Diamide derivatives containing a trifluoromethylpyridine skeleton:Design,synthesis,and insecticidal activity
XU Fang-zhou,WANG Yan-yan,GUO Sheng-xin,DAl A-li,WU Jian
State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering/Key Laboratory of Green Pesticide and Agricultural Bioengineering,Ministry of Education/Guizhou University,Guiyang 550025,P.R.China
Abstract: Diamide derivatives are biologically active molecules that have been widely applied in recent years in research on pesticides,especially insecticides.Using a simple and environmentally friendly scheme,a series of new diamide derivatives containing a trifluoromethylpyridine skeleton was designed,synthesized,and confirmed by 1H,19F and 13C NMR,and HR-MS.Their insecticidal activities against Plutella xylostella and Helicoverpa armigera were measured and the relationship between structure and activity was investigated.Eight of the title compounds (D2,D5,D10,D21,D28,D29,D30 and D33) showed 100% activity against P.xylostella at 500 mg L-1.One compound,D33,still showed 100% activity against P.xylostella at 100 mg L-1 and had the lowest LC50 (lethal concentration 50%,3.7 mg L-1) among the synthesized compounds.Molecular docking analysis revealed that D33 could be thoroughly embedded in the active pocket of the ryanodine receptor via hydrogen bonding in a manner similar to the commercial insecticide chlorantraniliprole.
Keywords: diamide derivatives,trifluoromethylpyridine,synthesis,insecticidal activity,molecular docking
1.lntroduction
The increasing resistance of pest insects to insecticides has created a major global challenge for the control of pests in crops (Vijay and Grewal 2018;Fanget al.2019;Fujiiet al.2019).For example,the diamondback mothPlutellaxylostella(L.) is one of the most difficult pests to manage,as it has acquired resistance to almost every class of insecticide and was the first species to develop resistance to theBacillusthuringiensis-based insecticides(Crickmore 2016).ControllingP.xylostellacosts up to US$4.5 billion annually (Zaluckiet al.2012) and losses due to this insect vary from~50% of the wheat crop to more than 80% of cotton production,so this pest is a threat to the needs of the expanding global population(Oerke 2005).Although numerous insecticides are available,their efficacy for controlling many pest insects is decreasing due to cross-resistance and other reasons(Ambethgar 2009).New insecticides with novel modes of action are urgently required to meet the ever-changing regulatory requirements and growing crop production,as well as to address increasing public concerns regarding the evolution of insecticide resistance.
Many recent reports have documented the isolation of bioactive compounds with excellent insecticidal activity and low toxicity (Deepaket al.2019;Paramasivamet al.2020;Cittrarasuet al.2021;Meenambigaiet al.2021).Diamide derivatives have become a major focus for the development of novel insecticides in recent decades(Karriet al.2018;Liet al.2019;Liuet al.2019) following the discovery and commercialization of several highly effective diamide insecticides,such as fluobendiamide(Liuet al.2010),chlorantraniliprole (Potaiet al.2018;Heet al.2019),cyantraniliprole (Luoet al.2019;Qiaoet al.2019) and SYP-9080 (Zhaoet al.2015;Renet al.2018).The database of commercially available pesticides is a useful starting point for the further development of this class,given that many novel diamide derivatives were developed through the modification of chlorantraniliprole and exhibit excellent insecticidal activities against various pests including diamondback moth,cotton bollworm,beet armyworm,and oriental leafworm moth (Ouet al.2012;Wanget al.2013;Liuet al.2017;Zhouet al.2018).Furthermore,our previous work has shown that some diamide derivatives exhibit excellent insecticidal activity against not only Lepidoptera but also Diptera and Homoptera (Wuet al.2012).Hence,compounds bearing a diamide moiety have attracted considerable attention as potential insecticidal agents.

Fig.1 The structures of some commercial pesticides containing a trifluoromethylpyridine group.
In light of these issues,this study used the commercially available insecticide chlorantraniliprole as a lead compound and introduced a trifluoromethylpyridine substructure,which enabled the design,synthesis and insecticidal testing of 33 novel diamide derivatives(Fig.2).The majority of the synthesized title compounds exhibited moderate to excellent insecticidal activity.In particular,compound D33showed 100% activity at the low concentration of 100 mg L-1againstP.xylostella,which was comparable to chlorantraniliprole (also 100%).Compounds D5,D10,D21,D28,and D29showed substantial insecticidal activity levels of over 85% againstP.xylostellaat 100 mg L-1.

Fig.2 The design of the title compounds.
2.Materials and methods
2.1.Reagents and instrumentation
All reagents were of analytical grade,and anhydrous solvents were dried and purified according to standard techniques.A series of substituted 2-aminobenzoic acids was purchased from Accela ChemBio Co.,Ltd.(Shanghai,China) and 3-chloro-5-(trifluoromethyl) picolinic acid was supplied by Ark Pharm,Inc.(Chicago,USA).Melting points of the title compounds were tested using a XT-4 binocular microscope (Beijing Tech Instrument Co.,Beijing,China).Using DMSO as the solvent and TMS as the internal standard,1H,19F and13C NMR spectra were recorded on an ECX 500 NMR spectrometer (JEOL,Tokyo,Japan) operating at room temperature.Highresolution mass spectra were recorded on a Q-Exactive Orbitrap LC-MS Instrument (Thermo Scientific,USA).All reactions were monitored by TLC.
2.2.General synthetic procedure for intermediate C
As shown in Fig.3,pyridine (0.7 g,8.87 mmol) was added to a solution of 3-chloro-5-(trifluoromethyl) picolinic acid (0.5 g,2.22 mmol) in 5 mL of acetonitrile,then methanesulfonyl chloride (0.51 g,4.43 mmol) was slowly added at -5°C and the solution was stirred for 10 min.The corresponding substituted 2-amino-benzoic acid(2.22 mmol) was added and stirred for a further 10 min,then pyridine (0.7 g,8.87 mmol) and methanesulfonyl chloride (0.51 g,4.43 mmol) were slowly added sequentially below 0°C.This mixture was then stirred at room temperature (RT) for 10 h and monitored by TLC.After completion of the reaction,5 mL of water was added to precipitate a crystalline solid that was filtered and recrystallized from ethanol.

Fig.3 The synthetic route of the title compounds D1-D33.
2.3.General procedure for preparation of title compounds D1-D33
As shown in Fig.3,the corresponding amine in acetonitrile (2 mL) was added dropwise to a suspension of intermediate C (0.51 mmol) in acetonitrile (4 mL) and stirred at RT for 1 h.The precipitate was filtered,washed with ethanol (3 mL) and dried to yield the crude product,which was then recrystallized from ethanol to produce a pure white solid (D1-D33).
2.4.lnsecticidal assay
All insecticidal assays were conducted at (25±1)°C using test organisms reared in the laboratory and repeated according to statistical requirements.The test organisms used in the bioassays were the generations descended from various generations and all of them remained nonresistant.Mortality was corrected by Abbott’s formula(Abbottet al.1925,1987),and evaluations were based on a percentage scale (0=no activity,100=complete eradication) at intervals of 5%.
lnsecticidal activity against P.xylostellaThe insecticidal activities of compounds D1-D33 against third instar larvae ofP.xylostellawere evaluated according to a previously reported procedure (Wanget al.2006;Fenget al.2010;Zhaoet al.2010).Fresh cabbage discs(2 cm in diameter) were dipped in solutions of compounds D1 to D33 for 10 s,air-dried,and placed in a 9 cm Petri dish lined with wet filter paper.Third instar larvae ofP.xylostellawere placed in the Petri dish and mortality was calculated after 72 h.Each assay was conducted three times.The blank control group was treated with distilled water containing TW-80 (0.1 mL L-1) and the commercial insecticide chlorantraniliprole was used as a positive control.
lnsecticidal activity against Helicoverpa armigeraThe insecticidal activities of the synthesized compounds againstH.armigerawere evaluated using Wang’s dietincorporation method (Wanget al.1999).A range of compound concentrations (3 mL solutions) was added to forage (27 g) in a 24-well plate and one larva was placed in each well.The mortality was calculated for each compound after 72-96 h.
2.5.Molecular docking study
AutoDock Molecular Modeling Software was used to study the molecular docking processes.The target compound was kept in a low-energy state (Luoet al.2020a,b) and the N-terminal domain crystal structure of the ryanodine receptor (PDB code: 5Y9V;Linet al.2017) downloaded from RCSB-PDB (http://www.rcsb.org) was dehydrated and hydrogenated.Molecular docking was carried out under default conditions using the Lib-Dock module.
And the second brother said to himself as he listened, Certainly he has managed very badly, but I should like to see if I can t do better, and win the princess for my own self
3.Results and discussion
3.1.Synthesis
The synthetic route of the title compounds D1-D33 is illustrated inFig.3.Intermediate C was obtainedviathe reaction at RT of 3-chloro-5-(trifluoromethyl) picolinic acid(A) with various substituted 2-aminobenzoic acids (B) in the presence of pyridine and methanesulfonyl chloride in acetonitrile.The desired compounds D1-D33 were conveniently obtained with satisfactory yieldsviathe treatment of intermediate C at RT with the various amines in acetonitrile.
Synthesized compounds D1-D33 were characterized by melting point,1H,13C and19F NMR,and high-resolution mass spectrometry (HR-MS).For example,in the1H NMR spectrum of D1,the singlet proton of -NHCObetween the benzene and pyridine rings was observed at shiftδ10.56 ppm and the proton of another -NHCOamide bridge presented atδ9.05 ppm as a doublet withJ=0.8 Hz.The proton adjacent to the pyridine N atom exhibited a singlet atδ8.63 ppm.Due to the coupling effect of Cl in the pyridine ring,the doublet atδ8.44 ppm with coupling constant 3.7 Hz represented the proton between -Cl and -CF3on the pyridine ring.The two protons on the benzene ring appeared atδ7.48-7.84 ppm as two doublets.The proton in the -CH-of the cyclopropyl group was seen atδ2.69 ppm as two triplets withJ=7.2 and 3.7 Hz because of the coupling coefficients from the two -CH2-moieties.Other cyclopropyl protons were observed atδ0.54-0.47 ppm andδ0.64-0.57 ppm.In the13C NMR spectra of the fluorine-containing compounds,the C of -CF3was observed atδ123 ppm as a quartet with coupling constant 273.4 Hz.The C atom adjacent to -CF3was also split to a quartet with coupling constant 33.3 Hz.
3.2.lnsecticidal activity and structure-activity relationship
Most of the title compounds showed moderate to excellent insecticidal activity against bothP.xylostellaandH.armigera(Table 1).At 500 mg L-1,compounds D2,D5,D10,D21,D28,D29,D30 and D33 exhibited 100% activity againstP.xylostellaand more than 80%activity againstH.armigera.Notably,at 100 mg L-1,D5,D10,D21,D28,D29 and D33 still displayed excellentactivity (86.3-100%) againstP.xylostella.Compounds D28-D33 had the same R substituent on the benzene ring as chlorantraniliprole (i.e.,4-chloro-6-methyl)but different substituents on the amide N,and they showed significantly different insecticidal activities as follows: D33 (R1=isopropyl)>D28 (R1=cyclopropyl)>D29(R1=methy l)>D 3 0 (R1=ethy l)>D 3 2 (R1=2,2-difluoroethyl)>D31 (R1=hydroxyethyl).It should be noted that D29 had the same R and R1substituent groups as chlorantraniliprole and did not show an improvement in insecticidal activity.However,when the R1group was changed from methyl to isopropyl,D33 (at 100 mg L-1) showed the highest activity which was similar to chlorantraniliprole.The insecticidal activities of the title compounds were dramatically affected by several factors,including the substituents on the benzene ring and amide N atom,and the chain length.
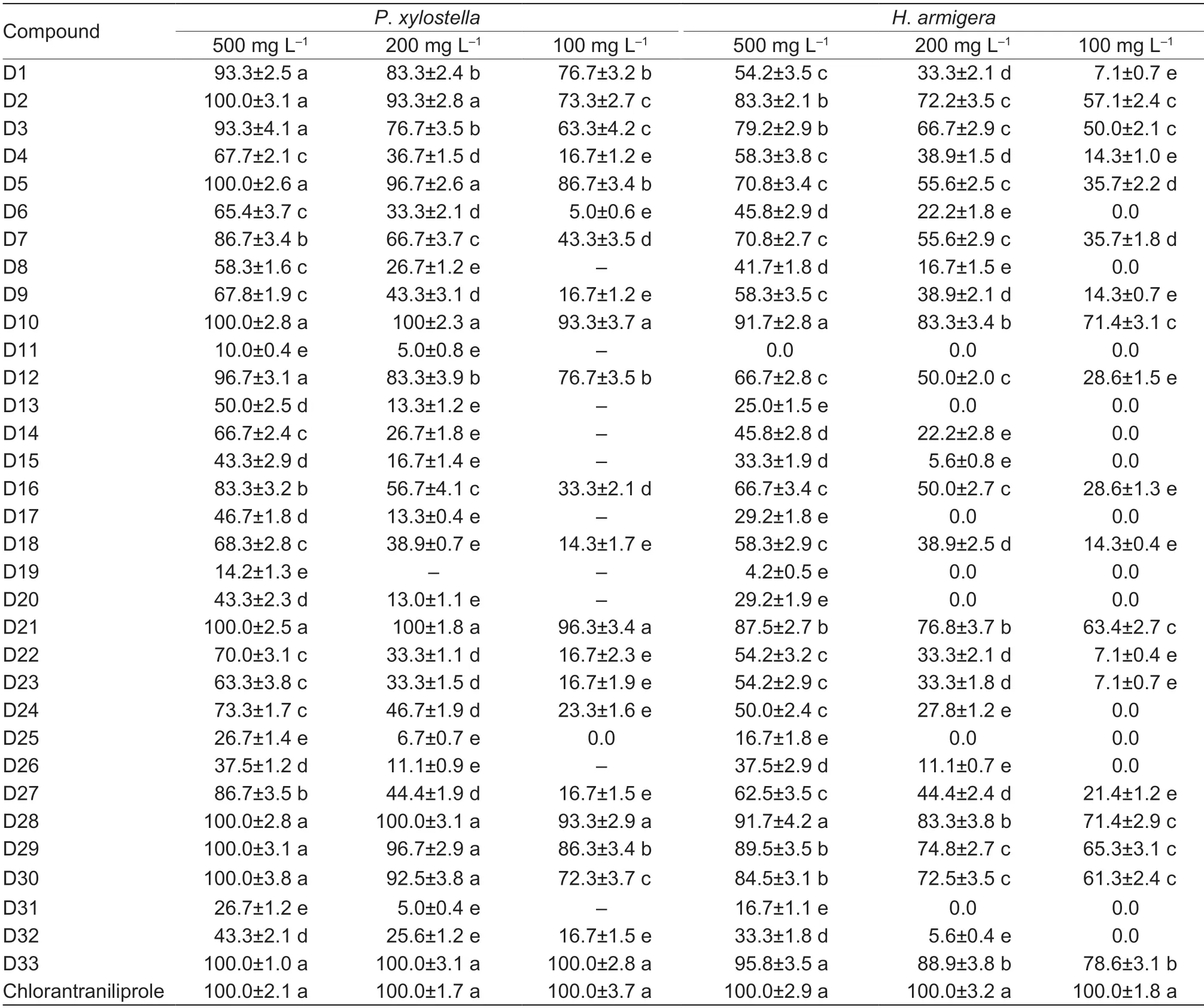
Table 1 Larvicidal activities of compounds D1-D33 and chlorantraniliprole against Plutella xylostella and Helicoverpa armigera
The median lethal concentrations (LC50) of the title compounds againstP.xylostellawere calculated(Table 2).Compounds D28 and D33 had LC50of 4.8 and 3.7 mg L-1,respectively.With a 4-chloro-6-methyl substituent on its benzene ring and its amide N atom substituted by isopropyl,D33 was thus confirmed as the synthesized compound with the highest insecticidal activity,although its LC50value of 3.7 mg L-1was still higher than that of chlorantraniliprole (LC500.68 mg L-1).However,further structural modification of the lead compound chlorantraniliprole is ongoing in order to find an insecticidal agent capable of completely controlling agricultural pest insects.

Table 2 The LC50 values (mg L-1) of the active title compounds and chlorantraniliprole against Plutella xylostella
Many active molecules containing a trifluoromethyl pyridine moiety and capable of controlling pest insects were obtained in our previous studies (Xuet al.2018;Luoet al.2020b).In the present study,the observed insecticidal activities of the synthesized title compounds (trifluoromethylpyridine-containing diamide derivatives) differed from those of previously reported molecules,including trifluoromethylpyridine containing 1,3,4-oxadiazole derivatives and anthranilic amide derivatives containing chiral thioether and trifluoromethylpyridine moieties.For example,in controllingP.xylostella,the LC50of the most active compound in this study,D33,was much lower than the 1,3,4-oxadiazole derivatives (54.5 mg L-1;Xuet al.2018) and slightly lower than the anthranilic amide derivatives (7.3 mg L-1;Luoet al.2020b).Other researchers have also structurally modified chlorantraniliprole to produce molecules that show excellent (>90%) insecticidal activity againstP.xylostellaat a low concentration of 10 mg L-1(Liet al.2008;Yanet al.2012).Further structural modifications of molecules containing the trifluoromethylpyridine moiety are ongoing in our laboratory in order to produce additional potential insecticidal candidates.
3.3.Molecular docking
The ryanodine receptor has been confirmed as the target receptor of the commercial insecticides chlorantraniliprole and cyantraniliprole (Davidet al.2008).The N-terminal domain crystal structure of the ryanodine receptor(PDB code: 5Y9V) has been reported (Linet al.2017).Interestingly,the crystal structure shows two potential species-specific insecticide-targeting sites (Linet al.2017),and one of them interacted strongly with our previously synthesized compounds that showed high insecticidal activity (Yuet al.2018).Thus,considering these findings and the insecticidal activity assay results presented above,molecular docking simulation was performed for D33 using Discover Studio 4.5 Software.The details of this process are available in Appendix A.
As shown in Fig.4,like chlorantraniliprole,compound D33 was thoroughly embedded in the pocket of the ryanodine receptor due to hydrogen bonds with amino acid residues R4563 and D4815,hydrophobic interactions between L4567,L4792 and Y4795,and other forces.Notably,this pocket is almost the same as the reported action pocket for chlorantraniliprole,in which residues D4815 and R4563 are the key factors affecting the interaction between molecule and pocket (Maet al.2020).D33 showed strong hydrogen bonding with amino acids D4815 (2.6 Å and 3.4 Å) and R4563 (2.9 Å),which was slightly weaker than the corresponding bonding between chlorantraniliprole and residues D4815 (2.7 Å and 2.8 Å)and R4563 (2.7 Å).These molecular docking results were consistent with the insecticidal activity data.
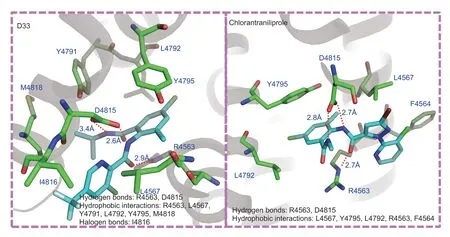
Fig.4 Molecular docking of the most active compound (D33) and chlorantraniliprole.
4.Conclusion
Building on the advantages of the commercial insecticide chlorantraniliprole,a trifluoromethylpyridine skeleton was incorporated to produce 33 novel diamide derivatives.These new derivatives exhibited excellent insecticidal activity againstP.xylostellaandH.armigera,and further studies are ongoing to develop new insecticides utilizing this novel mode of action.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21762012,32072445 and 21562012),the Program of Introducing Talents to Chinese Universities (D20023) and the S&T Planning Project of Guizhou Province,China ([2017]1402 and [2017]5788).The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn/) for the expert linguistic services provided.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
 Journal of Integrative Agriculture2022年10期
Journal of Integrative Agriculture2022年10期
- Journal of Integrative Agriculture的其它文章
- Responses of leaf stomatal and mesophyll conductance to abiotic stress factors
- Genetic dissection of the grain-filling rate and related traits through linkage analysis and genome-wide association study in bread wheat
- Expression profiling of transgenes (Cry1Ac and Cry2A) in cotton genotypes under different genetic backgrounds
- Linkage and association mapping of wild soybean (Glycine soja)seeds germinating under salt stress
- Genome-wide identification,expression and functional analysis of sugar transporters in sorghum (Sorghum bicolor L.)
- lnhibition of miR397 by STTM technology to increase sweetpotato resistance to SPVD
