Co-adsorption behavior of aggregated asphaltenes and silica nanoparticles at oil/water interface and its effect on emulsion stability
Gung-Yu Sun ,Ho Zhng ,Di-Wei Liu ,Chun-Xin Li ,b,Fei Yng ,b,Bo Yo ,b,Ze Dun , Xin-Y Chen , Fu-Jun Sheng
a College of Pipeline and Civil Engineering, China University of Petroleum (East China), Qingdao, 266580, Shandong, People's Republic of China
b Shandong Key Laboratory of Oil & Gas Storage and Transportation Safety, Qingdao, 266580, Shandong, People's Republic of China
Keywords:Asphaltene Silica nanoparticle Co-adsorption behavior Modification effectiveness Emulsion stability
ABSTRACT In petroleum industry, crude oil emulsions are commonly formed in oilfields. The asphaltenes and fine particles in crude oil may affect the stability of the emulsions by adsorbing at the water/oil interface. In this research,the effect of silica nanoparticles and asphaltenes on emulsion stability is explored first.The asphaltenes are proved to benefit emulsion stability.Unlike the asphaltenes,however,the modified silica nanoparticles may have positive or negative effect on emulsion stability, depending on the asphaltene concentration and aggregation degree in the emulsions.Further,it is confirmed by conducting interfacial experiment that the asphaltenes and particles can adsorb at the interface simultaneously and determine the properties of the interfacial layer. More in-depth experiments concerning contact angle and asphaltene adsorption amount on the particles indicate that the asphaltenes can modify the wettability of the particles.Higher concentration and lower aggregation degree of the asphaltenes can increase their adsorption amount on the surface of particles and then improve the modification effectiveness of the particles. Resultantly, the particles with good modification effectiveness can enhance the emulsion stability while the particles with poor modification effectiveness will weaken the emulsion stability.
1. Introduction
Crude oil emulsion is formed easily when the produced liquid experiences the shear actions of valves, pumps, etc. A series of problems will then come along,such as the waste of energy and the corrosion of equipment (Wang et al., 2019a). Hence, it is of great significance to explore the influencing factors and mechanisms of emulsion stability to guide the in-situ treatment of crude oil emulsions. Because crude oil has complex compositions, the emulsion stability is influenced by various factors, such as asphaltenes, resins, wax, clays, and grits (Lin et al., 2008; Abatai et al.,2020).
Asphaltenes are the inherent interfacially-active substances in petroleum and can adsorb at the interface due to their strong interfacial affinity (Kilpatrick, 2012; Kar and Hascakir, 2015). With the adsorption of asphaltenes, an interfacial layer can be formed and enhance the stability of the emulsion (Kumar et al., 2001;Kilpatrick, 2012; Liu et al., 2020a, 2020b, 2020c). Asphaltenes can adsorb at the interface irreversibly(Rond′on et al., 2006) and form an interfacial layer with solid-like properties (Yarranton et al.,2000; Fan et al., 2010; Langevin and Argillier, 2016). In our previous work, wrinkles were observed when the interfacial area was contracted (Liu et al., 2020a). Besides, the aggregation degree of asphaltenes can be easily affected by temperature, pressure, oil composition or any other external factors (McKenna et al., 2013;Salimi et al., 2016; Shojaati et al., 2017). We also found that the interfacial behavior of asphaltenes would vary with their aggregation degree. As a result, the emulsion stability will also be influenced (Liu et al., 2020a, 2020b; 2020c).
Fine particle is another key element to the stability of crude oil emulsions (Yan et al., 2001). Kaolin, montmorillonite, and quartz sand can be usually found in crude oil emulsions (Sullivan and Kilpatrick, 2002) and the main component of them is SiO2.Ramsden (1904) first found that emulsions could be stabilized by particles. Then, Pickering (1907) performed a systematic study about the emulsions doped with particles. In honor of him, the particle-stabilized emulsions are named as Pickering emulsions.After that, Pickering emulsions have been widely concerned by researchers because of their unique properties like the excellent emulsion-stabilizing ability.At present,the recognized stabilization mechanism of Pickering emulsion is that the particles with suitable wettability can adsorb and form a layer with protectiveness at the interface, preventing the coalescence of droplets (Binks, 2002;Aveyard et al., 2003). Many factors may affect the stability of Pickering emulsions.For example,the effects of particle wettability(Finkle et al., 1923; Stiller et al., 2004; Hunter et al., 2008) and concentration(Binks and Lumsdon,2000;Binks and Whitby,2004;Binks et al., 2005a; Leal-Calderon and Schmitt, 2008), electrolyte(Yang et al., 2006; Tan et al., 2011), pH of water phase (Lan et al.,2007),and temperature (Binks et al., 2005b) on emulsion stability have been extensively explored.
Due to the internal polar groups and heteroatoms, asphaltenes could also adsorb on the surface of particles and change their wettability indeed (Dud′aˇsov′a et al., 2008; Nassar, 2010; Nassar et al., 2011a, 2011b). The interactions that cause the asphaltenes to adsorb on the particle surface include van der Waals,acid-base,coordination, electrostatic and hydrogen bonding interactions(Wang et al., 2016). As mentioned above, asphaltenes and fine particles with suitable wettability can adsorb at the interface and influence the emulsion stability. On the other hand, asphaltenes and fine particles are very commonly seen in the produced fluid of oilfields. Therefore, it makes sense to carry out the relevant research. Sullivan and Kilpatrick (2002) used asphaltenes and clay particles to prepare emulsions and they found that the emulsions stabilized with montmorillonite were more stable than those stabilized with kaolin because montmorillonite could adsorb more asphaltenes. Sztukowski and Yarranton (2005) found that the emulsion could achieve the strongest stability when the proportion of asphaltenes and particles was equal to an appropriate value.Despite the efforts of these scholars,the stability of the emulsions is not systematically evaluated when the asphaltenes and particles both exist in the system and the mechanism of emulsion stabilization is still to be clarified.
In this study, the effect of asphaltenes and silica nanoparticles on emulsion stability is systematically studied.First,the bottle test and microscopic observation are performed to investigate the contributions of asphaltenes and particles to the emulsion stability under different conditions. Then, the co-adsorption behavior of asphaltenes and particles and the interfacial properties are studied by measuring the dynamic interfacial tension(DIFT)and dilational viscoelasticity, respectively. The crumpling ratio of the interfacial layer is calculated to evaluate the effect of particles and asphaltenes on the properties of the interfacial structure. The interactions between asphaltenes and particles are characterized by the measurement of contact angle and adsorption amount. Finally, the mechanism of emulsion stabilization is discussed.
2. Materials and methods
2.1. Materials
The hydrophilic silica nanoparticles were purchased from Beijing Deke Daojin Science and Technology Co. Ltd. The average particle size of the silica nanoparticles is 15 nm and its specific surface area is 250 m2·g-1. The analytically pure toluene, n-heptane, methylene chloride, and sodium chloride were all purchased from Sinopharm Chemical Reagent. The mineral oil was bought from Shanghai Macklin Biochemical Technology Co. Ltd and its carbon number distribution can be found in Fig. S1 in the Supplementary Information.The Tahe crude oil(supplied by Sinopec Tahe Oilfield Company, China) was used to extract asphaltenes. Its density is 0.968 g·cm-3at 30°C(measured with the pycnometry).The crude oil components are shown in Table 1 and the high content of asphaltenes is the reason for choosing it. The water phase was prepared with distilled water and sodium chloride with a concentration of 0.05 mol·L-1. The density of the water phase is 0.998 g·cm-3at 30°C.
2.2. Extraction of asphaltenes
Sufficient n-heptane was added to the Tahe crude oil(the mass ratio of n-heptane to crude oil was 40:1)and then the mixture was blended under reflux distillation for half an hour. After lowered to room temperature,the mixture was filtered.After that,the residue was washed with n-heptane to obtain the asphaltenes. Then, the asphaltenes in the residue were redissolved by methylene chloride.The asphaltene solution was dried out to obtain the asphaltenes in a nitrogen atmosphere.
2.3. Emulsion preparation and stability test
The solvents were prepared with toluene and mineral oil with different mass ratios.The mass fractions of toluene were set as 50%,67%, 75% and 100%, respectively. The asphaltenes and silica nanoparticles were added in sequence to the solvents to prepare the model oils. The asphaltene concentrations were controlled at 0.050 wt%and 0.300 wt%,respectively,and the concentration of the silica nanoparticles was fixed at 0.010 wt%. In order to ensure sufficient interactions between the asphaltenes and particles, the model oils were treated with 15 min sonication and 45 min continuous stirring at 30°C.
The model oils and water were then emulsified with a homogenizer (ANoni AD500S-H, China) in a 30°C water bath. The mass ratio of the model oil to the water was 7:3. The stirring speed was 18000 rpm and the process of the emulsification lasted for 10 min.After that, the emulsions were transferred into the calibrated colorimetric tubes to take bottle test and the temperature was kept at 30°C. The initial volume of the emulsions was 30 mL. The micromorphology of the bottom layer emulsions after 3 h quiescence was observed by a microscope (BX51, Olympus, Japan) at 30°C. The sizes of the droplets were measured with the Image J software (Version 1.52v).
2.4. Evaluation of asphaltene aggregation degree in model oil
The asphaltene aggregation degree was characterized by the conductivity measurement. The model oils were prepared in the same way as stated in Section 2.3. Then, the conductivity of the model oils was measured by a conductivity meter(DDS-307,INESA,China) and the test temperature was 30°C. Each experiment was conducted repeatedly and the average values are displayed.
2.5. Measurement of DIFT
The DIFT was measured with a tensiometer (Tracker H, TECLIS,France).An appropriative quartz vessel and a syringe were used to hold the water and oil phase, respectively. During the measurement, a CCD camera equipped at the tensiometer was used tocapture the shape of the oil droplet formed in the water phase.Then,the software could figure out the interfacial tension based on the hydrostatic calculation and Young‒Laplace equation. The pycnometer was used to obtain the densities of the model oils for interfacial tension calculation and the results are shown in Table S1 in the Supplementary Information.

Table 1 SARA components of the Tahe crude oil.
The measurement of the DIFT took for 3 h and the temperature of the experiment was fixed at 30°C by a water bath.The oil droplet volume was controlled at 8 μL.
2.6. Measurement of dilational viscoelasticity
The real-time response of the interfacial tension could be obtained during the experiment when the area of the droplet was changed sinusoidally.Then the interfacial dilational modulus could be calculated by the software according to Eq. (1).

where γ refers to the interfacial tension and A refers to the interfacial area of the droplet. The oscillation frequency was fixed at 0.1 Hz and the amplitude was set to be 10%of the droplet area.The volume of the droplet in the beginning was 8 μL and the duration of the measurement was 3 h. The experimental temperature was 30°C.
2.7. Measurement of crumpling ratio
The tensiometer was also used to form an oil droplet in the water phase and then the droplet was placed for 24 h to ensure the interfacial layer to develop adequately. After that, the droplet was contracted suddenly at the rate of 0.8 μL·s-1and the camera was used to record the whole process.Finally,the crumpling ratio could be calculated by Eq. (2).

where Vinitialrefers to the initial volume of the droplet before contraction and Vwrinklerefers to the droplet volume when the wrinkle just occurs. Each experiment was conducted repeatedly and the average values were used.
2.8. Determination on the wettability of silica nanoparticles modified by asphaltenes
The model oils containing asphaltenes and particles were prepared in the same way as stated in Section 2.3.Then,the solutions were filtered and dried out to obtain the modified silica nanoparticles. After that, the modified particles were collected and flattened into a layer. At last, the contact angle between the particles and the water phase was measured to evaluate their wettability. Each measurement was conducted repeatedly and the average values were used.
2.9. Measurement of the adsorption amount of asphaltenes on silica nanoparticles
First,the model oils were prepared in the same way as stated in Section 2.3. Then, the prepared model oils were centrifuged with 10000 rpm for 10 min. Afterwards, the absorbance of the supernatant was measured with a UV-visible spectrophotometer(A360,AOE Instrument Shanghai Company, China) and the asphaltene concentration could be obtained with a method of baseline calibration (as introduced in details in Section 2 of the Supplementary Information). At last, the adsorption amount of asphaltenes on the silica nanoparticles could be calculated according to Eq. (3).

where c0is the original asphaltene concentration in the model oil;m0is the mass of the model oil; c1and m1are the asphaltene concentration and mass of the supernatant, respectively. All the measurements were repeated three times and the average values were used.
3. Results and discussion
3.1. Asphaltene aggregation degree in model oils
Asphaltenes are the most polar components in crude oil and contain some heteroatoms and metal elements such as O, N, S, Fe,Ni, and V, forming unique molecular structures with polyaromatic rings and aliphatic side chains. Due to the strong interactions between the molecules, asphaltenes will aggregate spontaneously and the aggregation state can be easily affected by many external conditions like temperature, pressure, and oil composition. As a result, asphaltenes have different sizes and masses when they aggregate into different states in the oil.
As can be observed from Fig.1, the conductivity declines with decreasing the toluene content. Considering that the asphaltenes are the only conductive substances in the model oils,the decline of the conductivity should be attributed to the variation of the asphaltene aggregation degree.The absolute conductivity value can reflect the migrating rate of charged particles in a solution(Lesaint et al., 2013; Goual et al., 2014; Li et al., 2016; Wang et al., 2017;Moncada et al.,2019;Sun et al.,2019).Therefore,it can be inferred that the migrating rate of the asphaltenes slows down with the toluene content decreasing in the model oils. The reason may be that the asphaltene molecules aggregate and thus the size and mass of the aggregates get increased, resulting in the decline in the migrating rate of the asphaltenes. Hence, the asphaltene aggregation degree is increased when the toluene content decreases in the oils.
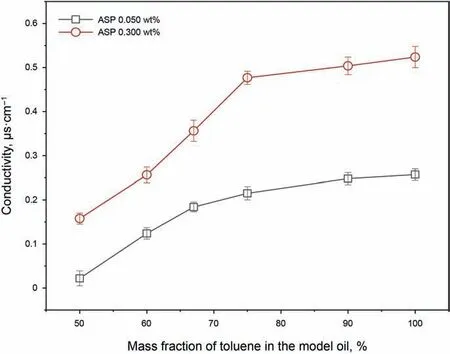
Fig.1. Conductivity of the model oils with different compositions.
3.2. Effects of asphaltenes and silica nanoparticles on emulsion stability
3.2.1. Bottle test results
The stability of model oil emulsions was evaluated by the bottle test.To prove whether the sole silica nanoparticle has the ability to stabilize the emulsion, an experiment was designed. Taking the model oil composed of pure toluene as an example, the emulsion with only the silica nanoparticles was prepared. The photograph was taken after 1 min since the emulsification completed and the photograph is shown in Fig. S2 in the Supplementary Information.It can be found that the emulsion cannot maintain stability. The model oil and water separate in a short time. This shows that the hydrophilic silica particles cannot stabilize the emulsion alone.
The emulsions containing only the asphaltenes were then prepared and the photographs are shown in Fig. 2. All the emulsions are W/O types and the method for identifying the emulsion type is explained in Section 5 of the Supplementary Information.As shown in Fig. 2a, the oil and water are completely emulsified in the beginning.However,the dispersed water droplets fall continuously with the extension of the time because of the low viscosities of the model oils.Two distinct layers can be observed clearly in each tube:the creaming oil at the upper layer and the residual emulsion at the bottom layer. As shown in Fig. 2a and b, the creaming oil layer cannot be found in the initial moment and its volume increases gradually with time. Hence, the volume ratio of the creaming oil can be recorded with time to evaluate the emulsion stability to a certain extent (Liu et al., 2021). According to Fig. 2c, the volume ratio at 3 h decreases with declining the toluene content in the model oils.In other words,the emulsion stability is enhanced with increasing the asphaltene aggregation degree. The reason is that the asphaltene aggregates with larger sizes can strengthen the structure of the interfacial layer when they adsorb at the interface.The in-depth discussion and analysis can be found in our previous study (Sun et al., 2019). In addition, the volume ratios decline markedly when the asphaltene concentration increases,indicating that the asphaltenes are beneficial to emulsion stability.
After that, the emulsions containing both the asphaltenes and silica nanoparticles were prepared. The photographs of the emulsions and the volume ratios are also recorded in Fig. 2. All the emulsions are also W/O types and the oil and water can be completely emulsified in the beginning as well.However,it can be found from the bottle test results that the effect of the particles on emulsion stability is different with the variation of the asphaltene concentration and aggregation degree. As shown in Fig. 2b and c,free water can be seen at the bottom of the tubes and the volume ratios of creaming oil are higher at the lower asphaltene concentration (0.050 wt%), no matter what the toluene content is in the model oils. It indicates that the particles play an important role in weakening the emulsion stability at low asphaltene concentrations.With the asphaltene concentration increasing, the stability of the emulsions doped with the particles is enhanced without doubt because of the positive effect of the asphaltenes on the stability.However,it is worth noting that the contribution of the particles to the emulsion stability is affected by the asphaltene aggregation degree.After adding the particles,the volume ratio of creaming oil increases when the mass fraction of toluene in the model oils is low but decreases when the mass fraction is high, as shown in Fig. 2c.This indicates that the particles can enhance the emulsion stability when the asphaltene concentration is high and the aggregation degree is low.However,even at high asphaltene concentrations,the particles still show a weakening effect on the emulsion stability if the asphaltenes are highly aggregated.
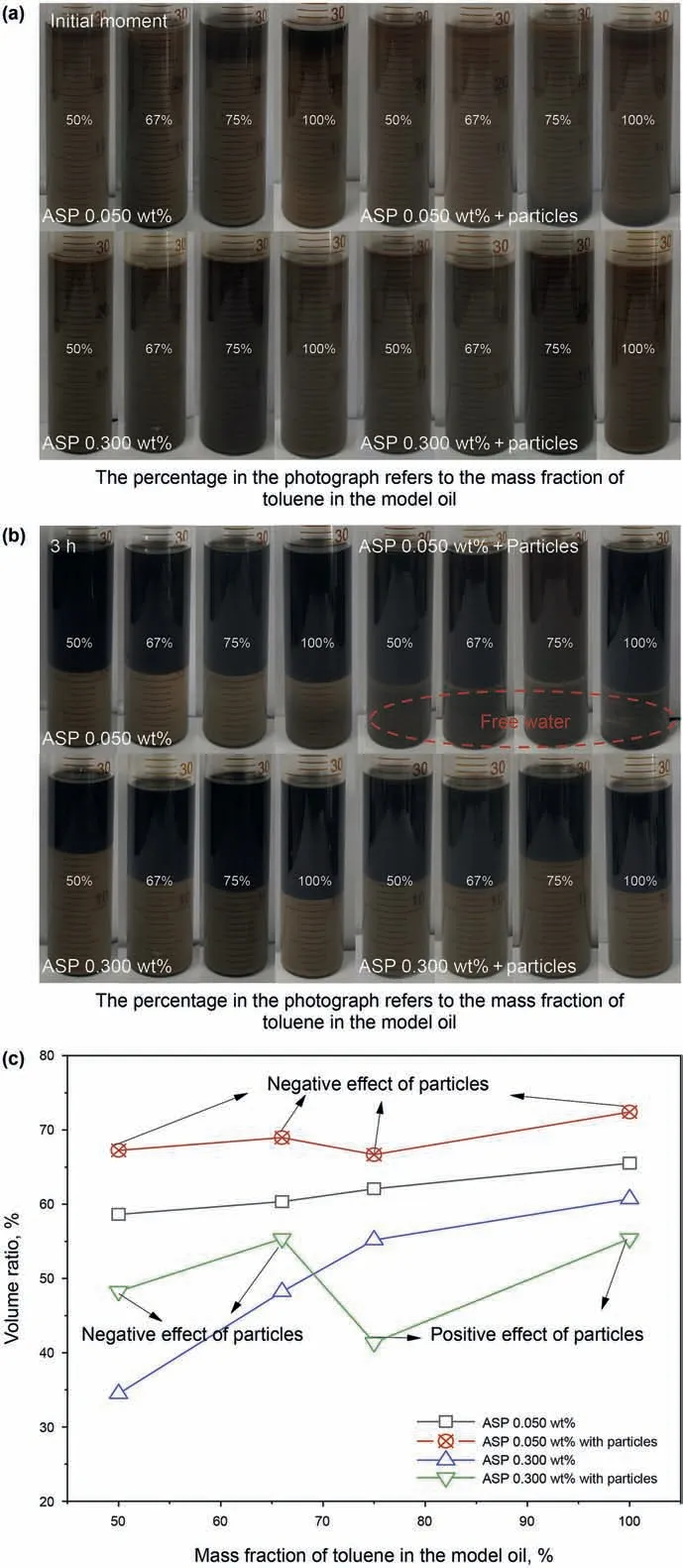
Fig. 2. Bottle test results of different emulsions: (a) Photographs of the emulsions at initial moment;(b)Photographs of the emulsions after quiescence for 3 h;(c)Volume ratios of the creaming oil for the emulsions at 3 h.
3.2.2. Micromorphology of the emulsions
In an emulsion with a poor stability, the droplets will coalesce easily and correspondingly the sizes of the droplets will increase quickly with the time. Therefore, the microstructure of the emulsions was photographed with the microscope to evaluate the emulsion stability. The microstructure photographs of the emulsions are exhibited in Fig. S4 to Fig. S7 in the Supplementary Information.
For further analysis, the Image J software is used to obtain the sizes of the droplets and the average sizes are shown in Table 2. It can be seen that the water phase is dispersed into similar sizes of droplets in the beginning after the imposition of the same shear strength and time. After that, the droplet size increases with the proceeding of time. The sizes of the droplets at 3 h can reflect the emulsion stability to some extent. When the model oil only contains asphaltenes,the average droplet size decreases monotonously with increasing the asphaltene aggregation degree,indicating that asphaltene aggregation is beneficial to emulsion stability. Moreover,the average droplet size in the emulsion doped with 0.300 wt% asphaltenes is significantly smaller than that with 0.050 wt% at 3 h, indicating that the emulsion with a higher asphaltene concentration is more stable.
Due to the severe instability of the emulsion containing 0.050 wt% asphaltenes and 0.010 wt% particles, the droplet size in the emulsion could not be obtained at 3 h, indicating that the presence of the particles is not beneficial to the emulsion stability.When the asphaltene concentration increases to 0.300 wt%and the mass fraction of toluene in the oil phase is low,the average droplet size increases after adding the particles. This manifests again the negative effect of the particles on emulsion stability. By contrast,when the mass fraction of toluene in the oil is high,the droplet size tends to decrease,indicating that the particles can also enhance the emulsion stability.
3.3. Co-adsorption behavior of asphaltenes and silica nanoparticles at the interface
The dynamic interfacial tensions between the model oils and water were measured and are shown in Fig.3 when the model oils only contain asphaltenes. The results are consistent with some published studies (Liu et al., 2020a, 2020b; 2020c). To be specific,the diffusion of asphaltenes from the oil phase to the interface causes a rapid decrease in the interfacial tension in the initial process. Then, the reorganization of the adsorbed asphaltenes at the interface results in the slow decline of the interfacial tension in the later process.
Taking the model oil composed of pure toluene as an example,the interfacial tensions were determined when the model oil contained neither the asphaltenes nor the particles or contained only the silica nanoparticles. As presented in Fig. 4, the silica nanoparticles themselves cannot alter the interfacial tension. However,the existence of the particles makes the interfacial tension show significant fluctuations,which may be attributed to the disturbance caused by the migration of particles through the interface. In the experiment, the particles were dispersed in the oil phase initially.Once the interface was formed, the particles would migrate from the oil phase to the water phase through the interface because of their strong hydrophilicity. The migration could bring disturbance to the interface, resulting in the measurement fluctuations of the interfacial tension. This phenomenon indicates that a part of the particles is unable to adsorb at the interface and tend to migrate to the water phase. However, it should also be noted that even hydrophilic particles could be adsorbed at the interface and then affect the emulsion stability according to the Pickering emulsion stabilization mechanism(Nawaz et al.,2017;Low et al.,2020).The hydrophilic particles may act as water bridges and weaken the emulsion stability when they adsorb at the interface. Hence, the hydrophilic particles cannot enhance the emulsion stability in either case.
Besides, the interfacial tension increases when the silica nanoparticles are added to the asphaltene-containing model oils. This should be attributed to the decrease in asphaltene adsorption amount at the interface because the particles cannot influence the interfacial tension alone. The reason behind the decrease may be that part of the asphaltenes adsorb on the surface of silica nanoparticles due to the complex interactions(Wang et al.,2016).Then,the asphaltene-adsorbed silica nanoparticles can occupy the interfacial area through adsorption because of the changes in their wettability. As a result, the adsorption and arrangement of the asphaltenes at the interface are disturbed and thus the interfacial tension is changed.The wettability modification of the asphaltenes on the particles will be confirmed in the following experiments.
When Vasilissa found herself left alone, she examined the hut, wondering to find it filled with such an abundance of everything. Then she stood still, remembering all the work that she had been bidden to do and wondering what to begin first. But as she looked she rubbed her eyes, for the yard was already neatly32 cleaned and the floors were nicely swept, and the little doll was sitting in the storehouse picking the last black grains and wild peas out of the quarter- measure of wheat.
3.4. Wettability modification of silica nanoparticles with asphaltenes
As presented in Fig. S8 in the Supplementary Information, the contact angle between water and the silica nanoparticles is 0°,meaning that the particles are infiltrated by water and are thus hydrophilic. In addition, the contact angle between water and the asphaltenes was also measured and the results are shown in Fig.S9 in the Supplementary Information.It can be found that the contact angle is about 150°, indicating the strong hydrophobicity of the asphaltenes. At first, the silica nanoparticles are modified by 0.050 wt% asphaltenes, but the modified-particles still show a certain hydrophilicity. In the process of measuring the contact angle,the water droplets infiltrate into the particles in a short time,so the contact angle cannot be obtained accurately. However, we can feel the extension of droplet penetration time obviously during the experiment. Therefore, it is undeniable that the particles are also modified to some extent even if the effectiveness is not obvious. After modification with 0.300 wt% asphaltenes, the contact angles between water and the particles are demonstrated inFig. 5. It is clearly seen that the contact angle has a great increase once the particles are modified by 0.300 wt% asphaltenes. Therefore, it is deduced that the modification effectiveness can be improved by increasing asphaltene concentration. In addition, the modification effectiveness is also related to the aggregation degree of asphaltenes and larger contact angles are obtained when the mass fractions of toluene are 75% and 100%.
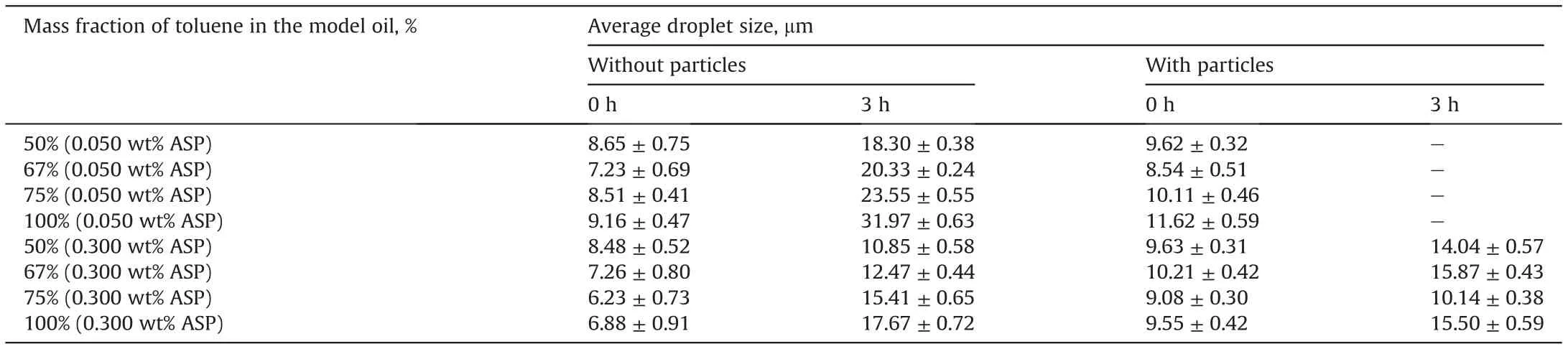
Table 2 Average droplet size in the emulsions.
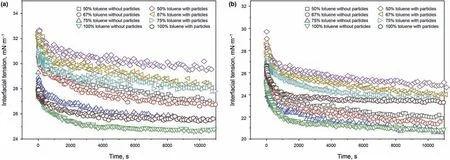
Fig. 3. Dynamic interfacial tension between different model oils and water: (a) Concentration of asphaltenes is 0.050 wt%; (b) Concentration of asphaltenes is 0.300 wt%.
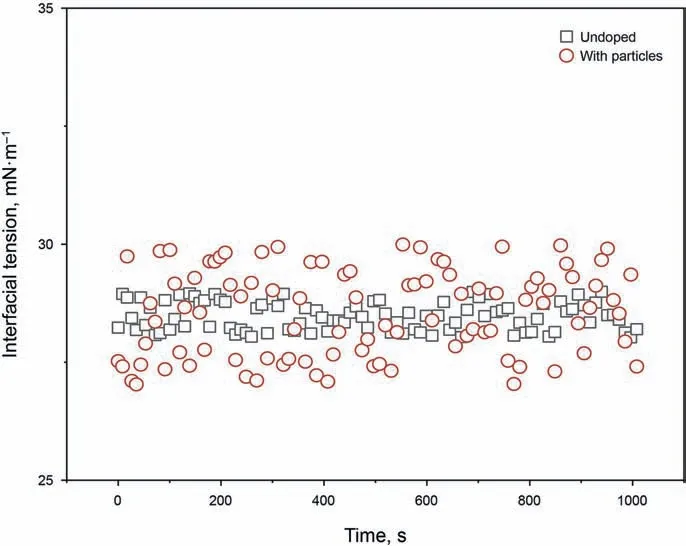
Fig. 4. Dynamic interfacial tension of the asphaltene-free model oil undoped/doped with the silica nanoparticles.
Because of the modification in the wettability, the particles are able to participate in the formation of the interfacial layer by adsorbing at the interface.Hence,the modified particles can change the interfacial properties and then affect the emulsion stability by adsorption.
3.5. Effect of silica nanoparticles on interfacial dilational viscoelasticity and structural properties
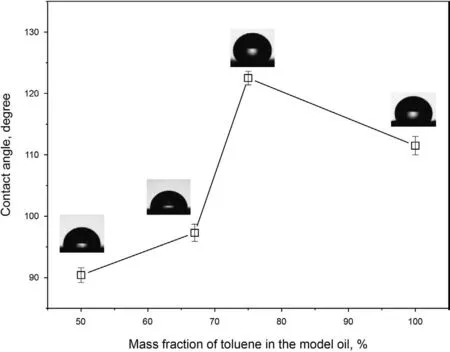
Fig. 5. Contact angle between water and the silica nanoparticles modified by asphaltenes.
According to Eq. (1), the interfacial dilational modulus can be considered as the changing rate of the interfacial tension when the interfacial area is changed by a unit. The change in the interfacial tension is caused by the migration of the adsorbed substances when the interfacial area has variations and the migration ability of the adsorbed substances determines the changing rate of the interfacial tension (Liu et al., 2019; Wang et al., 2019b). Therefore,the interfacial modulus value can reflect the migration ability of the adsorbed substances.
The dilational modulus of the interface was determined and the modulus values at 3 h are presented in Table 3.When the interface is adsorbed only by the asphaltenes, the dilational modulus increases as the toluene content decreases in the model oils. As mentioned above, the size and mass of the asphaltene aggregates increase with the toluene content declining in the model oils.As a result, the migration ability of the asphaltene aggregates is weakened because of their lager size and mass,resulting in the increase of the dilational modulus. Moreover, it can be found that the dilational modulus increases with the rise in the asphaltene concentration.This is mainly because the increase in the concentration can also promote the aggregation of the asphaltenes.
When the silica nanoparticles are added to the model oils with the asphaltenes, the interfacial dilational modulus has significant variations. As shown in Table 3, the dilational modulus with theparticles is lower than that without particles when the asphaltene concentration is 0.050 wt%. However, the dilational modulus increases after the addition of the particles when the asphaltene concentration is 0.300 wt%. As mentioned above, the asphaltenes and particles can adsorb at the interface together due to the modification. The asphaltene adsorption amount at the interface will decrease in the presence of the modified particles.It has been verified in the former section that the particles have a poor modification effectiveness by the asphaltenes when the asphaltene concentration is 0.050 wt%.It can be thus inferred that the modified particles may not affect interfacial tension obviously because of poor modifications. Hence, the decrease in the dilational modulus may result from the decrease in asphaltene adsorption amount at the interface. By contrast, the modification effectiveness is improved observably with the increase in asphaltene concentration. The particles will be provided with amphiphilic or even hydrophobic features when modified by 0.300 wt% asphaltenes, so they have the ability to affect the interfacial tension. The particles modified by the adsorption of the asphaltenes should have larger sizes and mass, so their migration ability should be weakened.Hence, the dilational modulus will increase when they adsorb at the interface (Liu et al., 2019).
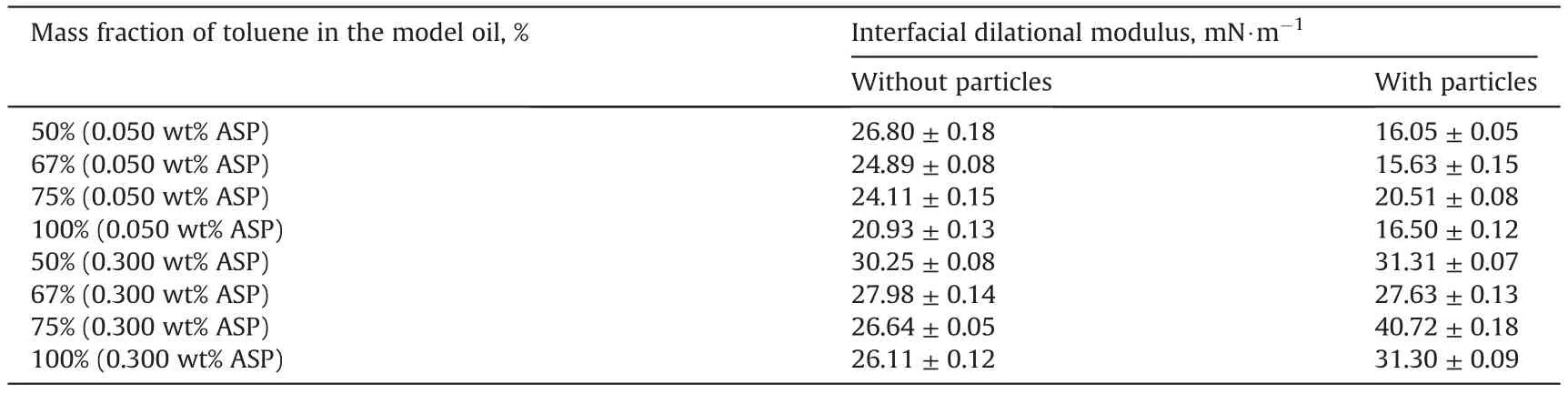
Table 3 Interfacial dilational modulus of the interfaces at 3 h for different oil compositions.
As shown in Fig. 6, the wrinkles are observed when the interfacial layer is contracted, indicating that the interfacial layer has rigid solid-like property. The crumpling ratio increases with the asphaltene concentration in Fig. 7, which may be attributed to the multilayer structure formed at the interface (Liu et al., 2020a). As recorded in Fig.7,the presence of the particles leads to a decrease in the crumpling ratio, indicating that the adsorption of the particles can change the properties of the interfacial structure and make the interface have more flexibility.
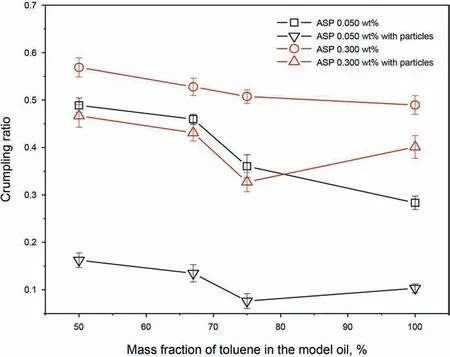
Fig. 7. Crumpling ratio of the interfacial layers.
3.6. Adsorption of asphaltenes with different aggregation degrees on silica nanoparticles
The adsorption amount of the asphaltenes on the silica nanoparticles was measured and the results can be seen in Fig. 8. It is shown that the adsorption amount increases significantly when the asphaltene concentration rises to 0.300 wt%. In addition, the adsorption amount is found to increase slightly and then decrease significantly with the toluene content decreasing in the model oils.This indicates that the adsorption amount of the asphaltenes on the particles is related to the asphaltene aggregation degree. Meanwhile,the turning point of the curves moves to the right side of the abscissa with the increase in the asphaltene concentration.

Fig. 6. Photographs of the wrinkles of the interfacial layers at 0.050 wt% asphaltenes: (a) Sole asphaltenes; (b) Asphaltenes and particles.
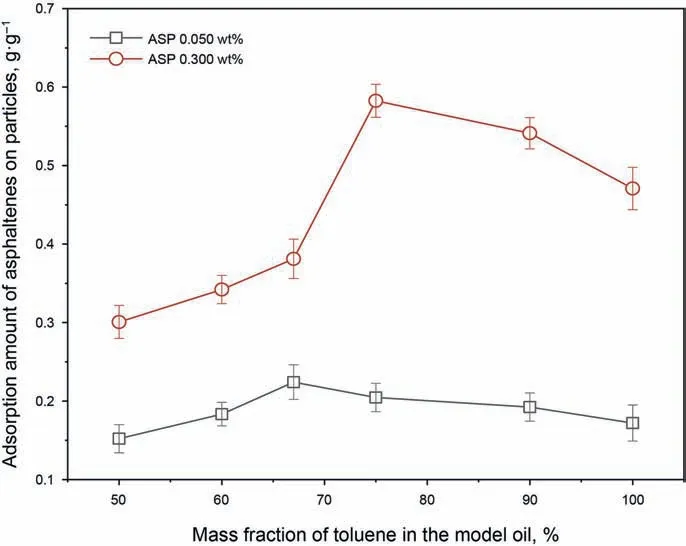
Fig. 8. Adsorption amount of asphaltenes on the silica nanoparticles.
It has been found in some articles that the modification effectiveness on particles is related to the adsorption amount of asphaltenes and the contact angle between water and the particles will increase when they adsorb more asphaltenes (Sullivan and Kilpatrick, 2002). Therefore, it is now understood why the modification effectiveness can be influenced by the asphaltene concentration and the toluene content in the model oils.
3.7. Mechanism discussion
The interfacial layer can prevent the coalescence of droplets and is thus crucial to emulsion stability.The emulsion can be stabilized by asphaltenes because asphaltenes can form a protective layer by adsorbing at the interface.The hydrophilic particles cannot adsorb at the interface and thus cannot affect the emulsion stability by preventing the coalescence of droplets.
The silica nanoparticles can be modified by the asphaltenes.The asphaltene-modified particles can co-adsorb with the asphaltenes and take part in the formation of the interfacial layer. The modification effectiveness of the particles is related to the concentration and aggregation degree of the asphaltenes. The increase in the asphaltene concentration and the decrease in the asphaltene aggregation degree can improve the modification effectiveness by promoting the adsorption of the asphaltenes on the surface of particles and resultantly the hydrophobicity of the particles is enhanced.
As the inherent interfacially-active substances, asphaltene molecules have the amphiphilic structures. When they disperse well in the oil doped with silica nanoparticles, the hydrophilic groups of the asphaltene molecules will adhere to the surface of the hydrophilic particles. The oleophilic groups cannot adhere to the surface of particles and can only stretch in the oil phase,resulting in the endowment of the hydrophobicity to the particles. However,instead of adsorbing on the surface of particles, asphaltene molecules tend to interact with themselves and form aggregates when the toluene content is low in the model oil. Hence, the adsorption amount of asphaltenes on the particles will decrease. This can explain why the turning point of the curves moves to the right side of the abscissa in Fig.8.The reason is that the increase in asphaltene concentration can also aggravate the aggregation degree of the asphaltenes to some extent.
The stabilization mechanisms of the emulsions containing the asphaltenes and solid particles are shown in Fig. 9. When the asphaltene concentration is low or the aggregation degree is high,the stability of emulsion will be weakened after adding the silica nanoparticles. The reason is that the modification effectiveness of the particles is poor and the modified particles are still hydrophilic to some extent on these conditions. When they participate in the formation of the interfacial layer,they will act as water bridges and weaken the stability of the emulsion.
By comparison, when the concentration of the asphaltenes is increased and the aggregation degree of the asphaltenes is decreased, the modification effectiveness and accordingly the hydrophobicity of the particles are enhanced. When the modified particles with strong hydrophobicity adsorb at the interface, they can prevent the droplets from coalescence through the barrier effect.Therefore,the emulsion stability can be enhanced and become even stronger than that only stabilized by asphaltenes.
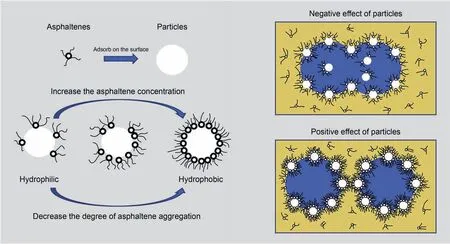
Fig. 9. Schematic of the stabilization mechanisms of the emulsions containing the asphaltenes and solid particles.
4. Conclusions
In this study,the impact of asphaltenes and silica nanoparticles on the emulsion stability is systematically explored, which can provide theoretical support for the treatment of crude oil emulsions containing solids in the field. The main conclusions are as follow.
1) When the asphaltene concentration is low or the asphaltene aggregation degree is high, the emulsion stability will be weakened after adding the silica nanoparticles. However, with increasing the asphaltene concentration and decreasing the asphaltene aggregation degree,the particles can be beneficial to emulsion stability.
2) Asphaltenes can adsorb on the surface of particles and enhance their hydrophobicity. The modified particles can adsorb with asphaltenes at the interface together and make the interface have more flexibility.
3) The concentration and aggregation degree of the asphaltenes can alter their adsorption amount on the particles. Lower aggregation degree and higher concentration of the asphaltenes help to increase the asphaltene adsorption amount on the surface of the particles. With the increase of the adsorption amount, the modification effectiveness is improved and the hydrophobicity of the particles is enhanced.When the modified particles with strong hydrophobicity adsorb at the water/oil interface, they can increase the emulsion stability through the barrier effect.
Acknowledgements
Support from the National Natural Science Foundation of China(Grant No.51704315) is gratefully acknowledged.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.petsci.2022.02.004.
- Petroleum Science的其它文章
- A fast space-time-domain Gaussian beam migration approach using the dominant frequency approximation
- Predicting gas-bearing distribution using DNN based on multicomponent seismic data: Quality evaluation using structural and fracture factors
- Reflection-based traveltime and waveform inversion with secondorder optimization
- Determination of dynamic capillary effect on two-phase flow in porous media: A perspective from various methods
- Settling behavior of spherical particles in eccentric annulus filled with viscous inelastic fluid
- Laboratory investigation on hydraulic fracture propagation in sandstone-mudstone-shale layers

