Trace amine-associated receptors at the cross-road between innate olfaction of amines, emotions, and adult neurogenesis
Evgeniya V. Efimova, Nataliia V. Katolikova, Evgeny V. Kanov, Raul R. Gainetdinov
Trace amines are the class of endogenous biogenic amines that traditionally include betaphenylethylamine, p-tyramine, tryptamine, octopamine, and others. Many trace amines represent products of amino acids decarboxylation by bacterial decarboxylases during tissue putrefaction or by endogenous decarboxylases in the body. Production of trace amines by gut microbiota is also known (Berry et al., 2017; Gainetdinov et al., 2018). Thus, trace amines are enriched during the decomposition of proteins and concentrated in certain bodily fluids. Their physiological action in mammals has been noted a long time ago, however, they were considered mostly as by-products of amino acid and monoamine metabolism. This was changed with the discovery in 2001 of trace amine-associated receptors (TAARs), a family of G protein-coupled receptors that are activated by trace amines. In humans, 6 types of functional TAAR receptors were identified - TAAR1, TAAR2, TAAR5, TAAR6, TAAR8 and TAAR9 (Berry et al., 2017; Gainetdinov et al., 2018). Since then, there is a growing interest in this family of receptors as possible new targets for pharmacotherapy. Indeed, several psychotropic substances have been shown to display high affinity to the most studied of the TAAR receptors - TAAR1, which has notable expression in the brain and some peripheral tissues (Berry et al., 2017). TAAR1 can modulate classical brain neurotransmitter systems - dopamine, serotonin, and glutamate, that are involved in the pathogenesis of many neuropsychiatric disorders. Indeed, the preclinical study of TAAR1 agonists showed them to be promising for the treatment of schizophrenia, drug dependence, depression and bipolar disorder (Berry et al., 2017; Gainetdinov et al., 2018). TAAR1 is already proven clinically as a novel pharmacological target. In clinical trials, TAAR1 agonist showed great promise for the treatment of schizophrenia with a unique mechanism of action not involving D2 dopamine receptor blockade (Koblan et al., 2020). At the same time, all other TAARs have been considered as exclusively olfactory receptors sensing innate odors encoded by volatile amines with no significant function in the brain or the periphery. However, we recently demonstrated that an “olfactory” TAAR5 receptor is present in the limbic brain areas and can regulate classical monoamine systems, emotional behavior, and adult neurogenesis (Espinoza et al., 2020; Efimova et al., 2021).
In the olfactory system, TAAR5 was found in the sensory neurons of the olfactory epithelium and their projections to glomeruli and considered to be important in sensing sociallyrelevant innate odors (Liberles, 2015). TAAR5 is activated by tertiary amines and the most selective and active of them is trimethylamine (TMA). Abnormal TMA metabolism can cause trimethylaminuria, a metabolic disorder that is characterized by a strong fish odor of the body. For mice, on the contrary, TMA odor is attractive. TMA is present in mouse urine and is thought to act as a male pheromone. While the involvement of TAAR5 in odor detection was well described, their function outside of the olfactory system has been neglected. Several previous studies showed the presence of TAAR5 mRNA in some areas outside the olfactory epithelium, including the amygdala, the ventromedial hypothalamus, and the spinal cord (Berry et al., 2017). However study of TAAR5 function was limited due to the lack of selective ligands. The only known to date is putative non-selective TAAR5 agonist - alpha-NETA. Studies showed that administration of alpha-NETA causes psychotic-like behavioral abnormalities and brain electrical activity as well as an alterations in brain monoamine levels (Belov et al., 2020).
The use of TAAR5 knockout (TAAR5-KO) mice allowed us to explore the expression pattern of TAAR5 in the brain and elucidate its neuronal functions. In TAAR5-KO mice, the TAAR5 gene was replaced with a cassette, containing LacZ insertion (Espinoza et al., 2020). By analyzing the expression of LacZ, we observed that in the olfactory bulb TAAR5 can be found not only in the glomerular layer but also in mitral and other cells projecting to the limbic brain areas. Furthermore, TAAR5 was observed in the regions of the limbic system, such as the amygdala, the hippocampus, the entorhinal cortex, the nucleus accumbens, the piriform cortex, the thalamic, and hypothalamic nuclei - the regions receiving olfactory input and known to regulate emotional behaviors (Figure 1). The localization of TAAR5 in these limbic areas, together with its presence in the olfactory system, suggests that TAAR5 is involved in the transduction of innate olfactory input into the limbic emotional system. Behavioral studies in TAAR5-KO mice showed that TAAR5 can be involved in the regulation of emotional behaviors. TAAR5-KO mice had significantly decreased anxiety levels in several tests. They also demonstrated increased exploratory behavior in the open field test, with no significant changes in general locomotor activity. Together with decreased anxiety, TAAR5-KO mice also showed decreased depression-like behavior in the learned helplessness test. Taken together, these data indicate that the lack of TAAR5 receptors resulted in a change in several aspects of the emotional behavior of mice (Espinoza et al., 2020). The presence of TAAR5 receptors both in the olfactory and the limbic systems, together with involvement in emotional behavior seems intriguing and yet very logical. Olfactory input is important in the function of the brain limbic system. It is known that removal of olfactory bulbs (olfactory bulbectomy) causes changes in the function of limbic brain areas as well as alterations in behaviors, similar to those that are observed in depressed patients (Morales-Medina et al., 2017).
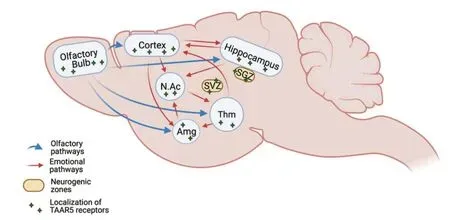
Figure 1|TAAR5 receptors are expressed in limbic brain areas involved in the transduction of olfactory information and neurogenic zones.
The lack of TAAR1 is known to affect the serotonin and the dopamine system. In TAAR5-KO mice, the serotonin level was also decreased in the striatum and hippocampus. At the same time, administration of 8-OH-DPAT, the selective agonist of the 5-HT1A receptor, showed a significantly greater change in body temperature in mutant mice (Espinoza et al., 2020). Alterations in the dopamine system of TAAR5-KO mice were also observed. TAAR5-KO mice had elevated by 30% level of dopamine and its metabolites in the striatal tissue (Efimova et al., 2021). Thus, the TAAR5 receptor is involved in the regulation of brain monoamine systems as it was shown previously for the TAAR1 receptor. It might be expected that not only TAAR1 and TAAR5 but all other TAAR receptors can act as modulators of classical monoamine systems in the brain through various mechanisms.
Surprisingly, not only the level of dopamine in the striatum was increased, but also the number of dopaminergic neurons in the Substantia Nigra. We observed that TAAR5-KO mice have an increased number of Tyrosine Hydroxylase-positive neurons in the Substantia Nigra pars compacta and pars lateralis (Efimova et al., 2021). Furthermore, TAAR5-KO mice have an increased level of glial derived neurotrophic factor (GDNF) in the striatum (Efimova et al., 2021). Neurotrophic factors, such as GDNF, are the proteins required for differentiation and survival of neurons during development, as well as maintenance of the adult nervous system. GDNF is known to be particularly important for dopaminergic neuron functioning. In embryonic midbrain cell cultures, GDNF promotes the survival and morphological differentiation of dopaminergic neurons while administration of GDNF into the adult rats Substantia Nigra could stimulate dopaminergic neuronal function (Barker et al., 2020). Taken together, these data suggest the increased dopamine neuron proliferation either during development or in adult TAAR5-KO mice. One highly debated possibility suggests that adult neurogenesis of the dopamine neurons might occur at potentially neurogenic zone surrounding the 3rdventricle (Jurkowski et al., 2020). It is well established that adult neurogenesis in mammals occurs primarily in two areas: the subventricular zone (SVZ), which is located in the walls of the lateral ventricles of the brain and connected through the rostral migration stream with an olfactory bulb, and the subgranular zone (SGZ) of the dentate gyrus of the hippocampus, that is mostly involved in memory formation. However, the recent evidence suggests that adult neurogenesis can also occur in other potentially neurogenic zones such as the striatum, the hypothalamus, the neocortex, and the areas surrounding the 3rdventricle (Jurkowski et al., 2020). Importantly, LacZ staining showed that TAAR5 is expressed in areas canonical for adult neurogenesis, SVZ, and SGZ, but also in areas surrounding the 3rdventricle. Some LacZ/TAAR5-positive cells were found also along blood vessels, known to be involved in the migration of proliferating neurons. These observations prompted us to evaluate adult neurogenesis events in these areas directly. Analysis of doublecortin-positive (neuroblastlike) and PCNA-positive (proliferating) cells showed that TAAR5-KO mice have an increased number of neuroblastoid and proliferating cells in the SVZ and SGZ zones. At the same time, no doublecortin- or PCNA-positive cells were found in the area surrounding the 3rdventricle in wild type or TAAR5-KO mice precluding us from making any conclusion on the status of neurogenesis in this area (Efimova et al., 2021). Further detailed studies with additional markers are needed to explain the mechanism of increase of the number of dopaminergic cells in TAAR5-KO mice. It would be important also to test the hypothesis if TAARs located in areas surrounding ventricles, as observed with TAAR5, may act as intra-brain “olfactory” sensors to detect trace amines in the cerebrospinal fluid thereby transmitting these signals of tissue damage from the cerebrospinal fluid to the neuronal tissue to regulate neurogenesis and related processes (Efimova et al., 2021).
Thus, “olfactory” TAAR5-mediated brain circuitry may represent a new type of monoamine neurotransmitter system that is involved in the transmission of innate odors into emotional responses and adult neurogenesis. Interestingly, a neurogenic theory of depression postulates impaired adult neurogenesis in the dentate gyrus as a trigger of the depression-like state, and that restoration of the neurogenesis by antidepressants may lead to recovery (Jacobs et al., 2000). Furthermore, in a rodent model of depression-like states a pronounced olfactory deficit accompanied by impairment of adult neurogenesis has been observed (Siopi et al., 2016). Thus, TAARs may play a key role in the junction that connects trace amines, emotional behavior (and, in particular, mood disorders), and adult neurogenesis. As many of the aspects of TAAR5 receptor functions and mechanisms of actions is yet to be determined, it is clear now, that it should not be considered as only an olfactory receptor, but also as a neuronal receptor involved in the regulation of brain neurochemistry, adult neurogenesis and emotional behavior. The olfaction and patterns of adult neurogenesis in the SVZ and SGZ are different between humans and rodents. It has been reported that TAAR5 is expressed in olfactory sensory neurons and amygdala in humans (discussed in Espinoza et al., 2020). Whether similar to mouse patterns of TAAR5 expression occur in other human brain areas (particularly limbic and neurogenic structures) requires further detailed studies. Nevertheless, one can propose that selective antagonists of TAAR5 may in the future become principally new psychotropic medications and expand options of pharmacotherapy of depression, anxiety, and/or neurodegenerative disorders such as Parkinson’s Disorder (Espinoza et al., 2020; Efimova et al., 2021). Finally, it would be important to explore if other TAARs will be acting as TAAR5 by being expressed in limbic brain areas and involved in the regulation of emotional behaviors and adult neurogenesis. However, we expect that since different TAARs are known to be activated by a selective set of products of amino acid decarboxylation (Gainetdinov et al., 2018) and project to discrete glomeruli (Liberles, 2015), their involvement in these processes will be likely variable. In fact, in preliminary studies in TAAR2-KO mice we did observe a similar, but not identical, pattern of brain TAAR2 expression in limbic areas as well as alterations in monoamine levels, emotional behaviors and adult neurogenesis (Kuvarzin et al., 2020). These studies eventually could provide the foundation of new pharmacological strategies based on targeting TAARs in general, that may represent a novel multifaceted approach for the treatment of a variety of psychiatric and neurodegenerative disorders.
The present work was supported by Russian Science Foundation grant 19-75-30008 (to RRG).
Evgeniya V. Efimova, Nataliia V. Katolikova, Evgeny V. Kanov, Raul R. Gainetdinov*
Institute of Translational Biomedicine and Saint Petersburg University Hospital, Saint Petersburg State University, Saint Petersburg, Russia
*Correspondence to:Raul R. Gainetdinov, MD, PhD, gainetdinov.raul@gmail.com.
https://orcid.org/0000-0003-2951-6038(Raul R. Gainetdinov)
Date of submission:March 12, 2021
Date of decision:April 26, 2021
Date of acceptance:June 29, 2021
Date of web publication:October 29, 2021
https://doi.org/10.4103/1673-5374.327338
How to cite this article:Efimova EV, Katolikova NV, Kanov EV, Gainetdinov RR (2022) Trace amineassociated receptors at the cross-road between innate olfaction of amines, emotions, and adult neurogenesis. Neural Regen Res 17(6):1257-1258.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- The importance of fasciculation and elongation protein zeta-1 in neural circuit establishment and neurological disorders
- Promoting axon regeneration in the central nervous system by increasing PI3-kinase signaling
- Microglial voltage-gated proton channel Hv1 in spinal cord injury
- Liposome based drug delivery as a potential treatment option for Alzheimer’s disease
- Retinal regeneration requires dynamic Notch signaling
- All roads lead to Rome — a review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease

