Spinning from Nature: Engineered Preparation and Application of High-Performance Bio-Based Fibers
Zongpu Xu, Mingrui Wu, Qi Y Dong Chen*, Ki Liu*, Ho Bi,*
a State Key Laboratory of Chemical Engineering, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
b Institute of Applied Bioresources, College of Animal Sciences, Zhejiang University, Hangzhou 310058, China
c State Key Laboratory of Industrial Control Technology, College of Control Science and Engineering, Zhejiang University, Hangzhou 310027, China
d Institute of Process Equipment, College of Energy Engineering, Zhejiang University, Hangzhou 310027, China
e State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China
Keywords:Bio-based fiber Hierarchical structure Bioinspired spinning Strengthening strategy Fiber applications
ABSTRACT Many natural fibers are lightweight and display remarkable strength and toughness. These properties originate from the fibers’ hierarchical structures, assembled from the molecular to macroscopic scale.The natural spinning systems that produce such fibers are highly energy efficient, inspiring researchers to mimic these processes to realize robust artificial spinning. Significant developments have been achieved in recent years toward the preparation of high-performance bio-based fibers.Beyond excellent mechanical properties,bio-based fibers can be functionalized with a series of new features,thus expanding their sophisticated applications in smart textiles, electronic sensors, and biomedical engineering.Here, recent progress in the construction of bio-based fibers is outlined. Various bioinspired spinning methods, strengthening strategies for mechanically strong fibers, and the diverse applications of these fibers are discussed.Moreover,challenges in reproducing the mechanical performance of natural systems and understanding their dynamic spinning process are presented. Finally, a perspective on the development of biological fibers is given.
1. Introduction
High-performance bio-based fibers hold promise for application in many fields, including the textile industry, building construction, automobile manufacturing, and biomedical engineering[1-9]. Over a long historical period that continues to the present day, humans have acquired fibers directly from nature, including animal fibers (e.g., silk, wool) and plant fibers (e.g., wood, cotton,and flax). Such fibers are lightweight, strong, biocompatible, and sustainable [10-13]. For example, silkworm fibers have been used as the basis of textiles in China for over 5000 years, whereas the first fully synthetic fiber, nylon, was invented in the 1930s. More surprisingly, silk fibers show unrivalled mechanical properties,with a toughness that is superior to that of steel, carbon fiber,and any synthetic polymer fiber (Table 1) [12,14-19]. The properties of natural fibers have attracted scientists and engineers to explore the relationship between fiber structures and mechanics,and to learn from nature for the preparation of high-performance fibers [2,16].
Like many other lightweight and strong biomaterials, such as nacre and bone, natural fibers display hierarchical structures assembled from the molecular to macroscopic scale [10,20,21].As the basic components,protein molecules in animal fibers or cellulose molecules in plant fibers form an aggregation state and then assemble into nanofibrils, which further gather into macroscale fibers (Fig. 1) [11]. Natural fibers usually combine stiff and soft domains, as in the case of spider silk, in which the internal βcrystalline region determines strength and the amorphous region determines toughness. Beyond their excellent mechanical properties, natural fibers are multifunctional. For example, spider silk webs can achieve water collection, due to their topological structures of periodic spindle-knots and joints [22], and the excellent thermal insulation of polar bears’hollow hair fibers keep the bears warm in an extremely cold environment[23].Over long-term evolution, natural fibers have been optimized in terms of their structures and functions, although they typically feature a verylimited number of components (i.e., proteins or polysaccharides)[20,24].Therefore,nature provides valuable and feasible strategies to create functional materials through the manipulation of multiscale structure [25]. In addition, compared with synthetic fibers,which require high temperature and pressure in their preparation process,silk fibers are produced at ambient temperature and from an aqueous solution, indicating the energy-efficient advantage of natural spinning systems [26,27].
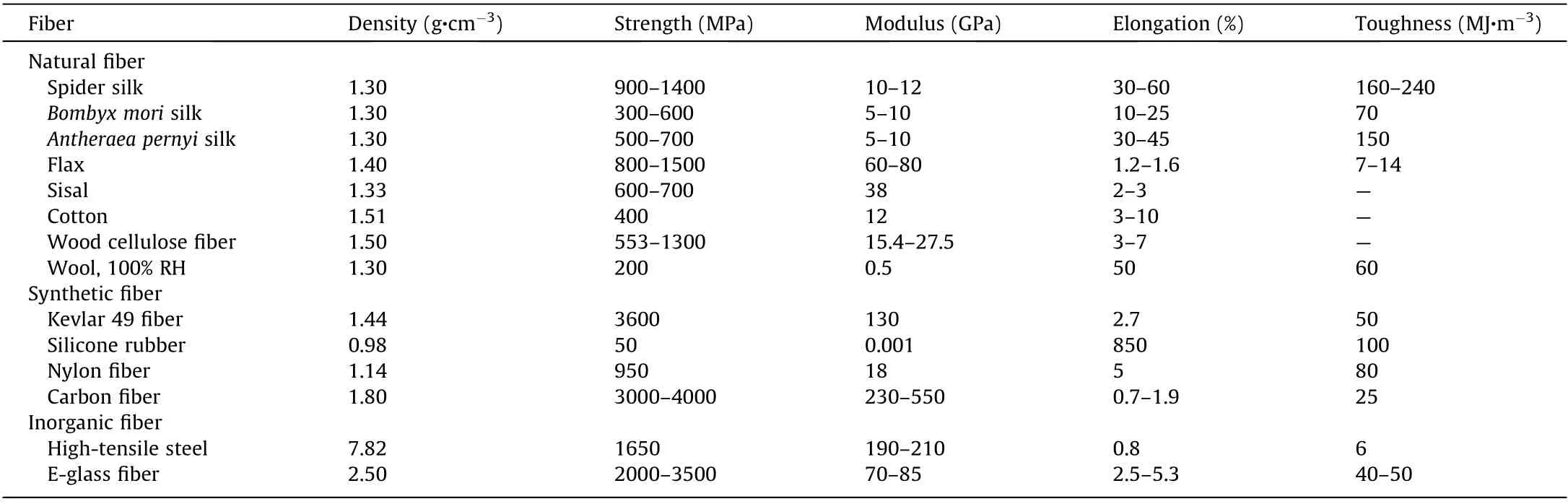
Table 1 Mechanical properties of natural and synthetic fibers [12,14-19].
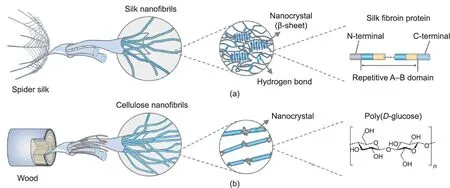
Fig. 1. Hierarchical structures of nanofibrils in (a) spider silk and (b) wood. Reproduced from Ref. [11] with permission.
Learning from nature, researchers have developed numerous mechanically strong bio-based fibers by mimicking natural fibers’structure and duplicating natural spinning processes[3,28].Moreover, these fibers can be functionalized to achieve enhanced thermal, magnetic, optical, electrical, and biological functions, further expanding their applications in smart textiles, electronic sensors,and biomedical engineering [29,30]. In this work, we present an overview of recent progress in the preparation and application of high-performance bio-based fibers. Starting with a brief explanation of the natural spinning process, we introduce various bioinspired spinning methods, including wet spinning, dry spinning,microfluidics spinning,and interface wiredrawing.We next discuss strengthening strategies to improve the fibers’ mechanics. Then,we illustrate the functional fibers that have been achieved by means of structure manipulation or nanomaterial incorporation and describe their potential applications. We also present current limitations and challenges in this field, and provide a perspective on the development of high-performance fibers.
2. Bioinspired spinning methods
Spiders and silkworms can produce mechanically strong silk fibers by means of their delicate and efficient spinning systems,in which the spinning dope undergoes chemical and physical changes [26,31,32]. The concentration of silk protein solution increases along the glands, along with a decrease in pH value and changes in cations (Ca2+and K+) [33]. Meanwhile, the gradually changed shape of the spinning duct provides shearing and stretching forces to the silk molecules, thereby promoting their conformation transformation[34].In the end,silk fibers are pulled out by the legs of the spider or the head movement of the silkworm, and then solidify in air. The mechanical properties of silk fibers can be influenced by the spinning conditions; for example,silks from the domestic silk moth,Bombyx mori,produced by faster,artificial reeling rates are stronger but more brittle than those produced by slower, natural reeling rates [27]. Inspired by natural spinning processes, various solvent-based spinning methods have been developed, including wet spinning, dry spinning, microfluidics spinning, and interface wiredrawing (Fig. 2). In addition, a non-solvent-based method, melt spinning, serves as an efficient method to produce bio-based fiber derived from bioresources with high carbon content, such as lignin [35,36]. In this section, we mainly focus on the abovementioned solvent-based spinning methods,briefly discussing their working principles and providing some related illustrations.
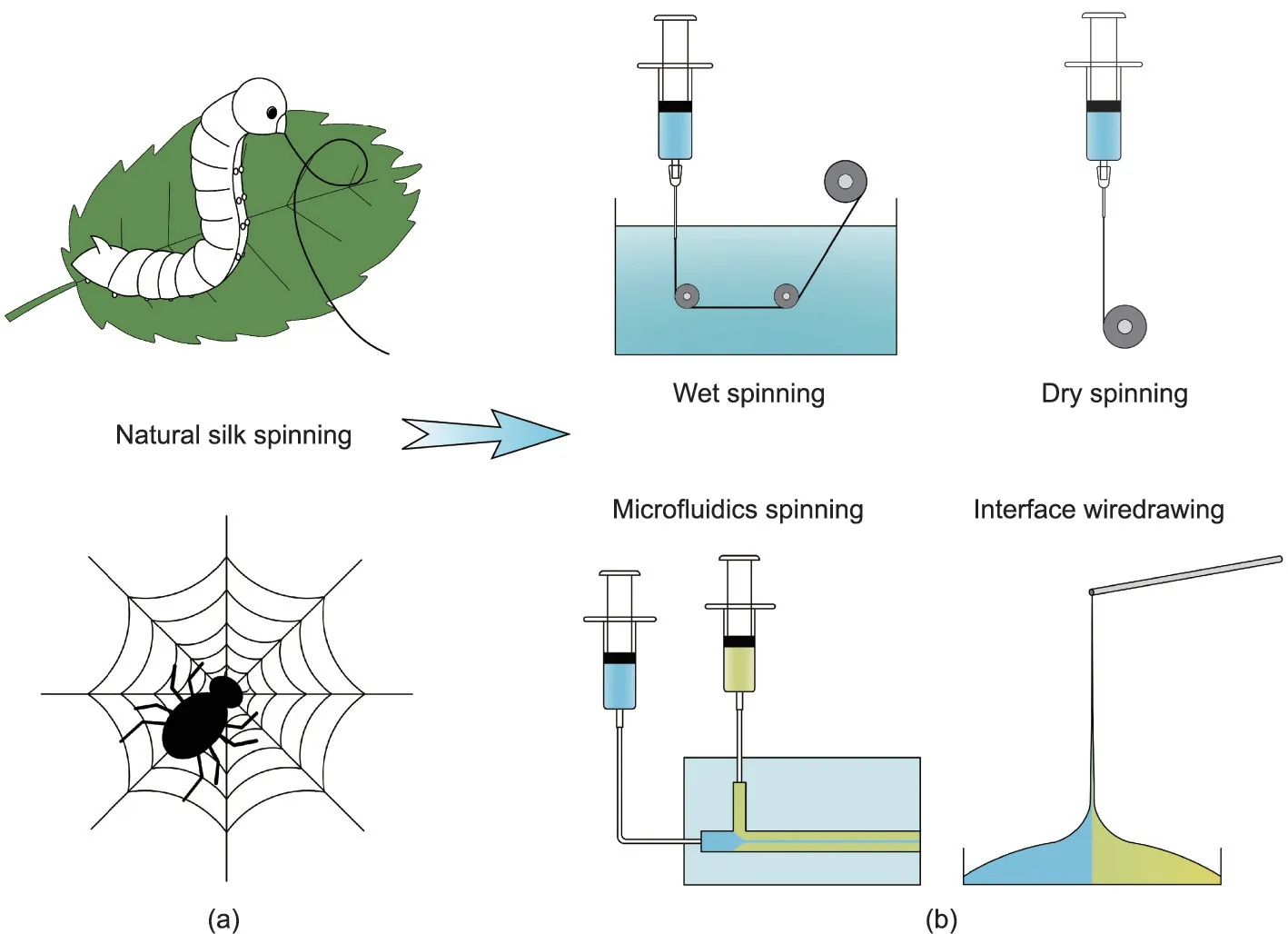
Fig. 2. Schematic illustrations of bioinspired spinning methods. (a) Schematic of natural silk spinning by a silkworm and spider; (b) various bioinspired spinning methods,including wet spinning, dry spinning, microfluidics spinning, and interface wiredrawing.
2.1. Wet spinning
In the wet spinning process, the pre-dissolved spinning dope is extruded into a coagulating bath;it is then solidified via the double diffusion effect between solvents.With choices of a proper solvent and corresponding coagulant, the spinning dope can form fibers under an appropriate extrusion speed. For silk spinning, fibroin protein is dissolved in an organic solvent such as 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), trifluoroacetic acid (TFA), or formic acid, and alcohols (methanol, ethanol, isopropanol) are usually selected as matched coagulants[37-40];however,these combinations result in undesired morphology and brittleness of the as-spun fibers due to rapid precipitation of the silk proteins.In comparison,a silk aqueous solution/ammonium sulfate combination is a better alternative due to its relatively moderate solidification rate[41,42].Another necessary procedure in wet spinning is post-drawing,which can promote conformation transition and increase the alignment of the molecules, thus significantly improving the mechanical properties of the resultant fibers [28]. For example, Zhou et al. [41] constructed a small-scale industrial wet spinning apparatus and prepared silk fibers with uniform diameter and a smooth surface from silk aqueous solution/ammonium sulfate systems(Fig. 3(a)). Moreover, the fibers that were obtained after being post-drawn six times exhibited a breaking stress of 450 MPa and a breaking strain of 27.7%,making them stronger and tougher than natural cocoon silk.
2.2. Dry spinning
In the dry spinning process, the spinning dope is directly injected into air and solidifies into fiber with the evaporation of the volatile solvent,in what is fairly similar to the natural spinning process. Artificial silk dry spinning was first reported in 2011 and generally requires a relatively high concentration of fibroin protein(>20 wt%)[43,44].Sun et al.[45]developed custom-built capillary dry spinning equipment and directly prepared silk fibers in air from a 50 wt% silk aqueous solution. Like wet spinning, dry spinning requires post-drawing. After being stretched via postdrawing four times, the breaking stress of the silk fibers increased from 45.7 to 326.7 MPa, although it was still lower than that of cocoon silk.
In addition to mimicking the natural dry spinning process,Ling et al. [46] prepared fibers that retained the hierarchical structures of natural silks(Fig.3(b)).A partially dissolved silk microfibril solution, which showed a liquid-crystal-like texture analogous to the nematic silk protein in silk glands, was selected as the spinning dope.These microfibrils were able to flow and align along the shear direction to fuse together into a long fiber,which allowed for easy hand reeling. As this fiber maintained the structural hierarchy of natural silks,its Young’s modulus reached 11 GPa,which was even higher than that of spider dragline silk. Similarly inspired by the natural formation of silk from liquid crystalline proteins, Ma et al. [47] obtained continuous fibers by directly drawing smectic engineered protein gels. The mechanical performance of the gel fibers could be manipulated by genetically tuning the charge density of the protein backbones, thus offering great possibilities of programmable design for biopolymeric fibers.
2.3. Microfluidics spinning
Microfluidics provides miniaturized channels to achieve precise control from individual fluids to fiber formation,and can mimic the hydrodynamic principles in natural spinning systems [48-53]. In this process, the spinning dope is extruded into a shear fluid via coaxial channels,and diffusion occurs at their interfaces.The inner dope remains at laminar flow and can be solidified into fibers in situ via polymerization, crosslinking, or solvent change. Using sodium alginate as the inner phase and calcium chloride as the outer stream, a range of hydrogel fibers with desirable features have been fabricated. For example, by adjusting the microfluidic channels design, Cheng et al. [49] and Yu et al. [51]obtained alginate fibers with different morphologies, including multicompartmental and hollow fibers, as well as spring-like helical fibers.
Apart from hydrogel fibers,microfluidics is a useful tool for the preparation of mechanically strong fibers[54-56].Mittal et al.[56]reported a flow-assisted technique to organize cellulose nanofibrils into macroscale fibers by means of microfluidics spinning(Fig. 3(c)). A charged cellulose nanofibril suspension was injected into the core channel and then successively surrounded by two sheath flows of deionized water and low-pH acid.In the core flow,cellulose nanofibrils exhibited poor alignment, as a result of Brownian motion and the electrostatic repulsions caused by carboxyl groups on the fibril surface.The first flow of deionized water supported electrostatic repulsion and aligned the cellulose nanofibrils in the flow direction; before this alignment was lost,the second flow of acid diffused into the dispersions and initiated a gel transition, thereby locking the well-organized structure of cellulose nanofibrils. The resultant fibers possessed nearly perfect unidirectional alignment of cellulose nanofibrils; thus, they had a Young’s modulus of about 70 GPa and a strength of about 1200 MPa, surpassing most reported nanocellulose-based fibers.
2.4. Interface wiredrawing
Interface wiredrawing uses the electrostatic interaction between components with opposite charges: The pulling process at their interfaces induces the orientation of molecules along the fiber axis [57-59]. Zou and Kim [57] found that graphene oxide and chitosan generated strong interactions, so they mixed a graphene oxide nanosheets suspension with chitosan solution and assembled them into long fibers by simply pulling the mixture upward in air.In another study,Grande et al.[58]deposited a drop of chitosan solution and a drop of a cellulose nanofiber suspension onto a plate, which placed the two liquids in lateral contact(Fig. 3(d)). The primary amino groups of the chitosan were protonated after dissolving in acidic aqueous media, while the surface of the cellulose nanofibers was modified with carboxyl groups.Due to complexation between the cationic macromolecules and anionic nanofibers, composite fibers could be continuously drawn from the drop interface. The drawing process ensured very good alignment of the cellulose nanofibers surrounded by chitosan,which endowed the compact fibers with a very high tensile modulus of about 22 GPa.
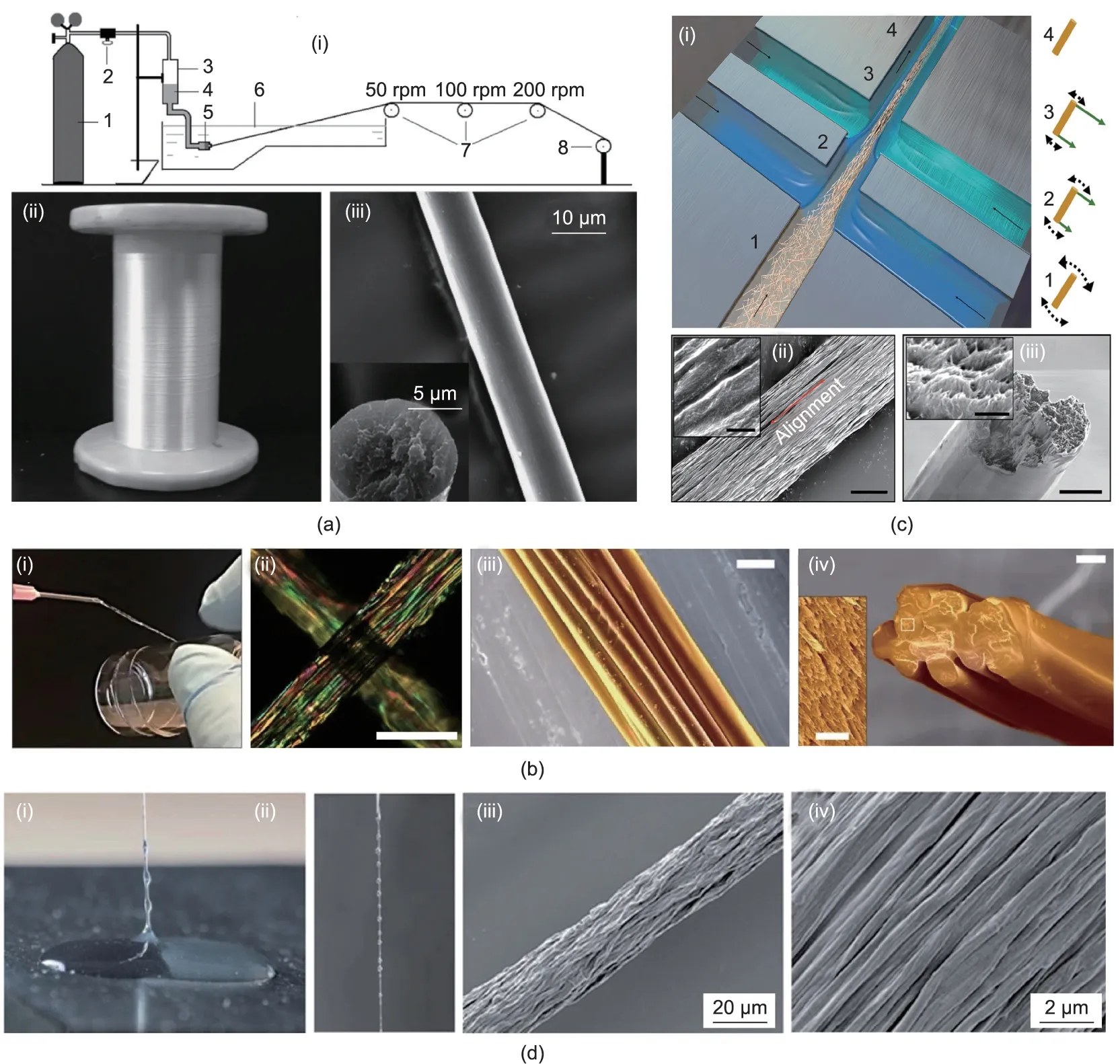
Fig. 3. Illustrations of bio-based fibers prepared via different spinning methods. (a) Preparation of silk fiber by wet spinning: (i) schematic of a small-scale apparatus(1: nitrogen gas cylinder; 2: pressure regulator; 3: dope storage cylinder; 4: spinning dope; 5: extrusion die; 6: heated coagulation bath; 7: draw rollers; 8: take-up roller;rpm: revolutions per minute); (ii) optical photograph of the lustrous silk fibers; (iii) scanning electron microscope (SEM) images showing the smooth surface and circular cross-section of the silk fibers.(b)Preparation of silk fiber by dry spinning:(i)optical photograph showing the hand-reeling process;(ii)polarized image of the as-spun fibers;(iii, iv) SEM images showing the fiber constituted by oriented silk microfibrils (scale bars are 100 μm for (ii), 20 μm for (iii) and (iv), and 2 μm for inset). (c) Preparation of cellulose fiber by flow-assisted microfluidics spinning: (i) schematic of cellulose nanofibrils flowing in the channel; (ii, iii) SEM images showing good alignment of the nanofibrils (scale bars are 3 μm for (ii) and (iii), and 400 nm for insets). (d) Preparation of cellulose nanofiber/chitosan fiber via interface wiredrawing: (i, ii) optical photographs of gently pulling the viscous thread from the interface of the cellulose nanofiber suspension (clear liquid) and chitosan solution (cloudy liquid); (iii, iv) SEM images showing fibrils aligned along the fiber direction.(a)Reproduced from Ref.[41]with permission;(b)reproduced from Ref.[46]with permission;(c)reproduced from Ref. [56] with permission; (d) reproduced from Ref. [58] with permission.
3. Strengthening strategies for mechanically strong fibers
Many natural fibers display remarkable mechanical properties,which originate from the ways in which the fibers’building blocks are assembled and arranged at multiple scales[20].In both animal and plant fibers,the orientations of molecular chains or fibrils have a significant influence on the fibers’ mechanical performance. For example, flax and sisal possess similar contents of cellulose and hemicellulose, but flax shows a higher strength and stiffness than sisal,mainly due to the lower fibril angel along the fiber axis of flax(8°-11°) than sisal (~20°) [12]—a finding that provides a valuable reference for the preparation of mechanically strong fibers. The nanofibrils in natural fibers are usually compactly connected by hydrogen bonds [10,60]; thus, enhancing inter-fibrillar interactions via physical or chemical crosslinking will significantly improve a fiber’s mechanical properties.As another example,wood is a tri-component biomaterial composed of cellulose, hemicellulose, and lignin. These three components are systematically integrated, which indicates that the natural strategy of combination can serve as an effective means of producing strong fibers. In this section,we briefly summarize the dominant strengthening mechanisms of natural fibers (Table 2) [10,12,20,60] and discuss various artificial strategies based on these mechanisms, including postdrawing, twisting, crosslinking, and incorporating fillers (Fig. 4)[56,61-63]. These strategies can be used alone or together, from the preparation of spinning dopes to the post-treatment of asspun fibers.
3.1. Post-drawing
As mentioned in the discussion on spinning methods, postdrawing plays an important role in producing mechanically strong fibers,because it can promote molecular orientation along the fiber axis [43,64,65]. The as-spun silk fibers produced by wet or dry spinning generally have poor strength but good extensibility, due to the disorder of the curly molecular chains. During the drawing process, the molecular chains are extensively stretched and arranged along the axis, which can increase the intermolecular interactions and fiber crystallinity. Zhang et al. [65] prepared silk fibers by wet spinning. The as-spun fibers had a Young’s modulus of 3.9 GPa and a strength of 95.1 MPa.After drawing the fibers four times, these values significantly increased to 6.9 GPa and 470.4 MPa. X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy(FTIR)results indicated that the amorphous structure of the silk had been transformed into a silk II structure,and Raman spectra changes revealed an increase in the alignment of protein chains.In another study,the tensile strength of silk fibers similarly improved from less than 100 to 420 MPa with increasing draw ratios (Fig. 4(b)) [61]. Post-drawing can drastically decrease fiber diameter, which may contribute to defect reduction and structure densification. However, excessive draw ratios will cause unexpected breakage of fibers, so the draw ratio should be controlled within a reasonable range to achieve the optimal outcome.
3.2. Twisting
Over the long history of fiber- and rope-making, twisting has been used as a simple yet useful method to produce mechanically strong textiles and ropes. Short fibers such as cotton can be integrated into continuous threads with a certain degree of strength by twisting,and long fibers such as silk can form strong yarns with a dense structure; both of these processes are very mature techniques in the textile industry. Mimicking this procedure, Kamada et al. [62] prepared strong and tough fibers based on protein nanofibrils by twisting two single fibers together into a tight combination (Fig. 4(c)). Cellulose nanofibers are also promising building blocks for strong fibers due to their extraordinary mechanical properties; however, their poor alignment, as well as the boundaries and voids among them, often leads to a significant loss of mechanics in the transition from nanoscale to macroscale[21,66-68]. Wang et al. [69] reported the creation of superstrong and super-stiff fibers from the direct assembly of bacterial cellulose nanofibers by means of drawing and twisting. These two steps not only achieved good alignment of the cellulose nanofibers but also reduced the inter-filament pores and induced strong inter-filament hydrogen bonding.The Young’s modulus and tensile strength of the obtained fibers reached 65.7 GPa and 826 MPa,respectively, and the specific tensile strength was as high as 598 MPa·cm3·g-1,substantially exceeding that of lightweight steel(227 MPa·cm3·g-1).
3.3. Crosslinking
Interactions between the building blocks of fibers can be strengthened by crosslinking via ions or covalent bonds, which can effectively improve the mechanical properties of fibers[70,71]. Yao et al. [71] used Fe3+ions to crosslink cellulose fibers,with the carboxyl groups on the fiber surfaces forming metalcarboxylate bonds with the metal ions. The Young’s modulus and tensile strength increased from 16.4 GPa and 248.6 MPa to 22.9 GPa and 357.5 MPa, respectively, as a result of stronger intra- and inter-fibrillar interactions. In another study, 1,2,3,4-butane tetracarboxylic acid (BTCA) was used to crosslink cellulose fibers by creating bridges between cellulose nanofibrils,replicating to some extent the interactions between cellulose and hemicellulose/lignin in natural plant fibers [56]. The tensile strength of the fiber increased from about 1200 to 1570 MPa after covalentcrosslinking(Fig.4(d)),achieving a record high for cellulose-based fibers.
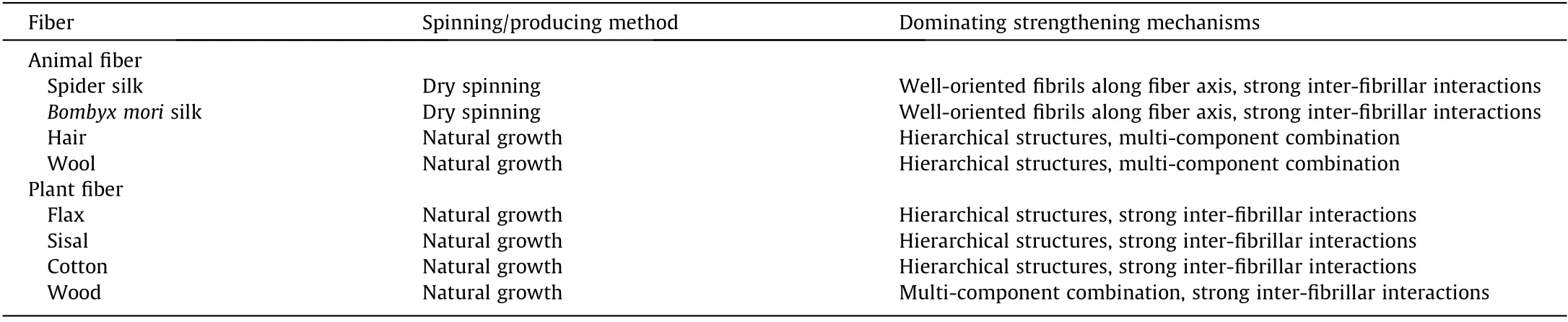
Table 2 Spinning methods and strengthening mechanisms of natural fibers [10,12,20,60].
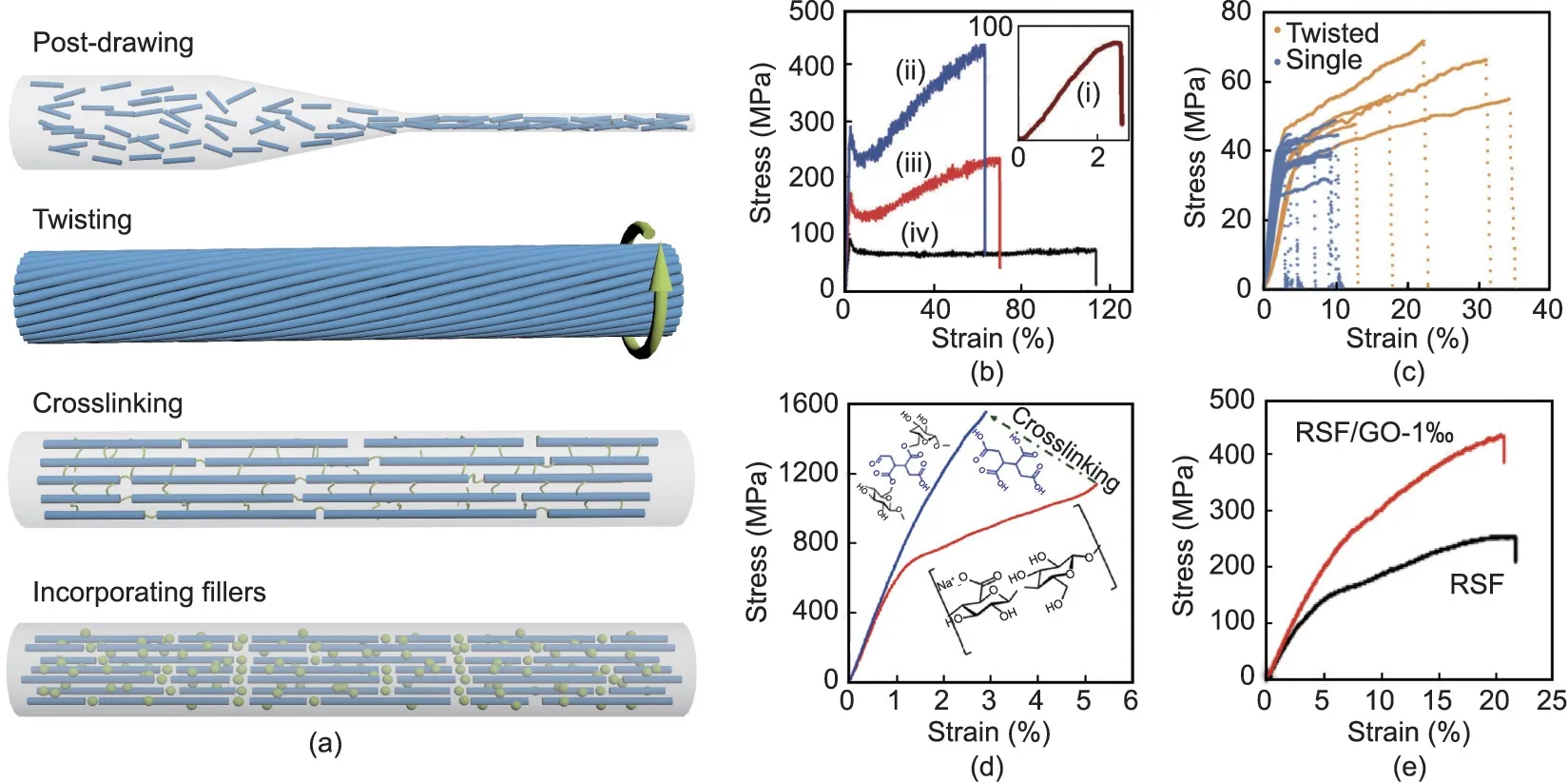
Fig.4. Strengthening strategies for bio-based fibers.(a)Schematic of various strengthening strategies including post-drawing,twisting,crosslinking,and incorporating fillers.(b) Stress-strain curves of silk fibers (i) without drawing and (ii-iv) with different draw ratios of (ii) 2, (iii) 6, and (iv) 9. (c) Stress-strain curves of single and twisted β-lactoglobulin fibers.(d)Stress-strain curves of cellulose fibers before and after crosslinking by 1,2,3,4-butane tetracarboxylic acid.(e)Stress-strain curves of silk fibers with and without graphene oxide (RSF: regenerated silk fibroin; GO: graphene oxide). (b) Reproduced from Ref. [61] with permission; (c) reproduced from Ref. [62] with permission; (d) reproduced from Ref. [56] with permission; (e) reproduced from Ref. [63] with permission.
Crosslinking is also an effective strategy for the mechanical enhancement of protein-based fibers. Recently, He et al. [72],Zhang et al. [73], and Li et al. [74] developed a series of mechanically strong fibers from both widely available proteins and recombinant chimeric proteins by means of the introduction of glutaraldehyde(GA)crosslinking.For example,bovine serum albumin (BSA) is generally not regarded as a good candidate for fiber production because of its intrinsic structural defects; however, a network of BSA molecules became highly condensed after crosslinking with GA. Compared with pristine BSA fibers, the breaking strength and toughness of the double GA-crosslinked BSA fibers improved significantly from only about 14 MPa and 0.17 MJ·m-3to 130 MPa and 143 MJ·m-3, respectively [72]. GA is a common protein crosslinker,so it was also used to enhance fibers based on recombinant chimeric proteins consisting of a squid ring teeth (SRT) segment and a cationic elastin-like polypeptide (ELP)sequence (Fig. 5) [74]. As in the study described above, GA crosslinking led to the formation of imine bonds among SRT-ELP molecules, thus strengthening their interactions. The breaking strength and toughness of 36mer SRT-ELP fibers,which are already as high as 550 MPa and 109 MJ·m-3, further increased to 603 MPa and 113 MJ·m-3after dimerization via disulfide linkages, making the fibers superior to many recombinant spider silks and even comparable to native spider silks.
3.4. Incorporating fillers
Nanomaterials can usually function as reinforcing fillers in composites due to their large surface area and outstanding intrinsic properties [75-77], and the same applies to nanomaterial fillers in bio-based fibers.Among them,carbon nanotubes[61],nanominerals [78], and graphene oxide [63,79] have been added to silk spinning dope with the aim of obtaining stronger and tougher fibers.Due to interactions between nanomaterials and silk protein through coordination complexes or intermolecular forces, the mechanical properties of the composite fibers were strengthened;this effect could be influenced by filler content and spinning method. For example, Zhang et al. [63] prepared silk fibers that incorporated graphene oxides by means of dry spinning:The fibers exhibited a breaking strength of 435.5 MPa at 0.1 wt% filler content, which was significantly higher than that of pure silk fibers(~252 MPa) (Fig. 4(e)). In another study, the breaking strength of wet-spun silk fibers increased from 439 MPa without filler to 697 MPa with 0.3 wt% of graphene oxide filler [79].
Aside from artificial spinning,silk fibers containing fillers can be directly produced by silkworms fed with nanomaterials[80-85].In this process, nanoparticle dispersions are generally sprayed onto mulberry leaves and then eaten by silkworm larvae.Although most of this diet is digested and excreted,a fraction of the nanomaterials is still incorporated into the silk fibers, which has been confirmed through elemental or spectral analysis.Because the silk fibroin and nanomaterials go through a natural spinning process, stronger interactions can be generated [85]. A study by Wang et al. [85]demonstrated significant improvement in the breaking strength of cocoon silk—from 360 MPa on a normal diet to 570 MPa on a diet containing 0.2 wt% carbon nanotubes and 590 MPa on a diet containing 0.2 wt% graphene. More importantly, this feeding procedure is easy to handle and scale up, thus paving a new path for the production of mechanically strong silk fibers by taking full advantage of a natural spinning system.
4. Applications of bio-based fibers
For thousands of years, people have directly utilized natural fibers for textiles, sutures, and building materials. With the development of industry and progress in technology, various highperformance synthetic fibers have been created, most of which are petrochemical-based products. Given the concerns of resource sustainability and environmental issues [86,87], bio-based fibers are gaining increasing attention. They are lightweight, mechanically strong, and biocompatible, and can be functionalized with a series of new features by means of structural manipulation or nanomaterial incorporation, which greatly expands their applications. In addition, some bio-based fibers can be carbonized into high-quality carbon fibers due to their carbon frameworks. For example,Xia et al.[35]and Ouyang et al.[36]were the first to prepare continuous precursor fibers based on modified lignin via wet spinning, which were then converted into carbon fibers through thermal stabilization and carbonization. In another study, Wang et al.[88]directly carbonized silk fibers through thermal treatment under an inert atmosphere and then fabricated a wearable strain senor with good electrical conductivity and high sensitivity.In this section, we illustrate the potential applications of bio-based fibers in terms of their enhanced thermal, magnetic, optical, electrical,and biological functions.
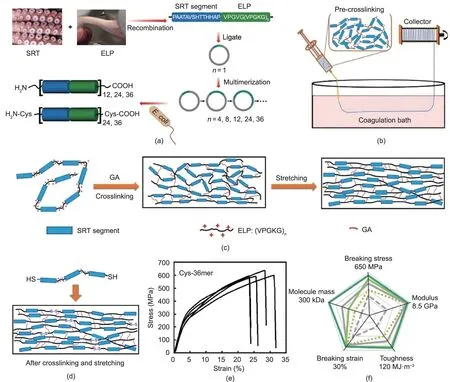
Fig. 5. Preparation of non-spider chimeric protein fibers with high strength and high toughness. (a) Schematic of the construction and expression of recombinant proteins consisting of an SRT protein segment and a cationic ELP sequence (E. coli: Escherichia coli). (b) Schematic of SRT-ELP fiber preparation via wet spinning. (c) Schematic of SRT-ELP fiber formation via GA crosslinking and post-stretching treatment: Amino groups on the ELP lysine residues are highly reactive sites for covalent crosslinking with GA. (d) Schematic of SRT-ELP chimera dimerization via disulfide linkage of the fibers. (e) Stress-strain curves of the fiber spun from SRT-ELP Cys-36mer. (f) Spider chart representing the mechanical performance evolution of SRT-ELP fibers formed from Cys-36mer chimeras. Reproduced from Ref. [74] with permission.
4.1. Thermal function
The use of bio-based fibers with excellent mechanical properties as yarns has been reported [66,70], including the twisting and dying of cellulose fibers, as shown in Fig. 6(a). However, biobased fibers generally exhibit high flammability due to their inherent nature,which severely limits their applications in certain areas[89,90]. Nechyporchuk et al. [91] prepared flame-resistant cellulose fibers by coating them with a shell of silica nanoparticles(Fig.6(b)).When burned directly by fire,pure cellulose fiber could only remain unburned for 0.3 s, whereas the silica/cellulose fiber remained unburned for 4.21 s. The silica shell provided a good thermal shield by acting as a barrier to heat transfer, which was important for the protection of the inner cellulose.
Efficient thermal insulation is very important for personal thermal management in a cold environment [92-94]. Inspired by the hollow structure of polar bear hairs, we recently developed a‘‘freeze-spinning” technique to fabricate biomimetic silk fibers with excellent thermal insulation [95]. The resultant fibers possessed an aligned porous structure (Fig. 6(c)), and the pore size could be regulated by choosing different freezing temperatures,as the temperature was closely related to the thermal performance.When the textile fibers had smaller pores and more layers, the absolute temperature difference between the stage and the fiber surface became greater,indicating a better ability to passively keep warm through dissipation via the structural design.A rabbit wearing the biomimetic porous textile became almost invisible under infrared camera, as its surface temperature became extremely close to that of the background,making it possible to achieve thermal stealth(Fig.6(d)).In addition,the textile was capable of active electroheating when doped with carbon nanotubes to induce a fast thermal response(Figs.6(e) and (f)). More importantly, the textile woven with biomimetic fibers showed good wearability and breathability for wearing, suggesting its great potential as a smart and multifunctional material for personal thermal management.
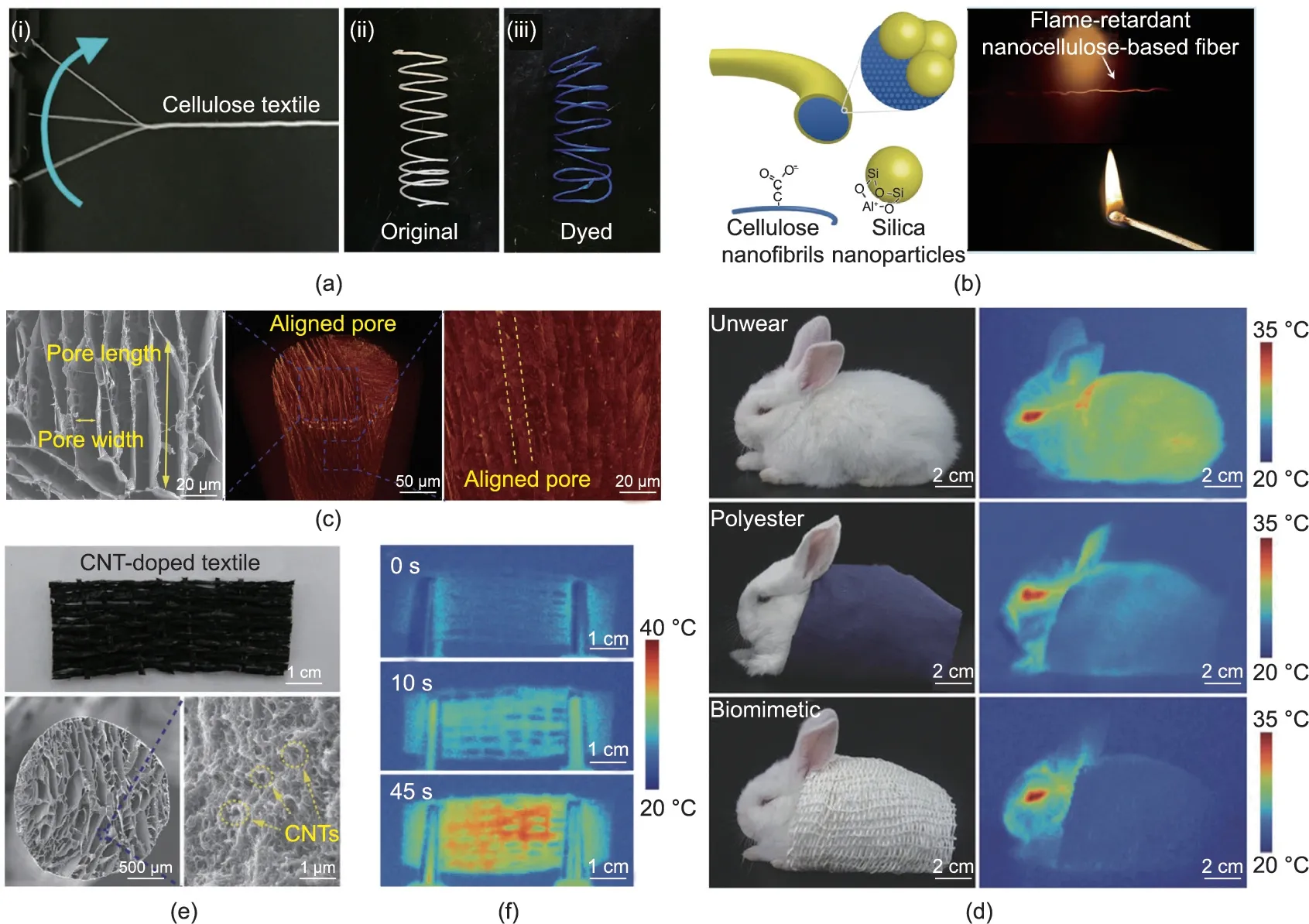
Fig.6. Illustrations of bio-based fibers with excellent thermal functions for textile use.(a)Strong bacterial cellulose fibers can be made into yarns and easily dyed into blue.(b) Cellulose fibers coated with silica nanoparticles can be used as flame-retardant materials. (c-f) Thermal insulating silk textile inspired by polar bear hair: (c) SEM and X-ray computed microtomography images of the porous biomimetic fiber showing the aligned lamellar pores along the axial direction;(d)optical photographs and infrared images of a rabbit before and while wearing different textiles,demonstrating the excellent thermal stealth function of the biomimetic porous textile;(e)optical photograph and SEM images of the porous biomimetic silk textile incorporated with carbon nanotubes (CNTs); (f) infrared images of the carbon nanotubes and silk textile during the electroheating process, indicating the material’s fast and active thermal response capability. (a) Reproduced from Ref. [70] with permission; (b) reproduced from Ref. [91]with permission; (c-f) reproduced from Ref. [95] with permission.
4.2. Magnetic, optical, and electrical functions
Magnetic fibers can be produced by incorporating magnetic components within the fibers, allowing the fibers to display stimuli-responsive behaviors.He et al.[96]prepared alginate fibers with a spider-silk-like structure via microfluidic spinning;spindleknots containing magnetic Fe3O4nanoparticles were generated via solvent evaporation.The size of the spindle-knots and the distance between them could be precisely adjusted. These functionalized fibers exhibited excellent stimuli-triggered responses, as they could move directionally following the rotation of external magnetic fields (Fig. 7(a)). Moreover, the fibers could be guided magnetically to form patterns and to assemble into various structures according to magnetic arrays,indicating their potential application as intelligent stimuli-responsive materials.
Fluorescent silk-based materials have been applied in biomedical engineering, optics, and photonics [97,98]. In general, fluorescent silk fibers can be produced by the genetic modification of silkworms or via dying treatment [99,100]. However, transferring the transgene to the next generation remains a problem, while dying often results in unstable fluorescence. Therefore, obtaining fluorescent silk fibers with both good mechanical properties and highly stable fluorescence has become an important issue. By directly feeding silkworm larvae with CdSe/ZnS core-shell quantum dots, Cheng et al. [101] obtained luminescent silkworm cocoons.Compared with normal or fluorescent-dyed silk,the resultant fibers had superior mechanical strength and toughness due to the reinforcing effect of the quantum dots. More importantly, the luminescent silk fibers exhibited decent fluorescent stability even after soaking in water for 720 h (Fig. 7(b)).
In recent years, wearable electronics have attracted increasing attention for their wide applications in signal sensing and health monitoring [102,103]. Due to their good flexibility and stitchability,bio-based textile fibers have become a promising base material in this area[46,104].Ling et al.[46]first prepared silk fibers via dry spinning and then coated carbon nanotubes onto their surface.The obtained conductive silk fibers responded very quickly to humidity and temperature changes; they could be woven into masks as a form of smart fabric in order to monitor touching and breathing(Fig.7(c)).Qi et al.[105]also used carbon nanotubes as the conductive component to fabricate functional cellulose fibers. According to scanning electron microscope (SEM) observations, the carbon nanotubes were well dispersed within the cellulose matrix rather than remaining on the fiber surface and thus promoted the formation of conductive networks.The electrical resistance of such fibers could change under external stimuli,including tensile strain,temperature,and environmental humidity;therefore,they have potential for use in the design of various sensors to perceive body movement or monitor body sweat.
4.3. Biological function
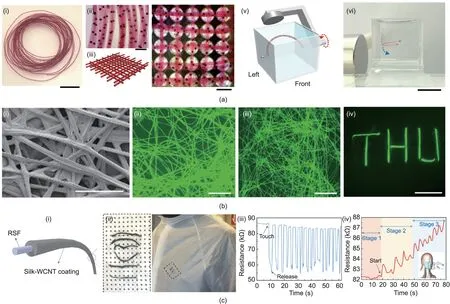
Fig.7. Illustrations of bio-based fibers with excellent magnetic,optical,and electrical functions for various applications.(a)Magnetic alginate fibers can be used as stimuliresponsive materials:(i-iv)optical photographs of hydrated fibers with magnetic oil cores and a 3D textile-like pattern constructed via magnetic arrays;(v,vi)schematic and photograph of the magnetic manipulation of a fiber(scale bars are 1 cm for(i)and(vi),and 1 mm for(ii)and(iv)).(b)Silk incorporated with CdSe/ZnS quantum dots can be used as fluorescent fibers:(i)SEM image of cocoon silk fibers;(ii,iii)photoluminescence images of silk fibers after soaking in water for 0 and 720 h;(iv)fluorescent image of the‘‘THU”logo(scale bars are 200 μm for(i),500 μm for(ii)and(iii),and 1 cm for(iv)).(c)Regenerated silk fibers with wall carbon nanotube(WCNT)coating can be used as wearable sensors: (i, ii) schematic and optical photographs of the conductive core-shell fiber; resistance response of conductive silk fibers to (iii) finger-touching and(iv) breathing. (a) Reproduced from Ref. [96] with permission; (b) reproduced from Ref. [101] with permission; (c) reproduced from Ref. [46] with permission.
One of the outstanding advantages of bio-based fibers is their good biocompatibility both in vitro and in vivo,which is important in biomedicine [7,50,106-108]. For example, microfluidics-spun collagen microfibers exhibited good cytocompatibility due to the low immunogenicity of collagen itself,and the well-oriented fibers were able to guide the migration of neuronal cells along the fiber axes (Fig. 8(a)). The axons grew up to 100 μm in length and were aligned along the fiber direction, suggesting the potential application of such fibers in peripheral nerve repair [109]. Apart from straight fibers, heteromorphic fibers could also be used in biomedicine. Alginate microfibers with gelatin methacrylate spindle-knots were able to work as cell carriers(Fig.8(b)),because the knots could be adjusted to suit the cell dimensions and promote cell adhesion and growth [110]. The spring-like helical alginate fiber was able to be stretched under external stimuli, so it could function as a mechanical sensor for cardiomyocytes.When connecting to an elastic hydrogel film seeded with cardiomyocytes,the fiber underwent cycles of elongation and contraction following the beating of the cardiomyocytes (Fig. 8(c)). The frequency of the transformation cycle of the helical fiber corresponded to the beating frequency of the cardiomyocytes. The mechanical behavior of the cardiomyocytes could be estimated from changes in the helical pitches of the fiber, which might be useful for visually monitoring the real-time bioactivity of cardiomyocytes [51].
Bacterial infection is another serious issue in biomedical applications. As a biopolymer with intrinsic antibacterial activity, chitosan can not only be used as an antibacterial coating but also be prepared in the form of antibacterial fibers. Wang et al. [111]reported a chitosan-based hydrogel fiber produced via microfluidics spinning (Fig. 8(d)); they found that the fiber exhibited good in vitro antibacterial activity against both Gram-positive and Gram-negative bacteria, especially when incorporated with Zn2+.In addition, bio-based fibers could be used in vivo for biomedical applications. For example, when chitosan/heparin fiber was used for wound suturing [59], the suture retained its effective tissueligation capability after two weeks,and no infection was observed,according to the stained sections. More interestingly, due to the high affinity of functional proteins with heparin, the composite fiber could bind adeno-associated virus (AAV) to achieve localized AAV-mediated gene delivery, thus further expanding the fiber’s applications in regenerative medicine. Zhang et al. [73] prepared alginate/BSA composite fibers, which were also applied as a surgical suture.As shown in Fig.8(e),the alginate/BSA suture closed the wound incision on the rat abdomen and liver without breakage,indicating its admirable mechanics and biocompatibility.
5. Conclusions and outlook
Nature provides not only abundant resources but also a great deal of inspiration for creating high-performance fibers. Table 3[45,46,55,56,58,59,61,63,65,71-74,78,79,91,96,105,109,112,113,117,123-125]presents a summary of various fibers in terms of their raw materials, spinning methods, mechanical properties, and potential applications. In recent years, great progress has been achieved in the preparation of mechanically strong and multifunctional biobased fibers by mimicking the structures of natural fibers and duplicating natural spinning processes. However, it is still challenging to produce bio-based fibers with mechanical properties that are close or even superior to dragline spider silk[28].Spider silk displays both very high strength and extraordinary toughness,two properties that are mutually exclusive in many artificial fibers. Therefore, the key issue is to achieve a favorable combination of these two properties.
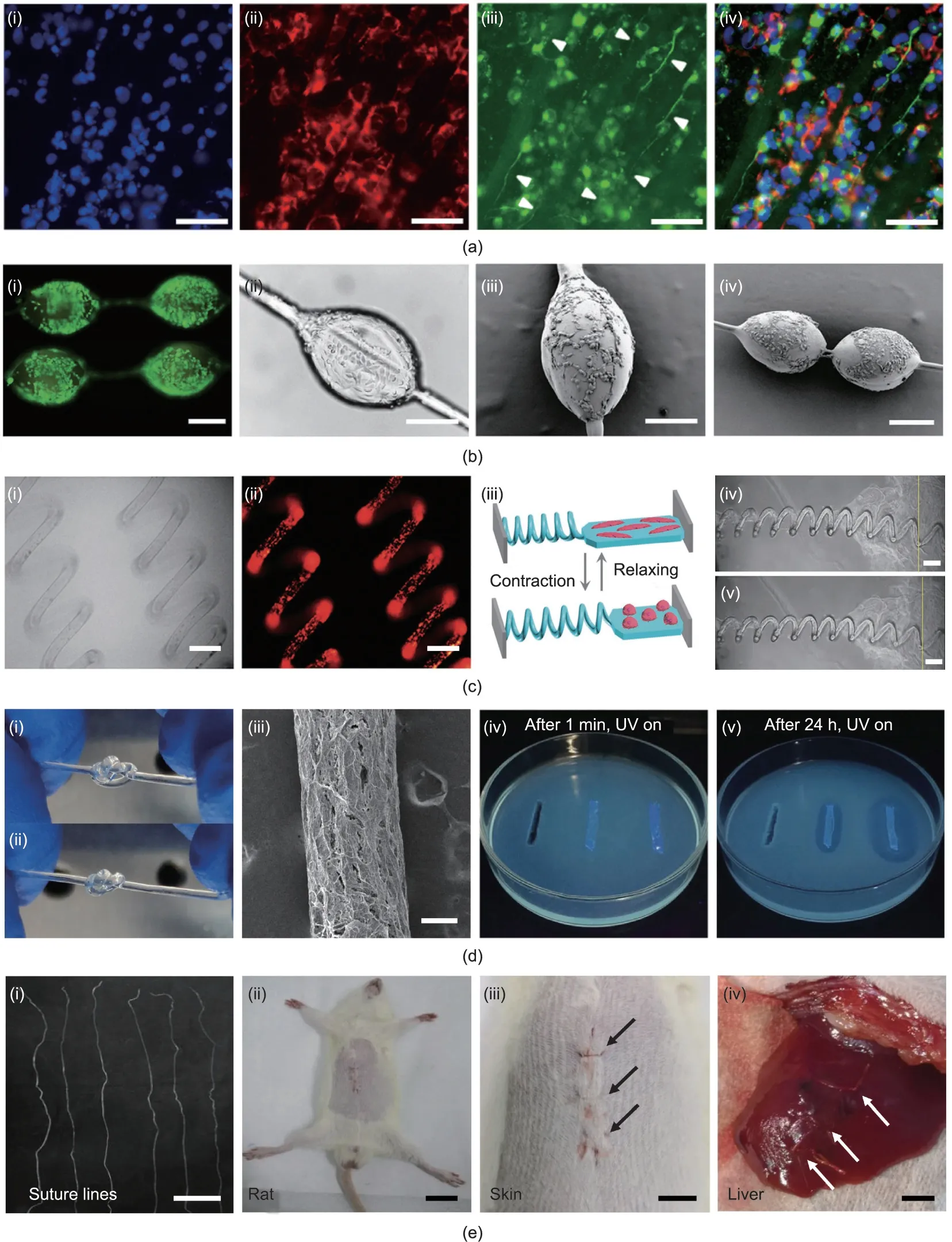
Fig. 8. Illustrations of bio-based fibers with excellent biological functions toward biomedical applications. (a) Collagen microfibers can be used for nerve repair:(i-iv)fluorescence microscope images of stained neuronal cells cultured on fibers showing axon growth along the fiber axes(white arrows;scale bars are 75 μm).(b)Alginate microfibers with gelatin methacrylate spindle-knots can be used as cell carriers: (i-iv) images of cell-loaded fibers showing their good biocompatibility (scale bars are 200 μm). (c) Helical alginate microfibers can be used as mechanical sensors for cardiomyocytes: (i-v) changes in helical pitches corresponding to the beating activity of cardiomyocytes cultured on a fiber-film complex (scale bars are 100 μm for (i) and (ii) and 150 μm for (iv) and (v)). (d) Chitosan-based hydrogel fibers show antibacterial properties:(i-iii)optical photographs and SEM image(scale bar is 200 μm;UV:ultraviolet);(iv,v)fluorescence photographs of the inhibition zones of E.coli in contact with fibers after 1 min and 24 h.(e)Alginate/BSA fibers can be used as surgical sutures:(i-iv)optical photographs of fiber bundles and wound suture on a rat skin and liver,with white and black arrows indicating the suturing positions (scale bars are 3 cm, 1 cm, 0.5 cm, and 0.3 cm, respectively). (a) Reproduced from Ref. [109] with permission;(b)reproduced from Ref.[110]with permission;(c)reproduced from Ref.[51]with permission;(d)reproduced from Ref.[111]with permission;(e)reproduced from Ref.[73]with permission.
To this end,more research efforts are needed in the whole fiber production procedure, from structural design and material selection to spinning devices, spinning methods, and post-treatments.A deeper understanding of the structure-mechanics relationship of natural biomaterials will provide new insights into the design of bioinspired fibers. As the spinning dope plays a basic and vital role in fiber performance, it is important to select and optimizerecombinant silk proteins by means of genetic engineering [112-114]. For example, engineered non-spider chimeric proteins can be regarded as a choice for mechanically strong fibers.In this strategy, diverse combinations of sequences and structures offer many options,aside from molecular weight regulation[1,74].Replicating the fine structure of a natural spinning system is also very important,as a great difference exists between current artificial spinning devices and the spinning ducts in spiders and silkworms [115-117]. To address this issue, ultrahigh-resolution 3D imaging and printing systems can be introduced to design and reconstruct a‘‘natural”spinning system.Rheology studies of the silk dope in natural spinning ducts [34,118-121] may lead to new discoveries,which will further guide and promote the preparation of highperformance fibers. Strengthening strategies are a conventional choice; for example, crosslinking and the incorporation of fillers can be expanded to different fibers.In addition,blending recombinant silk proteins with cellulose nanofibers for co-spinning would be a good trial, as such a complexation might offer a balance between structure and functional properties[55,122].Forced reeling is an effective approach to directly obtain silk fibers with surprising strength [27], but the industrial scale-up issues tend to be unsolvable.In this regard, feeding silkworms with nanomaterials may be promising for harvesting high-performance fibers on a large scale;however, the possible toxicity of excess nanomaterials should be considered. To sum up, there is still quite a long way to go in developing bio-based fibers. Nevertheless, after obtaining a better understanding of the spinning mechanisms that produce natural fibers and after a series of optimizations of spinning procedures,it will hopefully be possible to produce bio-based fibers that exceed their natural counterparts.
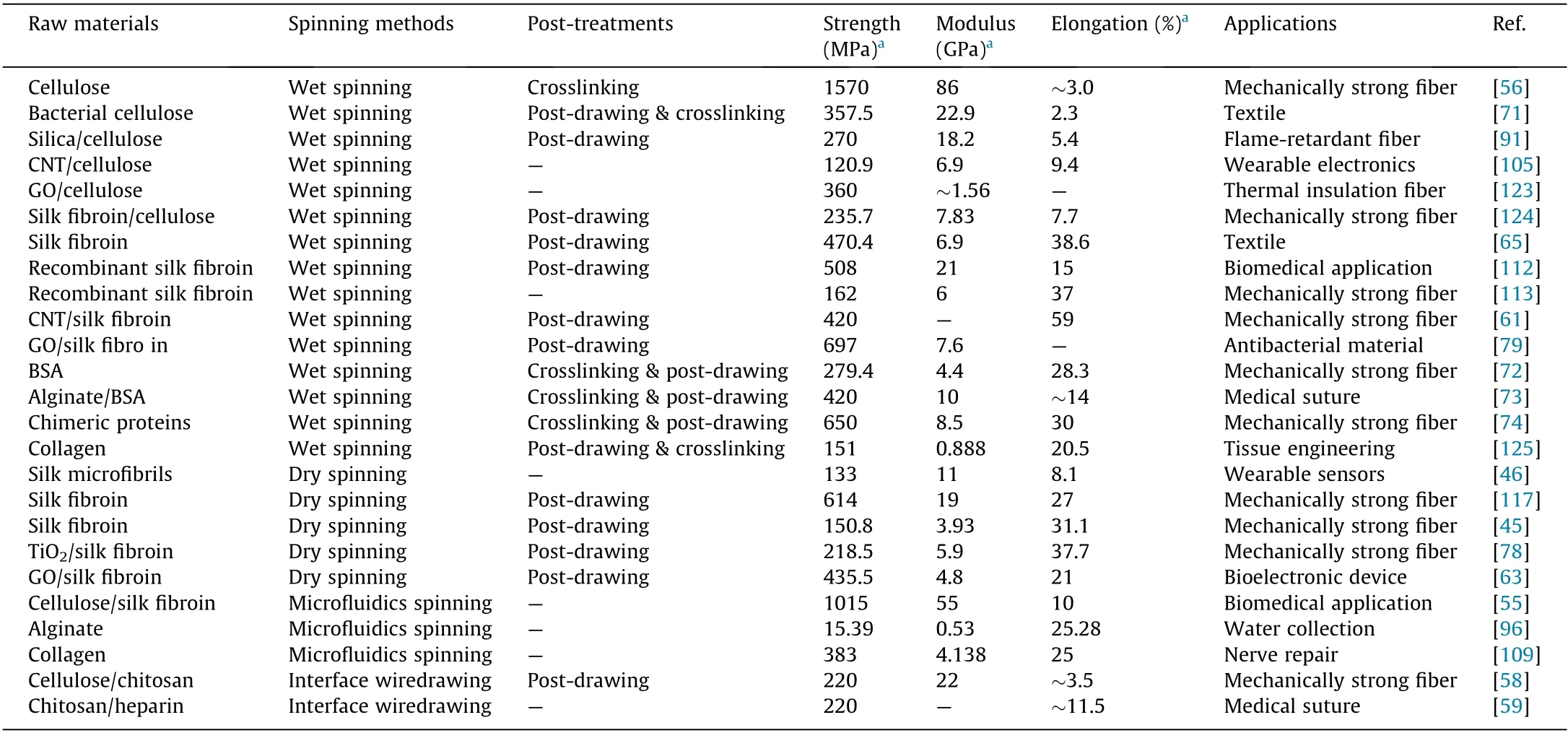
Table 3 A brief summary of bio-based fibers in terms of raw materials, spinning methods, post-treatments, mechanical properties, and potential applications.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFC1103900), the National Natural Science Foundation of China (22075244 and 51722306),Natural Science Foundation of Zhejiang Province (LZ22E030001),Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering (2021SZ-TD009).
Compliance with ethics guidelines
Zongpu Xu,Mingrui Wu,Qi Ye,Dong Chen,Kai Liu,and Hao Bai declare that they have no conflict of interest or financial conflicts to disclose.
- Engineering的其它文章
- The Pathway Toward Carbon Neutrality: Challenges and Countermeasures
- Weights-Based Gravity Energy Storage Looks to Scale Up
- New US Rules Promise to Unlock Hearing Aid Availability
- Flow in Porous Media in the Energy Transition
- Reactive Extrusion (REx): Using Chemistry and Engineering to Solve the Problem of Ocean Plastics
- A Future Perspective on In-Sensor Computing

