Effect of electroacupuncture at different time points on the recovery of gastrointestinal function after surgery for gastrointestinal malignant neoplasms
ZHOU Tianyi (周添奕), HUANG Siwei (黄思维), GU Chongying (顾翀颖), WANG Wenjia (王文佳), GU Qunhao (顾群浩),FENG Shouquan (冯寿全), SUN Xuqiu (孙栩秋), WANG Ke (王珂), LI Jing (李璟), ZHOU Jia (周嘉)
1 Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine,Shanghai 200437, China
2 Jinshan Hospital of Fudan University, Shanghai 201508, China
Abstract
Keywords: Acupuncture Therapy; Electroacupuncture; Acupuncture Analgesia; Acupuncture Anesthesia; Postoperative
Postoperative gastrointestinal dysfunction (PGID) is the leading cause of abdominal discomfort and long hospital stay after surgery[1]. The main clinical manifestations include decreased intestinal peristalsis and gastrointestinal dysfunction, e.g., diminished or disappeared bowel sounds, abdominal pain, bloating,nausea, vomiting, and absence of flatus or defecation in the early stage after surgery[2]. PGID is associated with longer hospital stays and increased perioperative complications, which will cause extra medical healthcare costs, suffering, and financial burden to the patients[3]. Western medications yet cannot produce satisfactory results in treating PGID[4]. More and more clinical evidence has recently been found to support the unique advantages and significant efficacy of acupuncture and electroacupuncture (EA) in improving PGID[5-8]. However, the intervention time of EA for PGID is varied. There is yet no report to study if EA intervention at different time points will affect the efficacy in treating PGID. Therefore, we observed the effects of EA during and after the surgery on gastrointestinal function recovery after operation for gastrointestinal malignant tumors to seek the optimal time to use EA to treat PGID.
1 Clinical Materials
1.1 Diagnostic criteria
The diagnostic criteria in Western medicine referred to theGuidelines of Chinese Society of Clinical Oncology(CSCO) Gastric Cancer[9]and theGuidelines of Chinese Society of Clinical Oncology (CSCO) Colorectal Cancer[10].
1.2 Inclusion criteria
Aged 18-80 years, gender unlimited; graded Ⅰ-Ⅲaccording to the American Society of Anesthesiologists grading system; diagnosed as gastrointestinal malignant tumors and met the necessary surgery requirements;volunteered to participate in the trial and offered written informed consent.
1.3 Exclusion criteria
Distant metastasis or widely spreading of tumor in the abdominal cavity; perforation or blocking caused by the tumor; skin infection around the points; nerve injury of either upper or lower limbs; coupled with severe diseases of the heart, brain, or lungs; severe mental disorders or cognitive impairments; pregnant or breast-feeding women; those who could not cooperate and failed to complete the trial, including difficulty speaking, infectious diseases, and other medical histories.
1.4 Statistical methods
All case data were recorded in a unified case report form and analyzed using the SPSS version 25.0 software by research staff uninvolved in protocol design or clinical implementation. The enumeration data[postoperative usage of patient-controlled analgesia(PCA) after the surgery] were processed using Pearson’s Chi-square test; Fisher’s exact test was applied when the theoretical number <5 and the total case number≤40 or the theoretical number <1. The measurement data [postoperative spontaneous flatus time, visual analog scale (VAS) score, consumption and times of additional analgesics, etc.] were described as mean ±standard deviation (±s) and analyzed by one-way analysis of variance (ANOVA) if normal distribution and homogeneity of variance were satisfied, and the least significant difference (LSD) test was used for betweengroup comparisons; if not meeting Mauchly’s test of sphericity, the data would be checked by Roy’s largest root test, and the LSD was adopted for pairwise comparisons. When not satisfying normal distribution or homogeneity of variance, the data would be expressed as median (lower quartile, upper quartile)[M (QL, QU)] and analyzed by Mann-WhineyU-test or Kruskal-WallisH-test. Bonferroni’s test was employed for pairwise comparisons.P<0.05 was recognized as statistical significance.
1.5 Blinding
The medical staff irrelevant to the program were invited as the third party to evaluate the efficacy, and the involved researchers, operators, and statisticians were separated to ensure blinding reliability and effectiveness.
1.6 General data
Sixty-three patients admitted to the Surgical Gastroenterology Department, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine,Shanghai University of Traditional Chinese Medicine were recruited between May 2019 and March 2020.They were diagnosed with gastrointestinal malignant tumors and required surgical treatment. The subjects were randomly divided into a control group, treatment group 1 (postoperative EA group), and treatment group 2 (intraoperative and postoperative EA group), with 21 cases in each group. Treatment group 1 and treatment group 2 each had one dropout case (the patients quit due to personal reasons), and 61 patients completed the trial, whose data underwent statistical analyses. The research process is illustrated in Figure 1. This trial had been approved by the Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine (Approval No. 2018-124).
2 Treatment Methods
All patients received conventional anesthesia and operation according to the tumor site. The patients all underwent surgery under general anesthesia and tracheal intubation. The patients stopped food intake 6 h and water intake 4 h before the surgery. The gastrointestinal decompression tube was placed prior to the surgery. An oxygen mask was provided during general anesthesia induction. Tracheal intubation was guided by the laryngoscope after the muscles relaxed.An anesthesia machine was connected to support mechanical ventilation. Anesthetics were added regularly to maintain anesthesia according to the operation duration and hemodynamic parameters.Anesthetics were terminated when the surgery finished,and the tracheal tube was removed when the patient awoke.

Figure 1. Study flow chart
2.1 Control group
After the surgery, patients in the control group received the conventional intervention, including fasting, gastrointestinal decompression, preventive antiinflammation, antacid treatment, parenteral nutrition support, and maintaining electrolyte and acid-base balances.
2.2 Treatment group 1
Patients in treatment group 1 received EA after the surgery in addition to the same intervention as in the control group.
Points: Bilateral Hegu (LI4), Neiguan (PC6), Zusanli(ST36), Shangjuxu (ST37), and Gongsun (SP4).
Methods:Disposable sterile acupuncture needles of 0.25 mm in diameter and 40 mm in length and G6805-2B EA apparatus were used. Hegu (LI4), Neiguan(PC6), Zusanli (ST36), Shangjuxu (ST37), and Gongsun(SP4) were punctured perpendicularly for 1.0-1.5 cm,0.7-1.0 cm, 1.5-2.0 cm, 1.5-2.0 cm, and 0.3-0.5 cm,respectively. Even reinforcing-reducing manipulation was performed to obtain needling sensations (Deqi).Afterward, the G6805-2B EA apparatus was connected with the frequency set at 2 Hz/10 Hz; Neiguan (PC6) to the negative electrode and Hegu (LI4) to the positive electrode, as one group; Zusanli (ST36) to the positive electrode and Shangjuxu (ST37) to the negative electrode, as another group. Gongsun (SP4) received no EA stimulation. The stimulation intensity was adjusted to the patient’s tolerance with the peak current around 5 mA. The EA treatment started 6 h after the surgery,30 min each time, once on the surgery day and twice on the second and third days of the surgery, 5 times in total.
2.3 Treatment group 2
Patients in treatment group 2 received EA during and after the surgery in addition to the same intervention as in the control group.
The acupuncture points, methods, and stimulation parameters were the same as in treatment group 1. But the EA stimulation started 30 min before anesthesia and continued till the end of the surgery. The 3M transparent tapes were used to fix the acupuncture needles. The postoperative EA treatment was the same as in treatment group 1.
3 Observation of Therapeutic Efficacy
3.1 Outcome measures
3.1.1 Primary outcome measure
The first flatus time after the surgery: The patient would be asked if they broke wind after the surgery and when it happened, which were kept a record.
3.1.2 Secondary outcome measures
Postoperative consumption of additional analgesics:Ketorolac tromethamine injection was offered to the patients according to their pain intensity. The consumption and frequency were properly recorded.group, showing statistical significance (P<0.05); the difference between the two treatment groups was statistically insignificant (P>0.05). The details are shown in Figure 2.
3.2.3 Comparison of the VAS score at different time points after the surgery
Taking measuring time as the intra-group factor and grouping as the between-group factor, the repeated measures ANOVA found a significant main effect of the measuring time (P<0.05) and grouping (P<0.05). In addition, the interaction of measuring time and grouping also showed a significant effect (P<0.05). The between-group comparisons showed that treatment group 1 was only significantly lower than the control group in comparing the VAS score at 72 h after the surgery (P<0.05). However, treatment group 2 was lower than the control group at multiple time points (6,12, 24, and 72 h after the surgery) in comparing the VAS score, all showing statistical significance (P<0.05).Compared with treatment group 1, the VAS score was notably lower in treatment group 2 at 6 h after the surgery (P<0.05). Please see Figure 3.

Table 1. Comparison of the baseline data of the three groups
Pain intensity: Pain intensity at 6, 12, 24, and 72 h after the surgery was scored using VAS. Zero for painless; 1-3 points for mild pain (not affecting sleep);4-6 points for moderate pain (slightly affecting sleep);7-10 points for severe pain (difficulty falling asleep or waking up due to the pain).
3.2 Results
3.2.1 Comparison of baseline data
There were no significant differences in the general characteristics (age, gender, height, and body mass) or the pertinent factors (operation duration, incision length, the American Society of Anesthesiologists grading, the surgery type, the type of gastrointestinal tumor, the stage of tumor, and pathological type) across the three groups (P>0.05), suggesting the comparability of the baseline data (Table 1).
3.2.2 Comparison of the first flatus time after the surgery
The average of the first postoperative flatus time was 73.67 h in the control group versus 69.50 h in treatment group 1 and 68.64 h in treatment group 2. The time was earlier in treatment groups 1 and 2 than in the control

Figure 2. Comparison of the first postoperative flatus time

Figure 3. Comparison of the VAS score at different time points
3.2.4 Comparison of the frequency of adding analgesics after the surgery
The comparison of using PCA after the surgery revealed insignificant differences among the three groups (P>0.05); the comparison of adding analgesics based on person-time also found that the differences were insignificant among the three groups. The details are shown in Table 2.
The comparison of the total frequency of using postoperative analgesics in 3 d after the surgery showed statistically equal across the three groups(P>0.05). Furthermore, at 12 h after the surgery,treatment group 1 was statistically equivalent to the control group in comparing the number of times analgesics were used (P>0.05); treatment group 2 had a lower frequency compared with the control group(P<0.05); the difference between treatment group 1 and treatment group 2 was statistically insignificant(P>0.05). The differences in the frequency of adding analgesics at 24 h, 48 h, and 72 h after the surgery were statistically insignificant among the three groups(P>0.05). The data are detailed in Table 3.
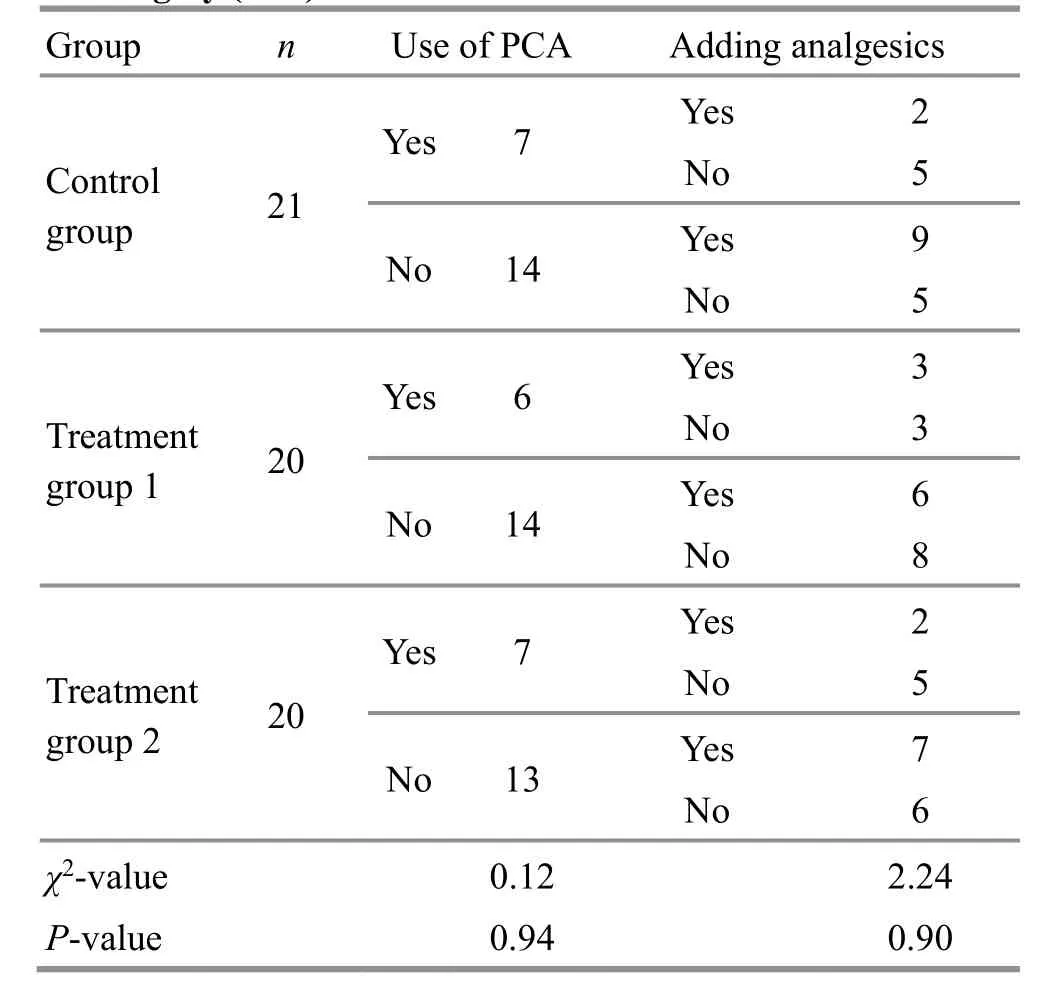
Table 2. Comparison of using PCA and adding analgesics after the surgery (case)

Table 3. Comparison of the frequency of adding analgesics after the surgery among the three groups [M (QL, QU), time]
4 Discussion
According to the clinical manifestations, PGID can be classified into “intestine Bi-Impediment”, “abdominal pain”, “bloating”, “stomach fullness”, or “stomachache”in traditional Chinese medicine (TCM). TCM holds that the scalpel injures the body during surgery, which consumes Qi and blood while expelling pathogenic evils.Besides, gastrointestinal surgery works directly on the stomach and intestines, thus more significantly affecting gastrointestinal function. Qi and blood are the sources of generation and transformation. A low level of Qiblood will result in insufficient healthy Qi, which in turn causes weakness and blockage in the stomach and intestines. Further, the hindered function of the spleen and stomach in ascending and descending and the failure of the liver in dredging will obstruct the middle Jiao (energizer) and the flow of Qi-blood and meridians.Moreover, blood insufficiency will prevent the Zang-Fu organs from getting enough nutrition. Vice versa,deficient Zang-Fu organs will affect the generation of Qi and blood. Then, a vicious cycle forms. Abdominal bloating and distending pain in severe cases are often caused by blockage due to Qi stagnation. Hence, PGID is a condition with a deficient root cause; however,patients often present with excess symptoms.
We selected bilateral Hegu (LI4), Neiguan (PC6),Zusanli (ST36), Shangjuxu (ST37), and Gongsun (SP4) in this study. Hegu (LI4) and Neiguan (PC6) are commonly used to treat gastrointestinal disorders and in acupuncture anesthesia. Patients with PGID experience blocked Fu-organ Qi. The stomach and large intestine share the same name as Yangming. Thus, Hegu (LI4)was used to unblock Yangming meridians in general to down-regulate Qi to cease vomiting. In addition, this point was also selected because of its notable analgesic effect[11-12]. Neiguan (PC6) is the Luo-Connecting Point of the Pericardium Meridian and one of the Eight Confluent Points and can modulate gastrointestinal function. Research also demonstrates that acupuncture at Neiguan (PC6) can protect cardiomyocytes, improve vascular endothelial cell function, and reduce the oxygen consumption of cardiomyocytes. Besides,stimulation to the median nerve near Neiguan (PC6)boosts the secretion of neurotransmitters[13-14]. Zusanli(ST36) and Shangjuxu (ST37), the Lower He-Sea Points of the stomach and large intestine, respectively, are commonly used to treat gastrointestinal diseases. It is found that stimulating Zusanli (ST36) can activate a variety of digestive enzymes, making gastrointestinal motility more powerful and regular[15-16]; EA at Shangjuxu (ST37) can improve the morphological injuries of colonic mucosae and reduce inflammatory cells to protect intestinal mucosae[17].
In general, acupuncture-moxibustion can help recover gastrointestinal function, prevent and treat nausea and vomiting, ease pain after surgery, and has minor adverse reactions, high safety rating, and convenient operation. Because it can reduce operative stresses and boost recovery, this therapy has become a common method of treating PGID[18-19]. Studies have found that preoperative EA or 6 h after the operation can achieve a satisfactory result[20-21]. However, it remains unknown if different intervention times can affect efficacy. As we know, acupuncture efficacy depends on various factors, including intervention time,acupoint selection, needling manipulations, etc.Acupuncture at different time points influences the accumulation of acupuncture effect and thus affects the final efficacy. Therefore, the intervention time of acupuncture is an essential factor influencing clinical efficacy.
Based on previous clinical reports, this randomized controlled trial designed two arms treated with EA 30 min before and 6 h after the surgery. The study results showed that EA’s effect on boosting gastrointestinal function recovery did not vary significantly due to different intervention time points.This demonstrates that EA, either starting 30 min before or 6 h after the surgery, can effectively promote the recovery of gastrointestinal function after gastrointestinal malignant tumor surgery. Regarding the postoperative analgesic effect, this study demonstrated that the pain intensity dropped with time after the surgery, and the preoperative EA intervention exerted significant effects on the pain intensity and the consumption of analgesics on the next day of surgery.Nevertheless, the analgesic effect of postoperative EA intervention was found to be notable on the third day after the surgery. Hence, the results suggest that the EA intervention should start 30 min before anesthesia rather than 6 h after the surgery to achieve a satisfactory postoperative analgesic effect.
According to this study, either starting 30 min before or 6 h after gastrointestinal malignant tumor surgery, EA can promote postoperative flatus and the recovery of gastrointestinal function. But the EA intervention starting 30 min before the surgery can effectively ease postoperative pain and reduce the consumption of analgesics to enhance recovery after the surgery.Multicenter, large-scale clinical research is expected to further optimize the EA intervention protocol for PGID and provide reliable practical and theoretical evidence.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Scientific Fund of Shanghai Municipal Health Commission (上海市卫生健康委员会科研基金, No. 201840011); Clinical Key Specialty Construction Project of Shanghai (上海市临床重点专科建设项目, No. shslczdzk04701); Traditional Chinese Medicine Evidence-based Capacity Building Project of National Administration of Traditional Chinese Medicine (国家中医药管理局中医药循证能力建设项目,No. 2019XZZX-ZJ0011); Shanghai Clinical Research Center for Acupuncture and Moxibustion (上海市针灸临床医学研究中心, No. 20MC1920500); Three-year Development Project for Traditional Chinese Medicine of Shanghai: Inheritance and Innovation Platform Construction Project for Traditional Chinese Medicine [上海市进一步加快中医药事业发展三年行动计划中医药传承创新平台建设项目, No. ZY(2018-2020)-CCCX-2004-04].
Statement of Informed Consent
Informed consent was obtained from all individual participants.
Received: 2 August 2021/Accepted: 29 January 2022
 Journal of Acupuncture and Tuina Science2022年5期
Journal of Acupuncture and Tuina Science2022年5期
- Journal of Acupuncture and Tuina Science的其它文章
- Efficacy of mild moxibustion combined with surgery for meniscal injury and its effect on TGF-β1 and PDGF levels in the fluid of knee joint
- Clinical study on tube moxibustion plus point-toward-point needling method in treating refractory facial paralysis
- Influence of buccal acupuncture on analgesic effect,immune indicators, and expression of Survivin and Livin proteins in patients with advanced-stage primary liver cancer
- Effect of acupuncture-like transcutaneous electrical nerve stimulation on labor pain in nulliparous women: a randomized controlled trial
- Influence of herbal cake-partitioned moxibustion on lumbar functions and inflammatory factors in patients with lumbar disc herniation due to kidney deficiency and blood stasis
- Observation on the therapeutic efficacy of Tuina plus “three-bridge” exercise for non-specific low back pain
