中药续断的化学成分研究
张成刚,杨昌贵,肖承鸿,凡迪,龚安慧,黄艳,周涛*
(1.贵州中医药大学, 贵州 贵阳 550025;2.贵州省农作物技术推广总站, 贵州 贵阳 550001)
续断始载于《神农本草经》,列为上品,是续断科植物川续断(DipsacusasperWall. ex Henry)的干燥根,具有补肝肾、强筋骨、续折伤、止崩漏之功效[1-2]。续断主要含有三萜皂苷类、环烯醚萜类、生物碱类等化学成分,其中三萜皂苷类、环烯醚萜类成分含量较高[3-4],药理研究表明续断具有抗骨折、抗骨质疏松、心肌保护、抗衰老及生殖系统保护等作用[5-6]。
“发汗”炮制法是常用的中药材产地加工方法,对于保证中药材质量具有重要作用[7]。《中国药典》规定续断等5种药材必须进行“发汗”加工处理[2,8],许多学者对续断“发汗”前后的化学成分及其含量变化做了大量研究,但结论各异。有的学者发现续断“发汗”后其川续断皂苷VI、川续断苷A、异绿原酸B、异绿原酸C等成分含量升高,有的学者实验得出续断“发汗”后其总皂苷、川续断皂苷VI、异绿原酸A含量降低[9-12],也有学者研究发现续断“发汗”前后其川续断皂苷VI的含量无变化[13],还有学者比较续断“发汗”前后的28种酚酸和脂类,发现顺-13-十八碳烯酸和十七烷醇仅存在于“发汗”前续断中,而“发汗”后检测到的9,12,15-十八碳三烯酸甲酯和9-亚甲基-9H-芴是“发汗”前续断中没有的新化学成分[14]。
为进一步阐释续断“发汗”前后化学成分及其含量的变化,并为续断“发汗”加工前后化学成分转化机制研究提供更丰富的化学成分物质基础,本文对续断药材70%乙醇提取物进行了研究,从中共分离得到13个化合物,包括5个三萜皂苷类化合物:川续断皂苷Ⅵ(1)、威严仙皂苷A(2)、续断皂苷A(3)、3-O-(2-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷(4)、3-O-(4-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷(5),6个环烯醚萜苷类化合物:马钱苷酸(6)、马钱子苷(7)、当药苷(8)、续断苷A(9)、续断苷B(10)、林生续断苷I(11),以及2个其他类成分:5-羟甲基-2-呋喃甲醛(12)和α-亚麻酸(13),其中化合物4、12、13为首次从续断中分离,也是首次从川续断属植物中分离得到。
1 仪器与材料
1.1 仪器
Bruker-400核磁共振波谱仪(德国布鲁克公司),Bruker Impact II高分辨飞行时间质谱仪(德国布鲁克公司),ACCHROM S6000高效液相色谱仪(华谱科仪(北京)科技有限公司),RIGOL L-3000四元泵(苏州普源精电科技有限公司),Flash System MP100中压系统(天津博纳艾杰尔科技有限公司);岛津LC-20AR液相色谱仪(日本岛津公司);BSA2202S-CW电子天平(德国赛多利斯公司);R-100旋转蒸发仪(瑞士步琦有限公司)。乙腈为色谱纯,95%乙醇、甲醇为分析纯(天津康科德科技有限公司);YMC-Pack ODS-A C18反相色谱柱(日本维美希公司);D101大孔吸附树脂(上海源叶生物科技有限公司);MCI小孔吸附树脂(日本三菱化学公司);水为去离子水。
1.2 材料
实验所用续断药材购自贵州省贵阳市太升中药材市场,经贵州中医药大学药学院江维克教授鉴定为川续断科植物川续断DipsacusasperWall. ex Henry的干燥根。
2 方法与结果
2.1 方法
干燥的续断药材10 kg,粉碎至适宜粒度后用70%乙醇回流提取3次,提取液回收溶剂后得到浸膏4 kg。浸膏用水溶解后注入D101大孔吸附树脂柱,分别用20%、50%、95%乙醇梯度洗脱,减压浓缩得到20%乙醇洗脱部位(Fr.1, 200 g)、50%乙醇洗脱部位(Fr.2, 500 g)以及95%乙醇洗脱部位(Fr.3, 200 g)。

2.2 结果
化合物1: 白色无定形粉末。1H-NMR (400 MHz, Piridine-d5)δ:4.22 (1H, m, H-3), 5.40 (1H, d,J= 3.0 Hz, H-12), 4.23 (1H, overlapped, H-23), 3.64 (1H, overlapped, H-23), 0.90 (3H, s, H-24), 0.95 (3H, s, H-25), 1.10 (3H, s, H-26), 1.16 (3H, s, H-27), 0.84 (6H, s, H-29, H-30)。4.91 (1H, d,J= 7.2 Hz, H-1′), 4.39 (1H, m, H-2′), 4.15 (1H, overlapped, H-3′), 4.30 (1H, overlapped, H-4′), 3.70 (1H, m, H-5′)。6.22 (1H, d,J= 7.8 Hz, H-1″), 4.08 (1H, m, H-2″), 4.16 (1H, overlapped, H-3″), 4.04 (1H, overlapped, H-4″), 4.05 (1H, overlapped, H-5″), 4.26 (1H, m, H-6″), 4.66 (1H, m, H-6″)。5.00 (1H, d,J= 7.8 Hz, H-1‴), 3.96 (1H, m, H-2‴), 4.29 (1H, overlapped, H-3‴), 4.15 (1H, overlapped, H-4‴), 3.83 (1H, m, H-5‴), 4.30 (1H, overlapped, H-6‴), 4.41 (1H, m, H-6‴)。13C-NMR (100 MHz, Piridine-d5)δ: 38.6 (C-1), 25.9 (C-2), 81.7 (C-3), 43.3 (C-4), 47.3 (C-5), 18.0 (C-6), 32.3 (C-7), 39.7 (C-8), 48.0 (C-9), 36.7 (C-10), 23.7 (C-11), 122.7 (C-12), 144.0 (C-13), 41.9 (C-14), 28.1 (C-15), 23.1 (C-16), 46.8 (C-17), 41.4 (C-18), 46.0 (C-19), 30.5 (C-20), 33.9 (C-21), 32.6 (C-22), 64.2 (C-23), 14.0 (C-24), 16.0 (C-25), 17.4 (C-26), 25.9 (C-27), 176.4 (C-28), 32.9 (C-29), 23.5 (C-30), 106.4 (C-1′), 72.8 (C-2′), 74.4 (C-3′), 69.4 (C-4′), 66.7 (C-5′), 95.4 (C-1″), 73.6 (C-2″), 78.4 (C-3″), 70.6 (C-4″), 77.7 (C-5″), 69.1 (C-6″), 104.9 (C-1‴), 74.9 (C-2‴), 78.1 (C-3‴), 71.3 (C-4‴), 78.1 (C-5‴), 62.3 (C-6‴)。以上数据与文献[15]报道基本一致,故将该化合物鉴定为川续断皂苷Ⅵ。
化合物2: 白色无定形粉末。HR-ESI-MS给出分子式C35H56O8(测量值m/z603.392 3 [M-H]-,计算值m/z603.390 2 [M-H]-)。1H-NMR (400 MHz, Piridine-d5)δ: 4.25 (1H, m, H-3), 5.46 (1H, br s, H-12), 3.68 (1H, overlapped, H-23), 4.26 (1H, overlapped, H-23), 0.90 (3H, s, H-24), 0.92 (3H, s, H-25), 1.00 (3H, s, H-26), 1.22 (3H, s, H-27), 0.92 (3H, s, H-29), 0.98 (3H, s, H-30)。4.96 (1H, d,J= 7.2 Hz, H-1′), 4.40 (1H, t,J= 8.0 Hz, H-2′), 3.27 (1H, dd,J= 3.0, 13.2, H-3′), 4.23 (1H, m, H-4′), 3.71 (1H, overlapped, H-5′), 4.27 (1H, overlapped, H-5′)。13C-NMR (100 MHz, Piridine-d5)δ: 38.6 (C-1), 25.9 (C-2), 81.7 (C-3), 43.3 (C-4), 47.4 (C-5), 17.9 (C-6), 32.7 (C-7), 39.6 (C-8), 49.9 (C-9), 36.7 (C-10), 23.6 (C-11), 122.4 (C-12), 144.6 (C-13), 41.9 (C-14), 28.1 (C-15), 23.6 (C-16), 46.4 (C-17), 41.7 (C-18), 46.2 (C-19), 30.7 (C-20), 34.0 (C-21), 33.0 (C-22), 64.3 (C-23), 13.4 (C-24),15.9 (C-25),17.3 (C-26), 26.9 (C-27), 180.0 (C-28), 33.0 (C-29), 23.5 (C-30), 106.4 (C-1′), 72.9 (C-2′), 74.5 (C-3′), 69.4 (C-4′), 66.70 (C-5′)。以上数据与文献[16]报道基本一致,故将该化合物鉴定为威严仙皂苷A。
化合物3: 白色无定形粉末。1H-NMR (400 MHz, Piridine-d5)δ: 4.18 (1H, m, H-3), 5.42 (1H, br s, H-12), 3.69 (1H, d,J= 10.4 Hz, H-23), 4.15 (1H, overlapped, H-23), 1.04 (3H, s, H-24), 1.00 (3H, s, H-25), 1.14 (3H, s, H-26), 1.16 (3H, s, H-27), 0.84 (3H, s, H-29), 0.85 (3H, s, H-30)。6.25 (1H, d,J= 8.0 Hz, H-1′) 4.12 (1H, m, H-2′), 4.19 (1H, overlapped, H-3′), 4.32 (1H, m, H-4′), 4.09 (1H, m, H-5′), 4.34 (1H, m, H-6′), 4.70 (1H, d,J= 10.0 Hz, H-6′)。5.02 (1H, d,J= 8.0 Hz, H-1″), 3.99 (1H, t,J= 8.4 Hz, H-2″), 3.87 (1H, m, H-3″), 4.19 (2H, overlapped, H-4″, H-5″), 3.18 (1H, dd,J= 3.6, 13.6 Hz, H-6″), 4.15 (1H, overlapped, H-6″)。13C-NMR (100 MHz, Piridine-d5)δ:38.6 (C-1), 27.5 (C-2), 73.2 (C-3), 42.7 (C-4), 48.4 (C-5), 18.4 (C-6), 32.7 (C-7), 39.7 (C-8), 48.0 (C-9), 37.0 (C-10), 23.6 (C-11), 122.7 (C-12), 144.0 (C-13), 41.9 (C-14), 28.1 (C-15), 23.2 (C-16), 46.8 (C-17), 41.5 (C-18), 46.0 (C-19), 30.5 (C-20), 33.8 (C-21), 32.3 (C-22), 67.7 (C-23), 12.9 (C-24), 15.9 (C-25), 17.4 (C-26), 25.9 (C-27), 176.3 (C-28), 32.9(C-29), 23.5 (C-30), 95.5 (C-1′), 73.7 (C-2′), 78.5 (C-3′), 70.7 (C-4′), 77.8 (C-5′), 69.2 (C-6′), 105.1 (C-1″), 74.9 (C-2″), 78.2 (C-3″), 71.3 (C-4″), 78.2 (C-5″), 62.4 (C-6″)。以上数据与文献[16]报道基本相同,故将该化合物确定为续断皂苷A。
化合物4: 白色无定形粉末。1H-NMR (400 MHz, Piridine-d5)δ:4.21 (1H, m, H-3), 5.39 (1H, m, H-12), 3.68 (1H, overlapped, H-23), 3.87 (H, d,J= 10.4 Hz, H-23), 0.90 (3H, s, H-24), 0.95 (3H, s, H-25), 1.10 (3H, s, H-26), 1.16 (3H, s, H-27), 0.84 (6H, s, H-29, H-30)。5.01 (1H, d,J=8.0 Hz, H-1′), 5.88 (1H, dd,J= 7.6, 13.2 Hz, H-2′), 4.21 (1H, overlapped, H-3′), 4.02 (1H, m, H-4′), 3.68 (1H, overlapped, H-5′), 4.24 (1H, m, H-5′)。6.24 (1H, d,J= 8.0 Hz, H-1″), 4.11 (1H, m, H-2″), 4.19 (1H, overlapped, H-3″), 4.32 (1H, overlapped, H-4″), 4.08 (1H, overlapped, H-5″), 4.33 (1H, overlapped, H-6″), 4.70 (1H, d,J= 10.0 Hz, H-6″)。4.95 (1H, d,J= 8.0 Hz, H-1‴), 4.00 (1H, m, H-2‴), 4.32 (1H, overlapped, H-3‴), 4.15 (1H, overlapped, H-4‴), 3.87 (1H, overlapped, H-5‴), 4.34 (1H, overlapped, H-6‴), 4.46 (1H, dd,J= 2.0, 11.6 Hz, H-6‴), 2.10 (3H, s, H-COCH3)。13C-NMR (100 MHz, Piridine-d5)δ:38.5 (C-1), 26.0 (C-2), 80.9 (C-3), 43.1 (C-4), 46.9(C-5), 17.9 (C-6), 32.5 (C-7), 39.7 (C-8), 47.9 (C-9), 36.6 (C-10), 23.6 (C-11), 122.7 (C-12), 143.9 (C-13), 41.9 (C-14), 28.1 (C-15), 23.1 (C-16), 46.8 (C-17), 41.4 (C-18), 46.0 (C-19), 30.5 (C-20), 33.7 (C-21), 32.3 (C-22), 63.4 (C-23), 13.3 (C-24), 15.9 (C-25), 17.3 (C-26), 25.8 (C-27), 176.3 (C-28), 32.9 (C-29), 23.5 (C-30)。105.0 (C-1′), 74.1 (C-2′), 69.5 (C-3′), 72.3 (C-4′), 66.9 (C-5′), 95.5 (C-1″), 73.7 (C-2″), 78.5 (C-3″), 70.7 (C-4″), 77.7 (C-5″), 69.1 (C-6″), 103.8 (C-1‴), 74.9 (C-2‴), 78.2 (C-3‴), 71.3 (C-4‴), 78.2 (C-5‴), 62.4 (C-6‴), 169.9 (C-COCH3), 21.0 (C-COCH3)。以上数据与文献[17]报道基本相同,故将该化合物确定为3-O-(2-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷。
化合物5: 白色无定形粉末。HR-ESI-MS给出分子式C49H78O19(测量值m/z993.501 8 [M+Na]+,计算值m/z993.503 5 [M+Na]+)。1H-NMR (400 MHz, Piridine-d5)δ: 4.29 (1H, m, H-3), 5.43 (1H, br s, H-12), 3.73 (2H, m, H-23), 0.90 (3H, s, H-24), 0.95 (3H, s, H-25), 1.10 (3H, s, H-26), 1.16 (3H, s, H-27), 0.84 (6H, s, H-29, H-30)。4.99 (1H, d,J= 7.2 Hz, H-1′), 4.37 (1H, overlapped, H-2′), 4.17 (1H, overlapped, H-3′), 5.54 (1H, br s, H-4′), 4.25 (1H, overlapped, H-5′), 4.34 (1H, overlapped, H-5′)。6.27 (1H, d,J= 8.0 Hz, H-1″), 4.14 (1H, overlapped, H-2″), 4.23 (1H, overlapped, H-3″), 4.33 (1H, overlapped, H-4″), 4.12 (1H, overlapped, H-5″), 4.37 (1H, overlapped, H-6″), 4.73(1H, d,J=10.0 Hz, H-6″)。5.05 (1H, d,J= 7.2 Hz, H-1‴), 4.01 (1H, dd,J= 7.6, 7.6 Hz, H-2‴), 4.21 (1H, overlapped, H-3‴), 4.21 (1H, overlapped, H-4‴), 3.90 (1H, m, H-5‴), 4.37 (1H, overlapped, H-6‴), 4.49 (1H, d,J= 11.6 Hz, H-6‴), 1.98 (3H, s, H-COCH3)。13C-NMR (100 MHz, Piridine-d5)δ: 38.6 (C-1), 25.9 (C-2), 82.0 (C-3), 43.3 (C-4), 47.4 (C-5), 18.0 (C-6), 32.9 (C-7), 39.7 (C-8), 48.0 (C-9), 36.7 (C-10),23.6 (C-11),122.3 (C-12),143.9 (C-13),41.9(C-14), 28.1 (C-15), 23.1 (C-16), 46.8 (C-17), 41.5 (C-18), 46.0 (C-19), 30.5 (C-20), 33.8 (C-21), 32.8 (C-22), 64.2 (C-23), 13.4 (C-24), 16.0 (C-25), 17.4 (C-26), 25.8 (C-27), 176.3 (C-28), 32.9 (C-29), 23.5 (C-30)。106.5 (C-1′), 73.1 (C-2′), 72.2 (C-3′), 72.3 (C-4′), 64.2 (C-5′), 95.4 (C-1″), 73.7 (C-2″), 78.5 (C-3″), 70.7 (C-4″), 77.7 (C-5″), 69.2 (C-6″), 105.0 (C-1‴), 74.9 (C-2‴), 78.2 (C-3‴), 71.3 (C-4‴), 78.2 (C-5‴), 62.4 (C-6‴), 170.6 (C-COCH3), 20.9 (C-COCH3)。以上数据和文献[15]报道的大体一致,从而将该化合物确定为3-O-(4-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷。
化合物6: 白色无定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.26 (1H, d,J= 5.5 Hz, H-1), 7.41 (1H, s, H-3), 3.09 (1H, dd,J= 7.6, 16.0 Hz, H-5), 1.65 (1H, m, H-6a), 2.03 (1H, m, H-6b), 4.06(1H, m,H-7),1.87(1H,m,H-8),2.24(1H,dd,J= 8.4, 14.0 Hz, H-9), 1.09 (3H, d,J=6.8 Hz, H-10), 4.67 (1H, d,J= 8.0 Hz, H-1′), 3.22 (1H, m, H-2′), 3.41 (1H, m, H-3′), 3.31 (1H, m, H-4′), 3.33 (1H, m, H-5′), 3.68 (1H, dd,J= 5.2, 12.0 Hz, H-6′a), 3.85 (1H, d,J= 12.0 Hz, H-6′b)。13C-NMR (100 MHz, CD3OD)δ: 96.3 (C-1), 150.9 (C-3), 112.7 (C-4), 31.0 (C-5), 41.5 (C-6), 73.8 (C-7), 41.0 (C-8), 45.3 (C-9), 12.2 (C-10), 169.7 (C-11), 98.6 (C-1′), 73.3 (C-2′), 76.6 (C-3′), 70.2 (C-4′), 76.8 (C-5′), 61.4 (C-6′)。以上数据和文献[18]报道的基本相同,从而将该化合物确定为马钱苷酸。
化合物7: 白色无定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.26 (1H, d,J= 5.5 Hz, H-1), 7.39 (1H, s, H-3), 3.10 (1H, dd,J= 8.0, 16.0 Hz, H-5), 1.61 (1H, m, H-6a), 2.02 (1H, m, H-6b), 4.04 (1H, m, H-7), 1.87 (1H, m, H-8), 2.22 (1H, dd,J= 8.0, 14.0 Hz, H-9), 1.09 (3H, d,J=6.8 Hz, H-10), 3.68 (3H, s, H-COOCH3), 4.65 (1H, d,J= 8.0 Hz, H-1′), 3.19 (1H, m, H-2′), 3.37 (1H, m, H-3′), 3.27 (1H, m, H-4′), 3.29 (1H, m, H-5′), 3.66 (1H, dd,J= 5.6, 12.0 Hz, H-6a′), 3.89 (1H, d,J= 12.0 Hz, H-6b′)。13C-NMR (100 MHz, CD3OD)δ: 96.3 (C-1), 150.7 (C-3), 112.6 (C-4), 30.8 (C-5), 41.3 (C-6), 73.3 (C-7), 40.8 (C-8), 45.1 (C-9), 12.0 (C-10), 168.2 (C-11), 50.3 (C-COOCH3), 98.7 (C-1′), 73.7 (C-2′), 77.0 (C-3′), 70.2 (C-4′), 76.6 (C-5′), 61.4 (C-6′)。以上数据与文献[19]报道大体一致,故将该化合物确定为马钱子苷。
化合物8: 白色无定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.55 (1H, overlapped, H-1), 7.60 (1H, d,J= 2.0 Hz, H-3), 3.15 (1H, m, H-5), 1.63~1.81 (2H, m, H-6), 4.45 (1H, m, H-7a), 4.37 (1H, m, H-7b), 5.55 (1H, overlapped, H-8), 3.71 (1H, m, H-9), 5.29 (2H, overlapped, H-10), 4.70 (1H, d,J= 8.4 Hz, H-1′), 3.22 (1H, m, H-2′), 3.40 (1H, m, H-3′), 3.29 (1H, m, H-4′), 3.33 (1H, overlapped, H-5′), 3.67 (1H, dd,J= 6.0, 12.0 Hz, H-6′a), 3.89 (1H, d,J= 10.8 Hz, H-6′b)。13C-NMR (100 MHz, CD3OD)δ: 96.7 (C-1), 152.7 (C-3), 104.7 (C-4), 27.0 (C-5), 24.5 (C-6), 68.4 (C-7), 131.9 (C-8), 42.4 (C-9), 119.6 (C-10), 167.2 (C-11), 98.4 (C-1′), 73.3 (C-2′), 76.5 (C-3′), 70.1 (C-4′), 76.9 (C-5′), 61.3 (C-6′)。上述数据与文献[20]报道大体相同,从而将该化合物确定为当药苷。
化合物9: 白色无定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.47 (1H, d,J= 4.8 Hz, H-1a1), 7.38 (1H, s, H-3a1), 2.70 (1H, m, H-5a1), 3.02 (1H, dd,J= 8.4, 7.8 Hz, H-6a1), 2.33 (1H, dd,J= 7.8, 6.6 Hz, H-6a1), 6.67 (1H, t,J=6.6 Hz, H-7a1), 5.68 (1H, ddd,J=7.8, 9.0, 9.6, 10.2, 16.8 Hz, H-8a1), 2.30 (1H, br m, H-9a1), 5.28 (1H, d,J= 16.8 Hz, H-10a1), 5.21 (1H, d,J= 10.8 Hz, H-10a1), 4.59 (1H, d,J= 7.8 Hz, H-1′a1), 3.10~3.29 (1H, H-2′a1), 3.10~3.29 (1H, H-3′a1), 3.10~3.29 (1H, H-4′a1), 3.10~3.29 (1H, H-5′a1), 3.54~3.58 (1H, H-6′a1), 3.78~3.82 (1H, H-6′a1)。5.39 (1H, d,J= 1.8 Hz, H-1a2), 7.45 (1H, s, H-3a2), 4.01 (1H, d,J= 4.2 Hz, H-5a2), 9.32 (1H, s, H-7a2), 5.54 (1H, ddd,J= 9.6, 7.2, 10.2, 9.6, 17.4 Hz, H-8a2), 2.48 (1H, br s, H-9a2), 4.96(1H,d,J= 17.4 Hz, H-10a2), 4.93(1H,d,J=10.2 Hz, H-10a2), 4.56 (1H, d,J= 7.8 Hz, H-1′a2), 3.10~3.29 (1H, H-2′a2), 3.10~3.29 (1H, H-3′a2), 3.10~3.29 (1H, H-4′a2), 3.10~3.29 (1H, H-5′a2), 3.54~3.58 (1H, H-6′a2), 3.78~3.82 (1H, H-6′a2)。5.06 (1H, br s, H-1b1), 7.34 (1H, s, H-3b1), 2.92 (1H, br q,J= 8.4 Hz, H-5b1), 1.67 (1H, ddd,J= 4.8, 3.6, 6.0 Hz, H-6b1), 2.07 (1H, m, H-6b1), 5.17 (1H, d,J= 4.8 Hz, H-7b1), 2.07 (1H, m, H-8b1), 1.97 (1H, m, H-9b1), 0.99 (3H, d,J= 6.6 Hz, H-10b1), 3.62 (3H, s, H-12b1), 4.57 (1H, d,J= 8.4 Hz, H-1′b1), 3.10~3.29(9H, H-2′b1,H-3′b1,H-4′b1,H-5′b1,H-6′b1,H-2′b2, H-3′b2,H-4′b2,H-5′b2),3.78~3.82(2H, H-6′b1, H-6′b2)。5.15 (1H, d,J=4.8 Hz, H-1b2),7.35(1H, s, H-3b2), 2.92(1H, br q,J=8.4 Hz, H-5b2), 1.62 (1H, ddd,J= 4.8, 4.2, 5.4 Hz, H-6b2), 1.97 (1H, m, H-6b2), 5.09 (1H, d,J= 6.0 Hz, H-7b2), 2.07 (1H, m, H-8b2), 1.87 (1H, m, H-9b2), 0.91 (3H, d,J= 7.2 Hz, H-10b2), 3.60 (3H, s, H-12b2), 4.53 (1H, d,J=7.2 Hz, H-1′b2), 3.54~3.58 (1H, H-6′b2)。13C-NMR(100 MHz, CD3OD)δ: 97.0 (C-1a1), 153.8 (C-3a1), 112.8(C-4a1), 32.6(C-5a1), 28.3(C-6a1), 155.7(C-7a1), 134.3(C-8a1), 45.3 (C-9a1), 119.3 (C-10a1), 166.9 (C-11a1), 99.1 (C-1′a1), 73.4 (C-2′a1), 76.7 (C-3′a1), 70.3 (C-4′a1), 77.1 (C-5′a1), 61.5 (C-6′a1)。96.9 (C-1a2), 151.4 (C-3a2), 110.0 (C-4a2), 29.4 (C-5a2), 142.4 (C-6a2), 196.8 (C-7a2), 134.0 (C-8a2), 44.0(C-9a2),118.1(C-10a2),167.0(C-11a2),98.5(C-1′a2),73.2(C-2′a2), 76.5 (C-3′a2), 70.2 (C-4′a2), 77.0 (C-5′a2), 61.4 (C-6′a2)。97.6 (C-1b1), 151.9 (C-3b1), 111.3 (C-4b1), 32.2 (C-5b1), 39.1 (C-6b1), 77.3 (C-7b1), 39.8 (C-8b1), 46.9 (C-9b1), 13.0 (C-10b1), 168.2 (C-11b1),50.6(C-12b1), 98.3(C-1′b1),74.5(C-2′b1), 76.9(C-3′b1),70.3(C-4′b1), 77.1 (C-5′b1), 61.4 (C-6′b1)。96.4 (C-1b2), 151.9 (C-3b2), 109.5 (C-4b2), 32.6 (C-5b2), 39.0 (C-6b2), 76.5 (C-7b2), 39.8 (C-8b2), 45.7(C-9b2), 12.9(C-10b2), 168.2(C-11b2), 50.5(C-12b2), 98.9(C-1′b2), 73.4 (C-2′b2), 76.7 (C-3′b2), 70.3 (C-4′b2), 77.1 (C-5′b2), 61.3 (C-6′b2)。上述数据与文献[21]报道的基本一致,从而将该化合物确定为续断苷A。
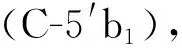
化合物11: 白色无定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.56 (1H, d,J= 6.4 Hz, H-1a), 7.50 (1H, s, H-3a), 2.84 (1H, m, H-5a), 1.86 (1H, m, H-6a), 1.74 (1H, m, H-6a), 3.57 (2H, m, H-7a), 5.78 (1H, m, H-8a), 2.65 (1H, m, H-9a), 5.26-5.31 (2H, overlapped, H-10a), 4.71/4.70 (1H, d,J=8.0 Hz, H-1′a/ H-1′b), 3.20 (2H, m, H-2′a, H-2′b), 3.31-3.38 (2H, m, H-3′a, H-3′b), 3.30 (2H, m, H-4′a, H-4′b), 3.31-3.38 (2H, m, H-5′a, H-5′b), 3.70 (2H, overlapped, H-6′a, H-6′b), 3.90 (2H, d,J= 15.6 Hz, H-6′a, H-6′b)。13C-NMRδ: 96.5 (C-1a), 152.2 (C-3a), 110.6 (C-4a), 30.0 (C-5a), 32.6 (C-6a), 59.9 (C-7a), 134.5 (C-8a), 44.1(C-9a), 118.0 (C-10a), 167.2 (C-11a), 98.8(C-1′a), 73.3(C-2′a), 76.9 (C-3′a), 70.2 (C-4′a), 77.0 (C-5′a), 61.4 (C-6′a)。96.2 (C-1b), 151.3 (C-3b), 111.8 (C-4b), 31.3 (C-5b), 38.9 (C-6b), 76.6 (C-7b), 39.7 (C-8b), 45.8 (C-9b), 12.5 (C-10b), 168.0 (C-11b), 50.4 (C-12b), 98.8 (C-1′b), 73.3 (C-2′b), 76.9 (C-3′b), 70.2 (C-4′b), 77.0 (C-5′b), 61.4 (C-6′b)。以上数据和文献[22]报道基本一致,故将该化合物确定为林生续断苷I。
化合物12: 棕色油状物。1H-NMR (400 MHz, CD3OD)δ: 7.38 (1H, d,J= 3.6 Hz, H-3), 6.58 (1H, d,J= 3.6 Hz, H-4), 4.62 (2H, s, H-6), 9.53 (1H, s, H-7)。13C-NMR (100 MHz, CD3OD)δ: 152.5 (C-2), 123.6 (C-3), 109.6 (C-4), 161.8 (C-5), 56.3 (C-6), 178.1 (C-7)。以上数据和文献[23]报道基本相同,从而将该化合物确定为5-羟甲基-2-呋喃甲醛。
化合物13: 棕色油状物。HR-ESI-MS给出分子式C18H30O2(测量值m/z277.216 8 [M - H]-,计算值m/z277.217 3 [M- H]-)。1H-NMR (400 MHz, CD3OD)δ: 2.27 (2H, t,J= 7.6 Hz, H-2), 1.59 (2H, m, H-3), 1.33 (8H, overlapped, H-4, H-5, H-6, H-7), 2.07 (4H, overlapped, H-8, H-17), 5.33-5.41 (6H, overlapped, H-9, H-10, H-12, H-13, H-15, H-16), 2.81 (4H, t,J= 8.4 Hz, H-11, H-14), 0.97 (3H, t,J= 7.2 Hz, H-18)。13C-NMRδ: 176.3 (C-1), 33.6(C-2), 25.2(C-3), 28.8(C-4), 28.9(C-5), 28.9(C-6), 29.3 (C-7), 26.8 (C-8), 129.7 (C-9), 127.5 (C-10), 24.7 (C-11), 127.8 (C-12), 127.8 (C-13), 24.7(C-14), 126.9 (C-15), 131.4 (C-16), 20.1 (C-17), 13.2 (C-18)。以上数据和文献[24]报道基本一致,从而将该化合物确定为α-亚麻酸(α-linolenic acid)。
3 讨论与结论
本研究对续断药材化学成分进行了进一步研究,分离鉴定了13个化合物,其中3-O-(2-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷、5-羟甲基-2-呋喃甲醛、α-亚麻酸为首次从续断药材中分离得到,同时也是首次从川续断属植物中分离得到。
以川续断皂苷Ⅵ为代表的三萜皂苷类化合物是续断发挥药效的主要化学成分群[12],3-O-(2-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷同川续断皂苷Ⅵ一样,也是具有相同化学骨架的三萜皂苷类化合物,由于化学结构的相似性推测其也具有类似于川续断皂苷Ⅵ的生物活性,该成分对于建立以质量标志物为药材优劣评价依据的续断药材质量标准提供了物质基础,同时为进一步阐释续断“发汗”加工前后化学成分及其含量的变化、化学成分转化机制研究提供了丰富的化学物质基础。

