as associations between PSC-morphology change and AchR cluster organization. The reliability of fluorescent co-labeling of S100-GFP and NG2dsRed of the PSC also allows for biochemical and genetic analysis through fluorescent-assisted cell sorting under normal and pathologic conditions.
The high expression of NG2 in PSCs brings up interesting questions concerning therapeutic effects in nerve injury and neuromuscular diseases and where the mechanism of action could be. Could NG2 be involved in some of the benefits observed through trophic factor therapy? For example, NG2 has been shown to bind trophic factors PDGF-A and FGF2, and such factors are components of multifactorial treatments such as stem cell-conditioned. The identification of NG2 as a marker in PSCs sheds light on possible peripheral therapeutic target effects and highlights the PSC as a structure of interest in investigating such therapeutic mechanisms. Many new questions can now be posed concerning PSCs’ specific biochemical and functional actions under normal and pathologic conditions (Figure 1).
T-Box transcription factor 21 (TBX21):TBX21, also known as T-bet, is mainly known for its roles in lymphocyte and immune cell function, though it has recently been shown to be expressed in various human body tissues (Zhang et al., 2020). TBX21 is a transcription factor known to exhibit multiple functions in both the innate and adaptive arms of the immune system, being expressed in dendritic and natural killer cells as well as Th1 cells, CD4+and CD8+T cells, respectively (Lazarevic et al., 2013). Surprisingly, TBX21 was recently found to be explicitly expressed by PSCs by Jablonka-Shariff et al. (2021) compared to axonal and muscular components associated with the NMJ. Like Castro et al. (2020), the S100-EGFP mouse was used, and the fluorescent NMJ band was isolated from sternocleidomastoid muscle via laser capture microdissection. The tissue, enriched with fluorescent PSCs, was analyzed by RNA microarray and qT-PCR, and a list of highly expressed genes was obtained. TBX21 is unique to the PSC and was expressed over nine times higher than in nerve or muscle tissue. Immunofluorescent labeling for TBX21 showed clear co-localization with S100-EGFP and alphabungarotoxin, which labels the post-synaptic AchRs.
Though TBX21 research has focused chiefly on immune responses, such responses can be critical in instigating or progressing inflammatory conditions or disease (Lazarevic and Glimcher, 2011). Given that neuromuscular diseases, including amyotrophic lateral sclerosis (ALS), have been linked to inflammatory and immune-associated responses at the level of the neuron and NMJ (Nardo et al., 2018), the expression of TBX21 in PSCs could be significant in its regulation of such processes. Though CD8+T cells are often associated with cytotoxic effects, the major immunohistocompatibility complex I protein triggered by CD8+T cells appears to be protective of NMJ innervation in some muscles in a SOD1G93Amouse model of ALS (Nardo et al., 2018). What role TBX21 may play in this response is not clear, but the finding of this PSC-specific marker at the NMJ will aid studies to elucidate this and many more questions that have remained unanswered.
Impact on future research:The PSC is a functionally important participant in the maintenance and plasticity of the NMJ, and these recent studies have highlighted specific markers to study them more clearly and deeply than previously possible. However, the physical and physiologic characteristics of the PSC revealed thus far have only begun to address questions that now may be addressed using these new approaches. A key benefit for these advances is an improved understanding of the role of the PSC in neuromuscular denervation and reinnervation under pathologic or therapeutic conditions.
Neuromuscular disconnection represents an early anatomical pre-symptomatic peripheral pathology in ALS. The loss of NMJ innervation through axon die-back occurs before axonal degeneration. For example, in the mutant superoxide dismutase 1 G93A (mSOD1G93A) mouse models of ALS, NMJ endplate denervation is observed approximately seven weeks into the mouse lifespan. By comparison, symptoms onset occurs at about 14-15 weeks of age. In slower progressing models, such as the mSOD1G37Rmouse, functional PSC alterations are observed much earlier than morphologic fluctuations. Specifically, PSC excitability is elevated pre-symptomatically and progressing throughout the lifespan of the mouse despite sustained structural integrity (breakdown of soleus NMJs in the G37R model occurs at approximately 55 weeks of age) (Arbour et al., 2015). This pronounced and prolonged neuromuscular endurance of excitability correlated with motor neuron hyperexcitability associated with motor neuron degeneration in ALS.
Thus, the PSC functional properties support dysfunctional neuromuscular motor signaling and promote progressive motor neuron pathology as integrity is broken down later in the disease. Pre-symptomatic loss of NMJ innervation is also observed in sporadic and some forms of hereditary ALS in humans. Despite two decades worth of research into the dying-back phenomenon of motor axons in ALS, the PSC response has remained relatively understudied. Recently, more findings have shed light on the anatomical and biological changes that the PSC undergoes following NMJ denervation in ALS. With the discovery that NG2 and TBX21 can help identify and isolate PSCs, many unanswered and new questions can be addressed to establish a stronger foundation of knowledge concerning the role of PSCs in normal conditions and disease.
This work was supported by the United States Department of Veterans Affairs, No. IK2RX002688, to CLW.
Chandler L. Walker*
Department of Biomedical Sciences and Comprehensive Care, Indiana University School of Dentistry, Neuromusculoskeletal Research Group, Richard L. Roudebush Veterans Affairs Medical Center, Indianapolis, IN, USA
*Correspondence to:Chandler L. Walker, PhD, chalwalk@iu.edu.
https://orcid.org/0000-0002-8616-8263(Chandler L. Walker)
Date of submission:May 13, 2021
Date of decision:July 7, 2021
Date of acceptance:July 19, 2021
Date of web publication:October 29, 2021
https://doi.org/10.4103/1673-5374.327334
How to cite this article:Walker CL (2022) Progress in perisynaptic Schwann cell and neuromuscular junction research. Neural Regen Res 17(6):1273-1274.
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- The importance of fasciculation and elongation protein zeta-1 in neural circuit establishment and neurological disorders
- Promoting axon regeneration in the central nervous system by increasing PI3-kinase signaling
- Microglial voltage-gated proton channel Hv1 in spinal cord injury
- Liposome based drug delivery as a potential treatment option for Alzheimer’s disease
- Retinal regeneration requires dynamic Notch signaling
- All roads lead to Rome — a review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease

