L-carnitine improves developmental competence of buffalo oocytes in vitro
Avijit Kumar Modak,Md Nuronnabi Islam,Asma Khatun,Md Hasanur Alam,Ireen Akter,AKM Ahsan Kabir,Md Abul Hashem,Mohammad Moniruzzaman
Department of Animal Science,Bangladesh Agricultural University,Mymensingh-2202,Bangladesh
ABSTRACT Objective:To investigate the effect of L-carnitine on in vitro maturation and subsequent in vitro embryo production of buffalo oocytes.Methods:Cumulus oocyte complexes (COCs) were aspirated from ovaries of slaughtered buffaloes.COCs were classified into good and fair qualities based on morphological observation of numbers and integrity of cumulus cells surrounding the oocyte.Both categories of COCs were placed in in vitro maturation medium with supplementation of different concentrations (0,0.250,0.375 or 0.500 mg/mL) of L-carnitine.Oocytes from both qualities were in vitro fertilized and in vitro cultured for 7 days,to examine the developmental competence.Results:Supplementation of L-carnitine to in vitro maturation medium increased the cumulus cell expansion rate of COCs to grade A,and reduced the cumulus cell expansion of COCs to grade B and grade C in both good and fair quality oocytes.Similarly,L-carnitine induced the in vitro meiotic progression of buffalo oocytes to metaphaseⅡ in both good and fair quality oocytes.Additionally,L-carnitine reduced the rate of oocyte degeneration in both good and fair quality oocytes.L-carnitine increased the rate of cleaved formation at day 2 and blastocyst formation at day 7 during in vitro culture in both qualities of oocytes.Moreover,a higher rate of blastocyst production was observed in L-carnitine-treated fair quality oocytes,which was higher than the results in the untreated good quality oocytes.Conclusions:L-carnitine enhances meiotic maturation and subsequent embryo development from both good and fair quality buffalo oocytes.
KEYWORDS: Buffalo;Cumulus oocyte complex;Embryo;In vitro maturation;In vitro fertilization;L-carnitine
1.Introduction
The scarcity of potential oocytes is one of the major limitations ofin vitroembryo production in buffaloes.The recovery rate of oocytes per ovary is low in buffaloes compared to bovine[1].A number of factors including the age of the donor,the time interval between slaughter and oocyte handling,aspiration procedure may affect oocyte quality that contributes to the development of oocytes.Moreover,slaughter-house derived buffalo oocytes have cellular damage that also results in their poor development[2].
In vitromaturation of oocytes,an important step ofin vitroembryo production is influenced by the soundness of oocyte cytoplasm and integrity of cumulus cells,size of the follicles,incubation time and culture medium[3].The quality of oocytes is a crucial factor that reflects the development of oocytesin vitro.Homogenous distribution of oocyte cytoplasm and intact cumulus cell layers are anticipating morphological criteria for subsequent development[4].Good quality oocytes are surrounded by several layers of dense cumulus cell layers.Fair quality oocytes have few number of loosely attached cumulus cell layers,where oocytes are often partially naked[5,6].However,sometimes fair quality oocytes along with good quality ones are included in the experiment ofin vitroembryo production to increase the availability of oocytes indeed[7].So,it is necessary to increase the competence of those fair quality oocytes to support assisted reproductive technologies in buffaloes.
In buffaloes,low fertility is associated with high intra-cytoplasmic lipid content in their oocytes[8].High lipid content causes degeneration of buffalo oocytes[1].On the other hand,lipids have a beneficial role during oocyte developmentin vitro.Intracellular lipid is a source of energy for the generation of mitochondrial adenosine triphosphate (ATP) through β-oxidation in oocytes and embryos[9].It has been reported that ATP enhances the developmental competence of oocytesin vitro[10].L-carnitine enhances ATP production in oocytes[11].It has also been reported thatL-carnitine improves the maturation of oocytes through increasing lipid metabolismin vitro[12,13].
L-carnitine is a water soluble and highly polarized amino acid.It is synthesized in the liver from lysine and methionine[14].Oocytes produce an excessive amount of reactive oxygen species (ROS)in vitro[15].Duringin vitrofertilization (IVF),ROS caused cell death through lipid peroxidation[16].Duringin vitromaturation,L-carnitine suppressed intracellular ROS and enhanced antioxidants in oocytes in mouse[17],bovine[18],porcine[19,20] and sheep[21].It contributes in total energy metabolism by enhancing oocyte maturation and subsequent development.Thus,it was thought thatL-carnitine might improve the developmental potentials of buffalo oocytes,specially less competent fair quality ones.Therefore,the present study was aimed to examine the influence ofL-carnitine in nuclear maturation and subsequent development of buffalo oocytes of both good and fair qualities.
2.Materials and methods
2.1.Chemicals
Unless otherwise stated,all chemicals were purchased from Sigma-Aldrich (St.Louis,MO,USA).
2.2.Collection of cumulus oocyte complexes (COCs)
Buffalo (Bubalus bubalis) ovaries were collected from a local slaughterhouse and transported to the laboratory in physiological saline [0.9% (w/v) NaCl] in a thermo flask.The ovaries were washed 5 times in physiological saline.The ovaries were transferred to Petri dishes containing the same saline solution and trimmed to remove the surrounding tissues and overlying bursa.Further,the ovaries were washed with physiological saline for five times.The COCs were collected by aspiration of 4-8 mm follicles using a syringe (Henke Sass Wolf,Tuttlingen,Germany) with the 18-G needle containing 1-2 mL of aspiration medium [Medium-199 supplemented with 3.2 mg/mL bovine serum albumin (BSA)and 250 μg/mL of gentamycin sulfate].Then,the COCs were examined under a zoom stereo microscope (CZM6,Labomed,CA,USA) at 40× magnification,and evaluated according to their integrity of cumulus cell layers.Oocytes with compact and dense cumulus cell layers were categorized as good quality and oocytes with lightly attached and sometimes partially naked cumulus cell layers were categorized as fair quality as described by Sureshet al[5].Total 730 COCs were collected from ovaries.Among the COCs,145 (19.86%) and 146 (20.00%) were classified as good quality and fair quality oocytes,respectively and used for the experiment.The rest of the COCs were discarded from the experiment.Both good quality and fair quality oocytes consisting of homogenous and healthy cytoplasm were washed subsequently three times in Medium-199 supplemented with 8% fetal bovine serum(Gibco BRL,Grand Island,NY,USA),0.8 mg/mL Na-pyruvate(29806-54;Nacalai Tesque,Inc.,Kyoto,Japan),1 mML-glutamine and 50 μg/mL gentamycin sulfate forin vitromaturation.COCs with cytoplasmic degeneration and complete detachment of cumulus cells from oocytes were classified as degenerated ones and excluded from the experiment as described previously[22].
2.3.In vitro maturation
COCs were cultured in TCM-199 with 10% fetal bovine serum,50 μM cysteamine,0.1 mg/mL Na-pyruvate,5 μg/mL 17 β-estradiol,5 μg/mL follicle stimulating hormone (Porcine FSH;NIDDK,Washington,DC,USA) and 0.08 mg/mL gentamycin sulfate forin vitromaturation of oocytes[22].To elucidate the effects ofL-carnitine,the medium was supplemented with either 0,0.250,0.375 or 0.500 mg/mLL-carnitine as described previously[18-21].Both good and fair quality oocytes were cultured with each concentration ofL-carnitine at 38.5 ℃ for 24 h under 5% CO2in humidified air.Afterin vitromaturation,oocytes were observed under an inverted phase contrast microscope (TCM 400;Labomed,CA,USA),and classified among three grades: A,noticeably expanded cumulus cells;B,moderately expanded cumulus cells;and C,less or zero expanded cumulus cells (Figure 1).
2.4.Assessment of oocyte nuclear maturation
For assessment of nuclear maturation,oocytes were washed in physiological saline and denudated using Pasteur pipette with the help of 0.1% (w/v) hyaluronidase enzyme.Then,the oocytes were fixed in aceto-ethanol (acetic acid: ethanol=1:3) for 48 h.The oocytes were stained with 1% (w/v) aceto-orcein for 5 min and washed with aceto-glycerol (glycerol: acetic acid: double distilled water=1:1:3).Oocytes were then examined to assess the nuclear maturation rate under a differential interference contrast microscopy(Olympus Corporation,USA) at 100× magnification and were classified on the basis of their chromosomal configuration in accordance with previous report of Alamet al[23].In this experiment,after meiotic resumption chromosomal configurations were classified as early diakinesis,late diakinesis,metaphaseⅠand metaphaseⅡ.Oocytes that showed cytoplasmic or nuclear abnormalities were considered as degenerated oocytes.
2.5.IVF
There was a lack of buffalo bull availability in the experimental site.Therefore,frozen semen of a high performing Murrah buffalo bull (No.107096) were collected from the Department of Livestock Services,Bangladesh.Before use,semen straws were retrieved from the liquid nitrogen container and thawed in a water bath at 38.5 ℃ for 30 s.The straw was wiped and cut off to pour semen into a 15 mL centrifuge tube.Then,semen were washed twice with working Brackett and Oliphant’s (BO) fertilization medium followed by the method described by Madanet alwith slight modifications[24].BO medium containing semen was centrifuged two times at 115×gfor 5 min.The supernatant was removed gently after the centrifugation.A number of 3-4 sperm droplets (100 μL) were prepared using a micropipette.The prepared sperm droplets were incubated for 5 h for capacitation and the motility was observed under a zoom stereo microscope at 100× magnification.Before insemination,COCs were picked up fromin vitromaturation medium and washed twice with the fertilization medium supplemented with 10 mg/mL fatty acid-free BSA.Then,the oocytes were added in sperm droplets and incubated for 18 h at 38.5 ℃ temperature with 5% CO2in air for IVF.
2.6.Assessment of embryo development
After 18 h of incubation,oocytes were washed at least 3 times in Research Vitro Cleave Medium (Cook Medical,Australia)[25].Then,putative zygotes were completely denuded from cumulus cells and spermatozoa by gentle pipetting with a fine glass pipette in preincubatedin vitroculture medium.The oocytes were examined with the help of a phase-contrast inverted microscopy at 100×magnification for evidence of cleavage.The embryos were cultured following the methodology described by Madanet alwith some modifications[24].The two-third amount of maturation medium was replaced by Research Vitro Cleave Medium supplemented with 10% fatty acid free BSA from the previously storedin vitromaturation droplets.Then,the zygotes were cultured up to 7 days at 38.5 ℃ temperature with 5% CO2in air.The day of IVF was designated day 0.In this experiment,observations were taken at days 2 and 7 ofin vitroculture to observe the cleavage (2-16 cells) and subsequent development up to blastocyst stage.The cleaved embryo was assessed on the basis of shape,sphericity,thickness of zona pellucida,size of perivitelline space and blastocoele.
2.7.Statistical analysis
Statistical software IBM SPSS version 22 was used to compute different statistic for comparing the treatment effects.Since the few observations of the data set were robust,median (IQR) were applied to evaluate the performance of the treatment at descriptive level,where IQR indicated the inter-quartile range.For generalizing the results,non-parametric test Kruskal-Wallis test was used to find the overall significance of the ANOVA model.For diagnosis the better treatment,Tukeypost-hoctest was applied.Data of normal distribution are expressed as mean±standard deviation (mean±SD)from at least four replicated cultures.APvalue less than 0.05 was considered as significantly different.
2.8.Ethics statement
All the experimental procedures of this study were approved and supervised by the committee for ethical standard of research in Bangladesh Agricultural University Research System (BAURES),Bangladesh Agricultural University,Mymensingh,Bangladesh(Reference number: BAURES/ESRC/691/2020).
3.Results
3.1.L-carnitine enhances in vitro maturation of buffalo oocytes
Representative morphologies of cumulus cell expansions are shown in Figure 1.Supplementation of 0.375 mg/mL and 0.500 mg/mLL-carnitine significantly increased cumulus cell expansion of grade-A category in good quality oocytes than other groups (P<0.05) (Figure 2).Significantly higher rate of grade-A expansion was recorded in 0.500 mg/mLL-carnitine treated fair quality oocytes than that of 0 and 0.250 mg/mL ofL-carnitine groups (P<0.05).Percentages of OCCs with grade-B expansion of cumulus cells were significantly higher in 0 and 0.250 mg/mL ofL-carnitine treated good quality oocytes than 0.500 mg/mL ofL-carnitine (P<0.05),while grade-B cumulus cell expansion did not differ in fair quality oocytes.Overall,good quality oocytes showed lower percentages of grade-C expansions than the fair quality oocytes.However,the proportion of grade-C cumulus cell expansion significantly decreased in 0.500 mg/mLL-carnitine treated good quality oocytes than 0 mg/mLL-carnitine treated group (P<0.05).In fair quality oocytes,the percentages of COCs with grade-C cumulus cell expansion decreased significantly in 0.500 mg/mLL-carnitine treated group than 0 and 0.250 mg/mLL-carnitine groups (P<0.05) (Figure 2).
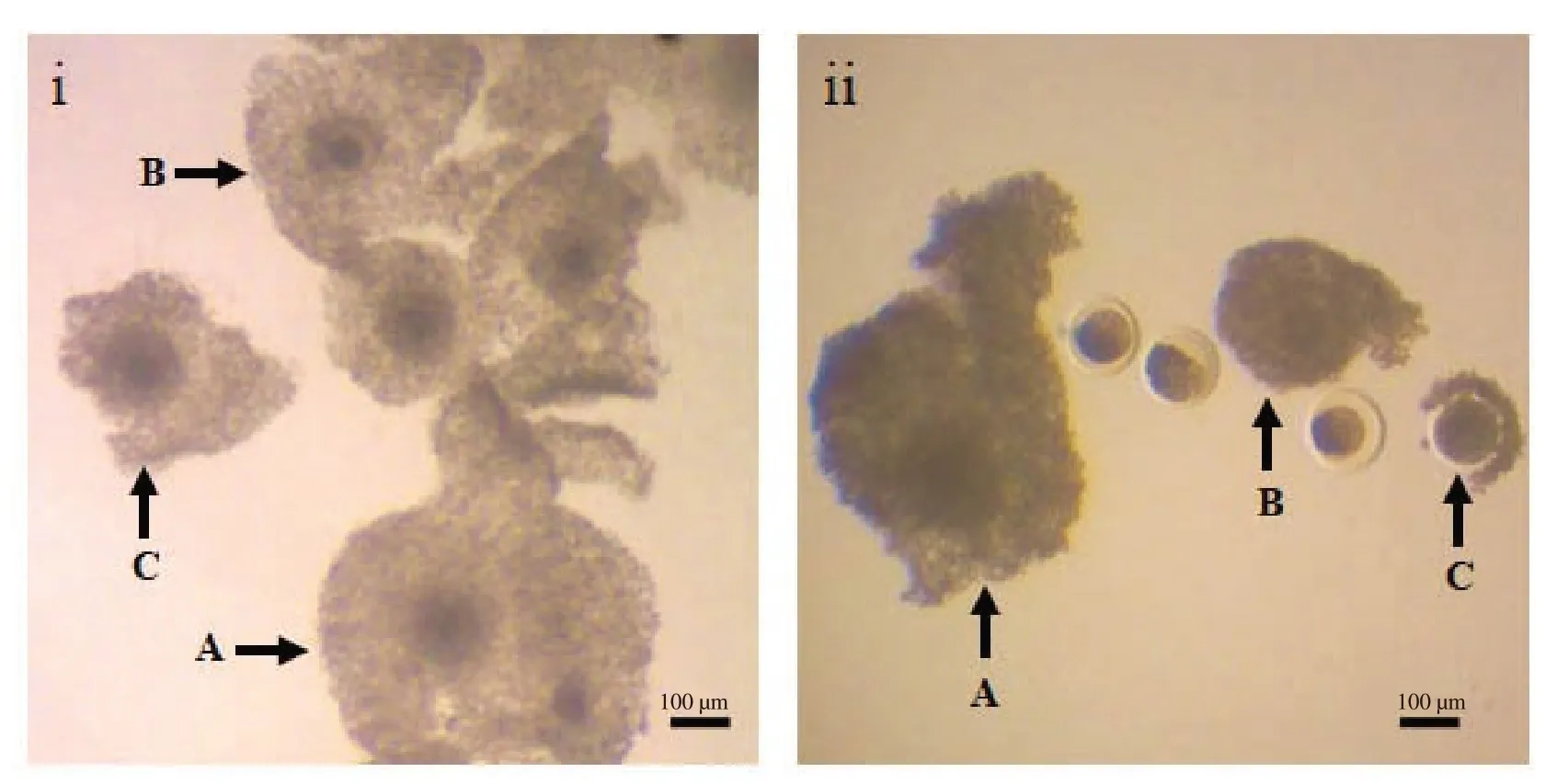
Figure 1.Cumulus cell expansion from buffalo cumulus oocyte complexes after in vitro maturation.The left panel (i) indicates good quality oocytes and right panel (ii) indicates fair quality oocytes in maturation medium.Both qualities of oocytes are cultured at 38.5 ℃ for 24 h under 5% CO2 in humidified air for in vitro maturation.The expansion of cumulus cells are categorized as grade A (noticeable expansion),grade B (moderate expansion) and grade C (less or zero expansions) which are represented in both images (i,ii) as A,B and C,respectively.Scale bars represent 100 μm.
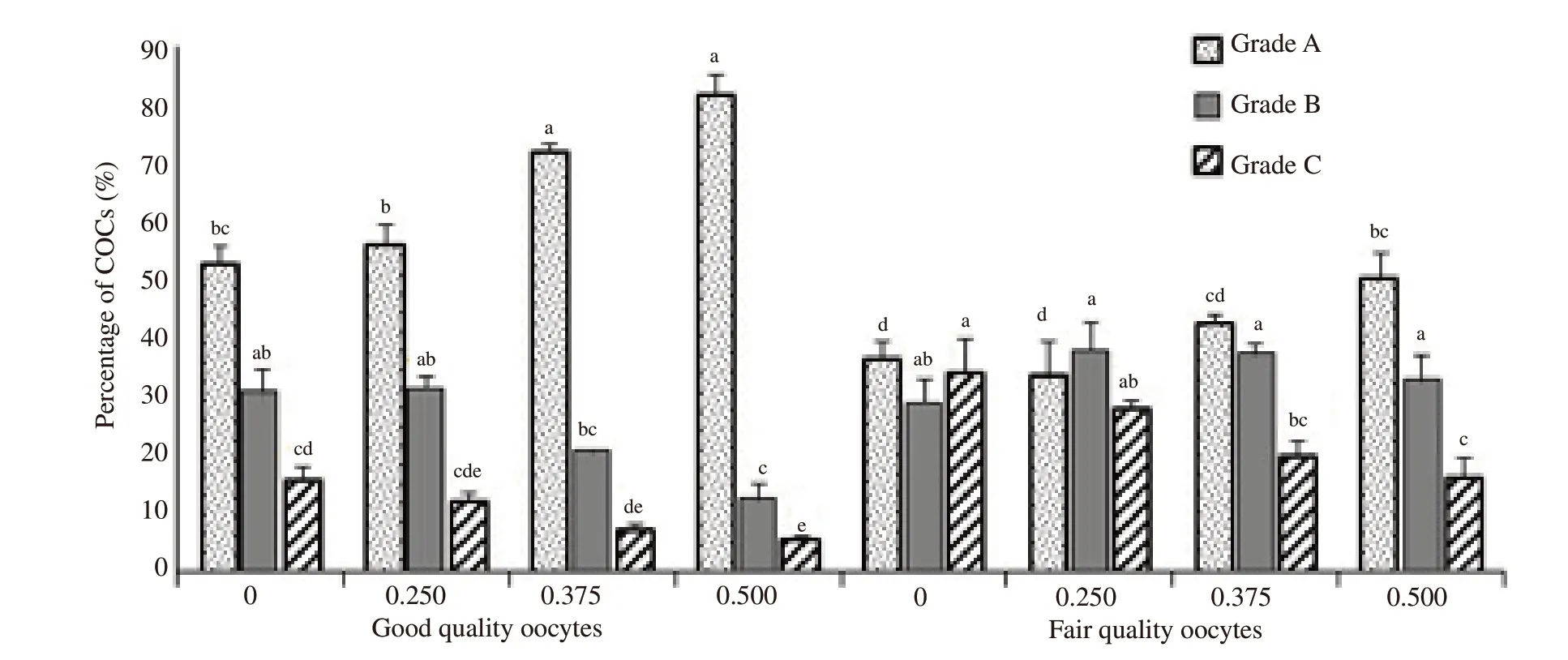
Figure 2.Influence of L-carnitine on cumulus cell expansion in good and fair quality oocytes.L-carnitine is supplemented with either 0,0.250,0.375 or 0.500 mg/mL concentrations of both types of oocytes.Oocytes are cultured at 38.5 ℃ for 24 h under 5% CO2 in humidified air for in vitro maturation.The cumulus cell expansions are categorized as grade A (noticeable expansion),grade B (moderate expansion) and grade C (less or zero expansions).All the data from the experiments are plotted in IBM SPSS Statistics (Version 22) and examined for normal distribution.Data are shown as the mean±SD from at least four replicated cultures.Bars with different letters (a-e) differ significantly (P<0.05).COCs: cumulus oocyte complexes.
The results fromin vitromaturation of oocytes with the supplementation ofL-carnitine are shown in Table 1.It was found that 0.500 mg/mLL-carnitine treated good quality oocytes showed a significantly higher rate of metaphaseⅡ (MⅡ) oocytes than that of 0.250 mg/mLL-carnitine and 0 mg/mL ofL-carnitine groups(P<0.05).A lower percentage of fair quality oocytes reached to the MⅡ stage withoutL-carnitine supplementation.This percentage increased when oocytes were treated with 0.500 mg/mLL-carnitine,comparable to the percentage of untreated good quality oocytes.However,we did not find any significant difference in oocytes at the MⅡ stage among the fair quality oocyte groups.Neither oocyte quality nor the concentrations ofL-carnitine influenced the percentages of oocytes at early diakinesis and late diakinesis stages.However,0.500 mg/mLL-carnitine treated good quality oocytes showed a significantly lower proportion of MⅠ oocytes than that of 0 and 0.250 mg/mLL-carnitine supplemented groups (P<0.05).Meanwhile,0.500 mg/mLL-carnitine treated both good and fair quality oocytes showed a significantly lower degeneration rate than that of the untreated groups.These findings suggested thatL-carnitine improved the meiotic competence and reduced the degeneration rate of buffalo oocytes.
3.2.Influence of L-carnitine on embryo development
The effects ofL-carnitine on the rate of cleavage and embryo development are shown in Table 2.The typical morphology of 2-cell embryo (A) and blastocysts (B) are presented in Figure 3.In this experiment,0.500 mg/mLL-carnitine treated good quality oocytes reached significantly higher percentage at cleavage stage than 0 and 0.250 mg/mLL-carnitine supplemented groups (P<0.05).However,we did not find any significant difference in cleavage rates among the fair quality oocyte groups.Similarly,the blastocyst yield was also significantly higher in 0.500 mg/mLL-carnitine treated good quality oocytes than 0 and 0.250 mg/mLL-carnitine treated groups(P<0.05).Moreover,0.500 mg/mLL-carnitine treated fair quality oocytes showed a higher blastocyst production than the untreated good quality oocytes group.
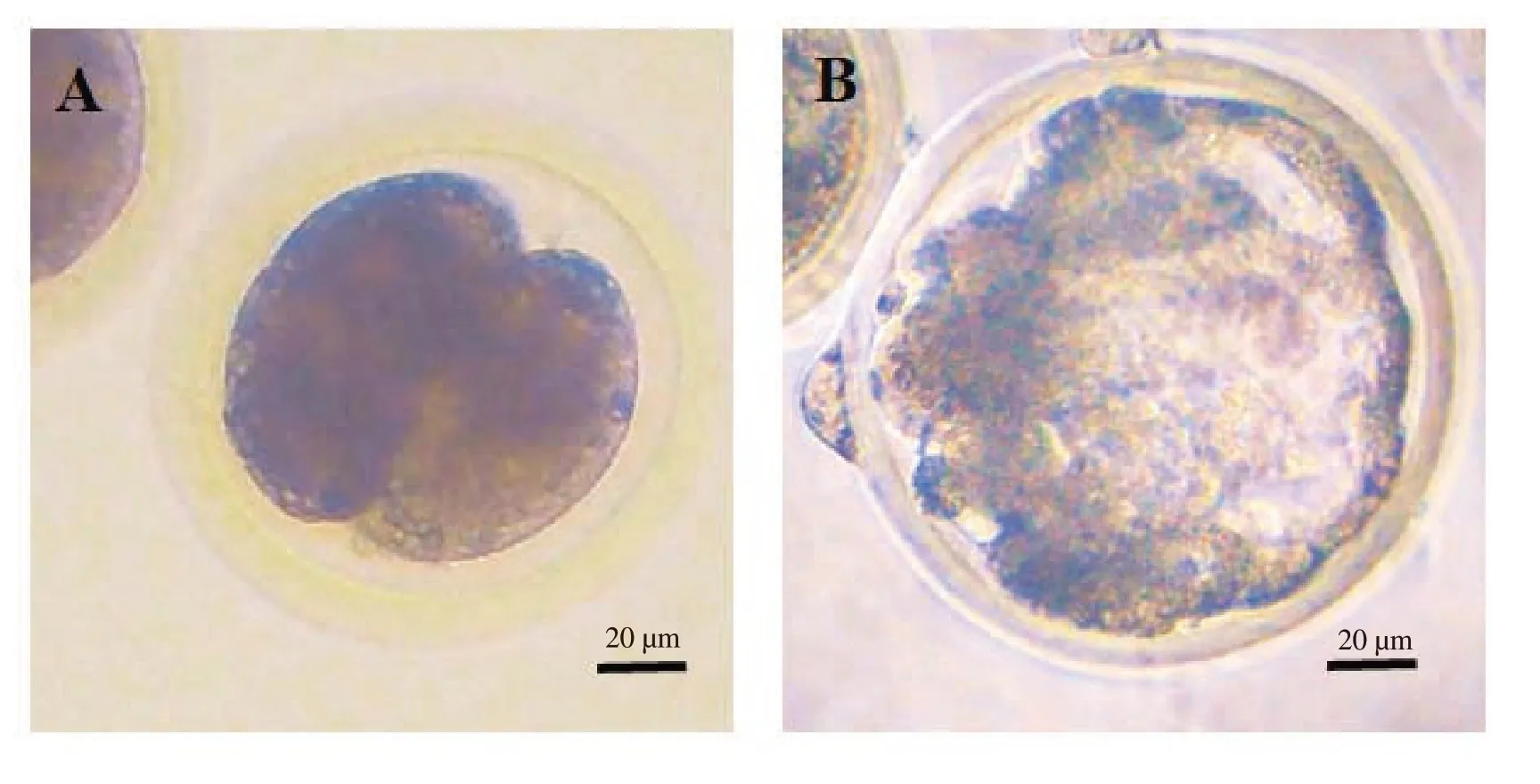
Figure 3. In vitro development of the buffalo embryos from oocytes in vitro matured with supplementation of L-carnitine.Oocytes are cultured at 38.5 ℃for 24 h under 5% CO2 in humidified air for in vitro maturation.Then,the oocytes are added in sperm droplets and incubated for 18 h at 38.5 ℃ with 5%CO2 in air for in vitro fertilization.Then,the zygotes are cultured for 7 days at 38.5 ℃ with 5% CO2 in air.The day of in vitro fertilization is designated as day 0.Observations are taken on day 2 and day 7 of in vitro culture to examine the cleavage and subsequent development to blastocyst stage of embryos.A:cleaved embryo;B: blastocyst.Scale bars represent 20 μm.
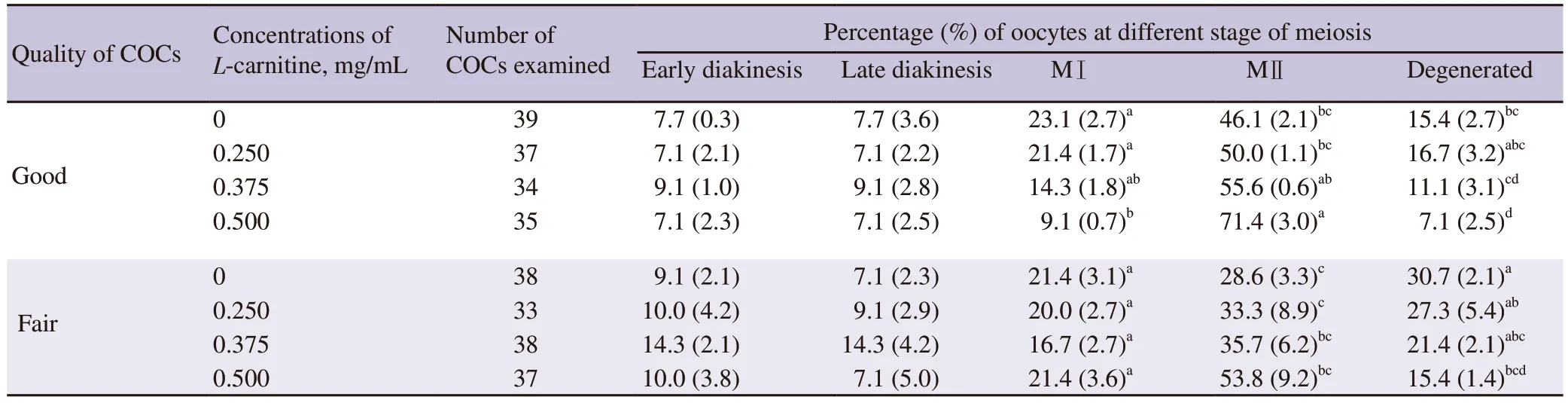
Table 1.Effect of L-carnitine on in vitro nuclear maturation of buffalo oocytes.
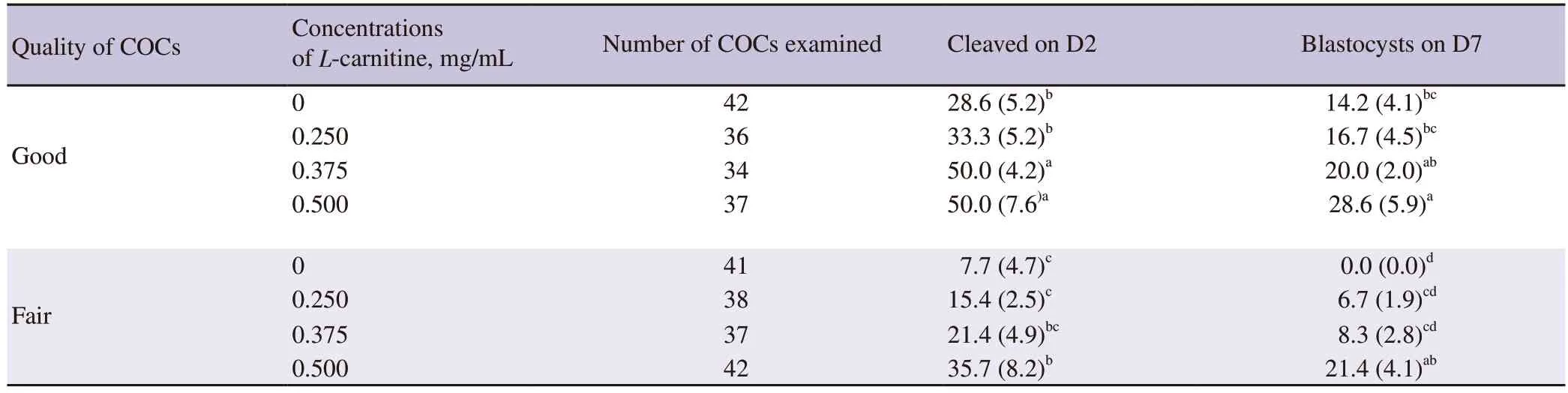
Table 2.Development of the buffalo embryos from oocytes in vitro maturated with L-carnitine.
4.Discussion
In the present study,L-carnitine enhancedin vitromaturation of buffalo oocytes.We found thatL-carnitine treatment resulted significantly higher rate of embryo development than untreated groups.L-carnitine dose-dependently increased the development of buffalo oocytesin vitro.In mammalian species,the soundness of cumulus cells is necessary for meiotic progression[26,27].Cumulus cells support oocyte development by providing nutrients and eliminating suppressive factors from the culture medium[28].Cumulus cell morphology and cytoplasmic conditions are two key parameters to assess oocyte quality[29,30].The classifications of COCs as good quality,fair quality and degenerated according to their morphologies of cumulus cells are vital factors for oocyte developmental competence.According to these criteria,buffalo oocytes were categorized as good quality and fair quality oocytes as bovine oocytes were reportedly classified as more competent and less competent oocytes[31].In the present study,19.86% oocytes belonged to good quality and 20.00% were of fair quality during collection.This proportion is comparable with the report of Sureshet alwhere 17% of fair quality oocytes were harvested[5].
The present study showed thatL-carnitine increased the development competence of both good and fair quality oocytes.The percentage of matured oocytes in high concentration(0.500 mg/mL) ofL-carnitine treated fair group was comparable to untreated good quality oocytes.A similar trend was observed in embryo development from fair quality oocytes.Knitlovaet alreported thatL-carnitine supplementation enhanced the development of bovine oocytes[31].They suggested that the proportion of MⅡoocytes obtained from meiotically less competent oocytes were comparable to the untreated more competent oocytes.Our results suggested that supplementingL-carnitine in culture medium buffalo embryo might be produced from fair quality oocytes rather than discarding those.Thus,an opportunity might be created to increase the availability of potential oocytes for embryo productionin vitro.
Our study demonstrated that the number of oocytes showing maximum (grade A) expansion was significantly higher inL-carnitine treated good quality oocytes than untreated ones.Results of the present study also revealed thatL-carnitine increased cumulus expansion in both good and fair categories of oocytes.However,treatment displayed no such differences in moderate expansion(grade B).A higher concentration ofL-carnitine (0.500 mg/mL),in both good and fair quality oocytes,exhibited a significantly lower rate of grade-C expansion.This might be due toL-carnitine induced increase in ATP production from intracellular lipid stores[32].
L-carnitine supplementation reduces the ROS level in oocytes and embryos[32].L-carnitine reduces H2O2level in MⅡ oocytes and prevents cell degeneration[19,33,34].Similarly,L-carnitine also inhibits embryo degeneration through development of the chromosomal structures[35].L-carnitine supplementation increases antioxidants and reduces free radicals,and thus prevents DNA damages[15,36].Our results suggest that supplementation ofL-carnitine inin vitromaturation medium decreases the degeneration rate and increases the subsequent development of buffalo oocytes,which supports the result of a previous study in mouse[35].However,further molecular study is needed to reveal the cellular signaling cascade ofL-carnitine on buffalo oocytes.
In conclusion,L-carnitine enhancesin vitrodevelopment,prevents degeneration and improves the developmental competence of buffalo oocytes.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research submitted.
Funding
This work was supported by Bangladesh Academy of Science(BAS-USDA;Project No.LS-16/2017),the International Foundation for Science (IFS;reference No B/5219),and Bangabandhu Science and Technology Fellowship Trust of Ministry of Science and Technology,People’s Republic of Bangladesh.
Authors’contributions
Avijit Kumar Modak and Mohammad Moniruzzaman designed and performed the experiments,analysed and interpreted the data.Ireen Akter,Md Nuronnabi Islam and Asma Khatun conductedin vitromaturation and IVF experiments.Avijit Kumar Modak,AKM Ahsan Kabir,Md Abul Hashem and Md Hasanur Alam cultured embryo.All authors contributed to designing the experiment,interpreting data and providing critical suggestions to improve the manuscript.
 Asian Pacific Journal of Reproduction2022年5期
Asian Pacific Journal of Reproduction2022年5期
- Asian Pacific Journal of Reproduction的其它文章
- Impact of gamete health on fertilization and embryo development: An overview
- An incidental presentation of Herlyn-Werner-Wunderlich syndrome with secondary infertility: A case report
- Evaluation of intratesticular chlorhexidine gluconate for chemical contraception in dogs
- Oxytocin improves testicular blood flow without enhancing the steroidogenic activity in Baladi goats
- Association of the microbial culture of follicular fluid,vaginal swab and catheter tip with β-hCG IVF positive and negative
- Anti-Müllerian hormone and antral follicle count predict ovarian response in women less than 45 years following GnRH antagonist multiple-dose protocol
