Preterm neonatal brain injury: are human amnion epithelial stem cells a pan-treatment for neuroprotection and neurorepair?
Joanne O. Davidson, Simerdeep K. Dhillon, Laura Bennet
Premature birth, defined as birth before 37 weeks completed gestation, represents 11.1% of all live births worldwide and the rate has increased in almost all countries over the past few decades (Dhillon et al., 2018). Although mortality after preterm birth has fallen steadily over time, preterm infants continue to have very high rates of neurodevelopmental disability, including severe motor disorders such as cerebral palsy (Dhillon et al., 2018; Yates et al., 2021). Currently there are no standard clinical neuroprotection treatments for preterm brain injury or impaired neurodevelopment. Development of treatments is a significant challenge given that the etiology is multifactorial and potentially synergistic in nature. Preterm birth itself acts as an intersect between potential adverse antenatal and postnatal factors including acute and/or chronic hypoxia and inflammation and clinical treatments such as antenatal steroids, with post-natal cardio-respiratory compromise, ventilation, infection, and ongoing clinical drug treatments including anticonvulsants, analgesics and postnatal corticosteroids (Bennet et al., 2018; Dhillon et al., 2018; Yates et al., 2021). Compounding this is the impact of injury on the stage of neural maturation leading to greater impaired neurodevelopment (Dhillon et al., 2018; Yates et al., 2021).
Infants born preterm are at high risk for neurodevelopmental disorders, such as cerebral palsy, with the greatest risk in extremely preterm infants (< 28 weeks of gestation), who have highest rates of poor neurological outcomes, including cognitive impairment, hearing loss and retinopathy of prematurity as reviewed in (Yates et al., 2021). When examined at term equivalent age, preterm infants show compromised white matter integrity, reduced cerebral cortical and deep nuclear grey matter volumes, and increased cerebrospinal fluid volumes (Yates et al., 2021). There are some unique characteristics that contribute to the vulnerability of the preterm brain to injury. Of particular note, pre-oligodendrocytes, which are the predominant oligodendrocyte lineage type present in the preterm brain a 23-32 weeks postconceptional age, have been shown to be particularly susceptible to hypoxic injury and death, contributing to the high burden of white matter injury seen in the preterm brain (Buser et al., 2010).
Neuroprotection and neurorepair treatments are feasible because neural injury evolves over time; hours, days, weeks and longer (Dhillon et al., 2018). For example, therapeutic hypothermia (TH) for hypoxic-ischemic encephalopathy in term newborns is an effective neuroprotective therapy if started within a specific window of opportunity (Dhillon et al., 2018; Yates et al., 2021). Preclinical animal studies demonstrate that this treatment is most beneficial started early; within 6 hours of the end of an insult, and continued for 3 days. TH is an example of a pantherapy, i.e., a treatment that is effective by working through many mechanisms of action, but with a very defined therapeutic window (Yates et al., 2021). It is notable, however, that many babies do not benefit from hypothermia even when started within 6 hours of delivery (Yates et al., 2021). For many cases this may be due to the insult having started well before birth and thus injury has evolved beyond the optimal period to start TH (Dhillon et al., 2018; Yates et al., 2021). Currently, TH is not used for preterm infants due to safety concerns, but as a population they are more likely to experience injurious insults before birth (Dhillon et al., 2018; Yates et al., 2021). For example, a recent large cohort of 115,502 preterm infants delivered in the USA between 2008 and 2011, reported that moderate to severe hypoxicischemic encephalopathy occurred at a rate of 37.3/1000 babies born before 37 weeks of gestation (Manuck et al., 2016). Further, diagnosing hypoxic-ischemic encephalopathy in the preterm infant is difficult, less is known about the evolution of injury in preterms with hypoxic-ischemic encephalopathy, and overall with inflammation/infection, the synergistic effects of multiple insult hits, the additive effects of other factors such as clinical treatments, gestational age and sex (Dhillon et al., 2018; Yates et al., 2021). The challenge of developing treatments for preterm babies is further complicated by a lack of robust diagnostic and prognostic biomarkers to determine those at risk of injury and different phases in the evolution of injury.
Thus, in developing neuroprotective, and indeed neurorepair therapies for preterm babies a pan-therapy approach that has a wide window of opportunity is advantageous. Such therapies ideally target key mechanisms of injury through anti-inflammatory, anti-oxidant and anti-apoptotic actions for example, to create a less toxic environment for proliferating cells to survive and develop as well as foster neurorepair through release of factors supporting cell proliferation, development and integration into and optimization of the neural network. Current candidates for preterm neuroprotection being tested include melatonin, creatine and recombinant erythropoietin (rEPO) (Dhillon et al., 2018; Yates et al., 2021). The most detailed trial to date is with rEPO, which reported that repeated high doses of rEPO in extremely preterm infants starting on average 24 hours after birth was not associated with improved neurodevelopmental outcomes at 24 months of age (Juul et al., 2020). Given the promise of pre-clinical data, it is not yet clear why this trial did not have better outcomes. It may be that the delay in starting the treatment suggests a defined window of opportunity to start this therapy. Equally, rEPO may be a treatment suited to a much later start time (Dhillon et al., 2018; Yates et al., 2021). Further, the protocol of repeated but intermittent use of rEPO was perhaps not an optimal approach given the relatively fast clearance of rEPO out of the circulation, or it may be that treatment duration needs to be longer.
Stem cells are also being evaluated (Bennet et al., 2018; Nair et al., 2020; Yates et al., 2021). They have utility as a neuroprotection agent in a variety of ways through the release of chemokines and cytokines, trophic factors and extracellular vesicles which reduce inflammation and programmed cell death and promote cellular proliferation, development and cell-cell communication, they also have mitoprotective effects that act to reduce oxidant release and stabilize cellular metabolism (Bennet et al., 2018; Nair et al., 2020; Yates et al., 2021). A variety of different types of stem cells are being tested (Bennet et al., 2018; Nair et al., 2020), but in this brief review we examine the effects of human amnion epithelial cells (hAECs).
hAECs offer a number of practical advantages (Bennet et al., 2018; Yates et al., 2021). They can be readily harvested from the amnion membranes, which surround the fetus in utero and are discarded at birth, therefore they do not require invasive extraction, making them readily available for rapid treatment in the early postnatal phase. hAECs are pluripotent, are capable of differentiating into cell types of all three germ layers, are nontumorigenic and non-immunogenic (Bennet et al., 2012). Further, they have significant immunomodulatory properties, including increasing anti-inflammatory cytokines and decreasing pro-inflammatory cytokines as well as increasing the release of neurotrophic factors including brain derived neurotrophic factor, glial derived neurotrophic factor and neurotrophin-3, making them ideal as a generic pan-therapy (Bennet et al., 2012) (Figure 1).
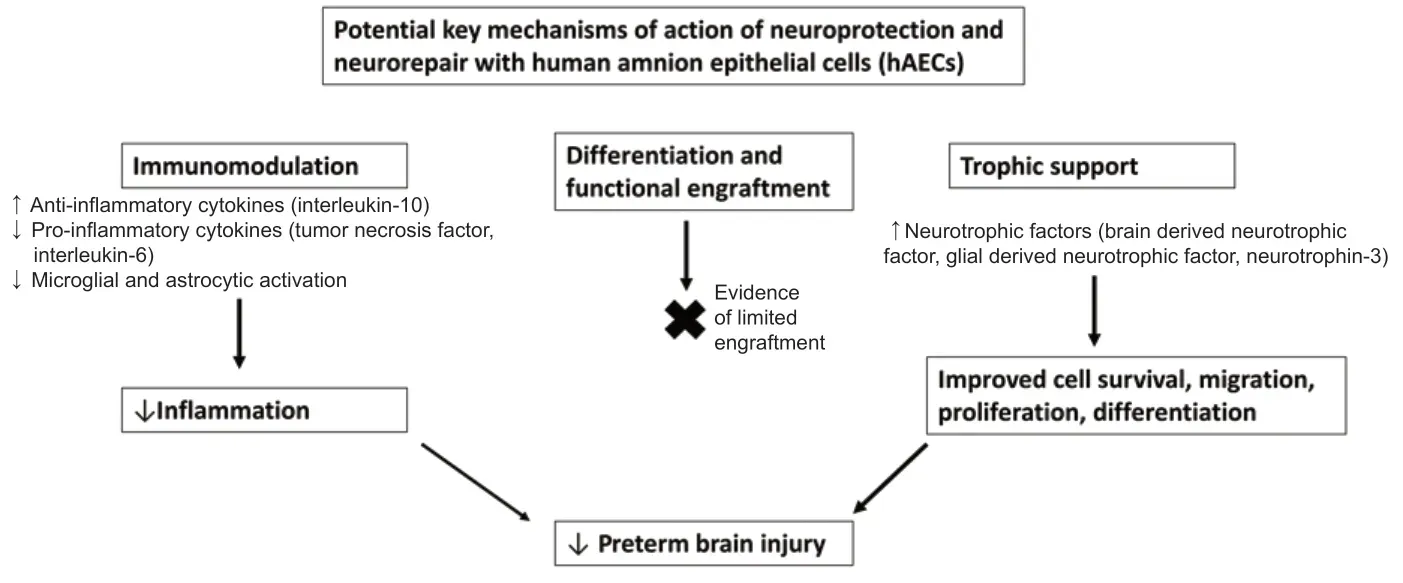
Figure 1|Schematic diagram outlining the potential key mechanisms of action of neuroprotection and neurorepair with human amnion epithelial cells (hAECs), which include immunomodulation and trophic support, with little evidence of functional engraftment of cells into the brain.
Utilizing a neural injury model in preterm fetal sheep, we have recently shown that hAEC treatment is neuroprotective after hypoxiaischemia (HI) and hAECs have a relatively long therapeutic window of opportunity for delayed treatment (van den Heuij et al., 2019; Davidson et al., 2021). We demonstrated that a one hour administration of 1 × 106hAECs into the lateral ventricles either at 2 hours or 24 hours resulted in similar anti-inflammatory, anti-gliotic and neuroprotective effects when assessed at 7 days post-HI (Davidson et al., 2021). hAEC treatment was associated with a significant reduction in microglia in the white and grey matter and astrocytes in the white matter and partial improvement in myelination, but no effect on oligodendrocyte survival. Further, neuronal survival was increased in the hippocampus by both infusion protocols but only increased in the striatum and thalamus when hAECs were administered from 24 hours after HI.
Surprisingly, however, despite the significant anti-inflammatory effects of hAEC treatment seen in this study, there was no improvement in oligodendrocyte cell survival. This highlights the complex relationship between inflammation and cell death that may relate to the dynamic changes in microglial phenotype and function over time, which determines their relative proinflammatory and reparative roles (Bennet et al., 2018). It is important in future studies that we better understand the dynamic nature of microglia and the changing nature of the pathogenic landscape relative to insults so that we can optimize the benefits of hAEC treatment and minimize any negative synergistic relationships.
We have also recently demonstrated that repeated doses of hAECs at 24 hours, 3 and 10 days after HI was associated with improved brain weight, improved oligodendrocyte maturation and myelination, reduced microglia and astrocyte number and reduced neuroinflammation after 21 days recovery (van den Heuij et al., 2019). Notably in this study, hAECs were successfully given intranasally, which is a more clinically relevant mode of delivery.
In mice exposed to the gram-negative bacteria outer membrane component lipopolysaccharide (LPS) on embryonic day 16 and hyperoxia (65% oxygen) on postnatal day zero, hAEC treatment at P4 rescued the decreased body weight, reduced apoptosis and astrocyte aerial coverage in the white matter but interestingly, increased the density of total and activated microglia (Leaw et al., 2017). However, when primary microglia were stimulated with LPS for 24 hours, followed by co-culture with hAEC conditioned medium for 48 hours, the hAEC conditioned medium increased microglial phagocytic activity, decreased microglia apoptosis and decreased expression of the M1 activation marker CD86, suggesting that hAECs are capable of directly immunomodulating microglia (Leaw et al., 2017).
Encouragingly, early and delayed treatment with hAECs has been shown to be neuroprotective in a fetal model of inflammation induced by intravenous administration of LPS (Yawno et al., 2017). When LPS was given for 3 consecutive days (109-111 gestational days), with 3 intravenous doses of 60 million hAECs given starting 24 hours later (110-112 days), there was significant attenuation of activated microglia, reduced number of pyknotic cells and significantly more oligodendrocytes and myelin basic protein compared to animals treated with LPS only (Yawno et al., 2017).
Evidence suggests that hAECs may also have utility in reducing injury related to ischemic stroke and restoring functionality even if treatment is delayed by up to 3 days in young and old mice (Evans et al., 2018). When hAECs were given acutely after stroke there was a reduction in neural numbers of immune cells and M1 polarized macrophages, while treatment at 24 hours modified the presence of both myeloid and lymphoid cell lineages (Evans et al., 2018). Thus, delayed treatment may be beneficial for recovery from stroke via modulation of inflammation through both innate and adaptive immune responses (Evans et al., 2018). hAECs are being investigated in other inflammatory conditions such as ventilation induced neonatal brain injury and bronchopulmonary dysplasia, which itself is associated with preterm brain damage (Yates et al., 2021).
In conclusion, a variety of studies of injury related to different causes has demonstrated that hAECs can be protective, even when the onset of treatment is delayed (at least to 24 hours). This is of significant benefit in the treatment of preterm newborns who are exposed to multiple mediators of injury, but where it is even difficult to determine risk for acute HI at an early stage (Yates et al., 2021). Significant further work is required to determine optimal timing of doses relative to the pathogenic and repair neural landscapes and dose as well as dosing regimen for multiple doses as a variety of studies have shown that stem cells induce differential effects on neurorepair with single and repeated doses as both injury and repair evolve (Bennet et al., 2018; Nair et al., 2020). Inflammation is a key common target and in this regard it is important that we increase our understanding of the complex and dynamic changes in microglial phenotype and function during the evolution of preterm brain injury to improve our ability to target immunomodulatory treatments such as hAECs, to further optimize their efficacy.
The present study was supported by the Health Research Council of New Zealand (17/601, 12/613) (to LB).
Joanne O. Davidson, Simerdeep K. Dhillon, Laura Bennet*
Department of Physiology, University of Auckland, Auckland, New Zealand
*Correspondence to:Laura Bennet, PhD, l.bennet@auckland.ac.nz.
https://orcid.org/0000-0002-4336-7596(Laura Bennet)
Date of submission:March 25, 2021
Date of decision:May 11, 2021
Date of acceptance:July 8, 2021
Date of web publication:October 29, 2021
https://doi.org/10.4103/1673-5374.327339
How to cite this article:Davidson JO, Dhillon SK, Bennet L (2022) Preterm neonatal brain injury: are human amnion epithelial stem cells a pantreatment for neuroprotection and neurorepair? Neural Regen Res 17(6):1261-1262.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- The importance of fasciculation and elongation protein zeta-1 in neural circuit establishment and neurological disorders
- Promoting axon regeneration in the central nervous system by increasing PI3-kinase signaling
- Microglial voltage-gated proton channel Hv1 in spinal cord injury
- Liposome based drug delivery as a potential treatment option for Alzheimer’s disease
- Retinal regeneration requires dynamic Notch signaling
- All roads lead to Rome — a review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease

