Biosynthesis of artemisinic acid in engineered Saccharomyces cerevisiae and its attractiveness to the mirid bug Apolygus lucorum
TENG Dong ,LIU Dan-feng ,Khashaveh ADEL ,SUN Pei-yao ,GENG Ting ,ZHANG Da-wei ,ZHANG Yong-jun
1 State Key Laboratory for Biology of Plant Diseases and Insect Pests,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,P.R.China
2 Laboratory of Ecology and Evolutionary Biology,Yunnan University,Kunming 650091,P.R.China
3 Langfang Scientific Research Trial Station,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Langfang 065000,P.R.China
4 Institute of Plant Protection,Gansu Academy of Agricultural Sciences,Lanzhou 730070,P.R.China
Abstract Artemisia annua is an important preferred host of the mirid bug Apolygus lucorum in autumn.Volatiles emitted from A.annua attract A.lucorum.Volatile artemisinic acid of A.annua is a precursor of artemisinin that has been widely investigated in the Chinese herbal medicine field.However,little is known at this point about the biological roles of artemisinic acid in regulating the behavioral trends of A.lucorum.In this study,we collected volatiles from A.annua at the seedling stage by using headspace solid phase microextraction (HS-SPME).Gas chromatography-mass spectrometry (GC-MS) analysis showed that approximately 11.03±6.00 and 238.25±121.67 ng h-1 artemisinic acid were detected in volatile samples and milled samples,respectively.Subsequently,a key gene for artemisinic acid synthesis,the cytochrome P450 gene cyp71av1,was expressed in engineered Saccharomyces cerevisiae to catalyze the production of artemisinic acid.After the addition of exogenous artemisinic alcohol or artemisinic aldehyde,artemisinic acid was identified as the product of the expressed gene.In electroantennogram (EAG) recordings,3-day-old adult A.lucorum showed significant electrophysiological responses to artemisinic alcohol,artemisinic aldehyde and artemisinic acid.Furthermore,3-day-old female bugs were significantly attracted by artemisinic acid and artemisinic alcohol at a concentration of 10 mmol L-1,whereas 3-day-old male bugs were attracted significantly by 10 mmol L-1 artemisinic acid and artemisinic aldehyde.We propose that artemisinic acid and its precursors could be used as potential attractant components for the design of novel integrated pest management strategies to control A.lucorum.
Keywords: artemisinic acid,CYP71AV1,biosynthesis,Apolygus lucorum,electrophysiological responses,behavioral trends
1.Introduction
In the last century,the cotton bollwormHelicoverpa armigeraHübner (Lepidoptera: Noctuidae) was considered the main pest of cotton in China (Liet al.2015).Due to the widespread cultivation ofBacillus thuringiensis(Bt) transgenic cotton,many lepidopteran insects have been effectively controlled,including cotton bollworms,which has led to a large reduction in the use of broad-spectrum chemical pesticides in cotton fields(Malaquiaset al.2021).As a consequence,some mirid bugs (Hemiptera: Miridae) that were historically only minor pests,have emerged as the new dominant insect pests in cotton fields.The population ofApolyguslucorumhas increased dramatically and this mirid has become the most important pest of economic crops in China (Wuet al.2003).Apolyguslucorumis a polyphagous piercingand-sucking insect that has more than 200 host plants from 50 families.In addition,this mirid bug has strong migration and spreading abilities (Luet al.2008,2010;Yinet al.2021).More importantly,A.lucorumis seasonally transferred among different host plants,such as fruit trees in spring,cotton in summer andArtemisiaannuain autumn (Panet al.2017).
Host shifts are an important process for allowing insects to cope with climate change and habitat destruction (Mazzi and Dorn 2012).Artemisia annua,annual wormwood,has been identified as one of the main hosts ofA.lucorumadults in autumn (Panet al.2017),and the population density ofA.lucorumadults onA.annuain autumn was significantly higher than that on other plants (Panet al.2018).Several volatile compounds emitted fromA.annuahave been reported to affect the behavior ofA.lucorumadults.For example,m-xylene,butyl acrylate,butyl propionate and butyl butyrate demonstrate significant attractiveness to both male and femaleA.lucorum(Panet al.2021).However,little is known about the biological roles of artemisinic acid released fromA.annuain regulating the behavioral trends ofA.lucorum.
Volatile artemisinic acid,a precursor of artemisinin,is produced in the glandular hairs ofA.annua(Liet al.2021).Artemisinin was first extracted fromA.annua,and is currently the most effective drug for treating malaria worldwide (Tu 2011).However,the commercial yield of artemisinin directly extracted fromA.annuais limited by the supply ofA.annua,and the total synthesis of artemisinininvivois complicated and expensive.Therefore,theinvitrosynthesis of artemisinin’s precursor substance,artemisinic acid,is considered to be a potential method to boost commercial production and has been extensively investigated (Liu and Yeh 1996;Levesque and Seeberger 2012).
The cytochrome P450 genecyp71av1is the key gene responsible for the synthesis of artemisinic acid (Weatherset al.2016).In 2006,the amorpha-4,11-diene oxidase genecyp71av1was cloned from the glandular hairs ofA.annua(Roet al.2006).When CYP71AV1 and cytochrome P450 reductase (CPR) were co-expressed inSaccharomyces cerevisiaeat the same time,CYP71AV1 could catalyze the three-step oxidation reaction from amorpha-4,11-diene to artemisinic acid production.Subsequently,the first engineered strain ofS.cerevisiaeproducing artemisinic acid was designed (Paddonet al.2013).
In the current study,artemisinic acid fromA.annuaplants was collected and analyzed using gas chromatography-mass spectrometry (GC-MS).The pYES2 expression vector and INVScI yeast cells were used to express CYP71AV1 in order to develop attractants for the control of bugs (Liuet al.2018).Electroantennogram (EAG) recordings and behavioral assays were carried out to evaluate the behavioral trends ofA.lucorumin response to artemisinic acid,artemisinic alcohol and artemisinic aldehyde.Our data will reveal the biological effects of artemisinic acid and its analogs on the behavior ofA.lucorumand aid in the development of attractants for the control of these bugs.
2.Materials and methods
2.1.Plants and insects
Wild typeA.annuaand cotton (Zhongmian 12) were planted at the Langfang Scientific Research Trial Station(39.53°N,116.70°E),Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Hebei,China.Apolyguslucorumadults were also bred at the Langfang Scientific Research Trial Station.The laboratory bug colony was established in plastic containers (20 cm×10 cm×6 cm) at (28±1)°C,(60±10)%relative humidity,and a 14 h light:10 h dark cycle.The adults and newly emerged nymphs were reared on green beans (PhaseolusvulgarisL.) and 10% sucrose solution.Three-day-old adults were used in the study.All adults were starved for 6 h prior to the electrophysiological and behavioral experiments.
2.2.Collection and identification of volatiles
Headspace solid phase microextraction (HS-SPME) was performed to collect volatile samples.A pot containing oneA.annuaplant at the seedling stage was placed into a glass jar (10 cm in diameter×25 cm in height),and the container was tightly sealed with metal clamps on the lid (Huanget al.2015).An SPME (SAAB-57330U,Sigma-Aldrich,Bellefonte,USA) handle was inserted into the plastic jar,and the fiber was placed at a distance of 2 cm from the plant to collect volatile samples for 8 h.After absorption,the collected samples were analyzed on a GC-MS-QP2010SE (Shimadzu,Japan) equipped with an SH-Rtx-5MS dimethylpolysiloxane column(0.25 μm×0.25 mm×30 m,Shimadzu,Japan).The SPME fiber was directly inserted into the GC injector.Purified helium was used as the carrier gas at a constant flow rate of 0.8 mL min-1.The injector,transfer line and ion source temperatures were set at 250°C.The GC oven temperature was initially maintained at 50°C for 3 min and then increased to 120°C at a rate of 30°C min-1,held for 2 min and finally increased to 280°C at a rate of 20°C min-1and held for 5 min.Compound identification was accomplished by comparisons of recorded mass spectra with the mass spectral libraries of NIST version 17.0(National Institute of Science and Technology Software,USA) (Liuet al.2018).
To fully collect volatile artemisinic acid,2.0 g of topA.annualeaves were collected and ground in 1.8 mL of 5 mol L-1CaCl2at room temperature.The milled samples were placed into PTFE/Silicon Septa screw cap glass vials (Agilent Technologies,USA),respectively,at 50°C in a shaking incubator at 200 r min-1for 30 min.The SPME fiber was inserted into a glass vial to 1.0 cm from the top of the liquid level in order to collect volatile samples for 30 min.After absorption,the analysis step was the same as described above.Products were identified by comparison of their retention times and mass spectra with those of authentic standards analyzed under the same conditions.Three biological replicates were performed in the SPME-GC-MS analysis.The external standard method was used to calculate the relative contents of artemisinic acid in the collected samples.Standard artemisinic acid solutions of 1,10,50,100 and 1 000 ng μL-1were prepared.Based on an external standard method,three injections of each concentration of standard solution were analyzed to determine the linearity and range(Fig.1).The content of a target compound was determined according to its peak area (Rajanaet al.2019).
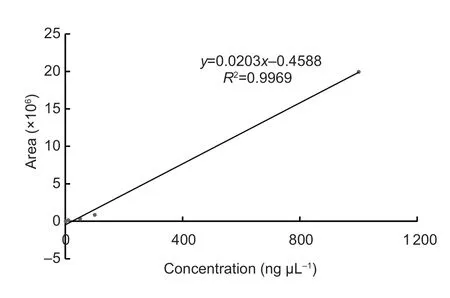
Fig.1 Linear relationship between peak area and concentration of artemisinic acid.
2.3.RNA extraction and cDNA synthesis
Total RNA ofA.annuatop leaves was extracted using the RNAprep Pure Kit (Tiangen Biotech Beijing Co.,Ltd.,China) following the manufacturer’s protocol.The RNA quality and quantity were checked using 1.2%agarose gel electrophoresis and a NanoPhotometer®N60(IMPLEN,Munich,Germany).cDNA was synthesized using the FastQuant RT Kit (Tiangen Biotech Beijing Co.,Ltd.) according to the manufacturer’s instructions.The synthesized cDNA templates were stored at -20°C until further use.
2.4.Vector construction and heterologous expression
The open reading frame (ORF) of targetcyp71av1was amplified using gene-specific primers containing restriction sites (XbaI-F: GCTCTAGAATGAA GAGTATACTAAAAGCAATGG;BamHI-R: CGGGATCC CTAGAAACTTGGAACGAGTAACA).The amplified product was then inserted into the pYES2 plasmid containing thecprgene (Fig.2).The empty vector pYES2 and the recombinant construct (cpr-pYES2-cyp71av1)were transformed into the INVSc1 strain ofS.cerevisiae,and then the transformed cells were screened on SC-U selection medium.A single colony containing the recombinant construct was inoculated in 10 mL of SC-U liquid medium at 30°C in a shaking incubator at 280 r min-1until the OD600of the culture reached 1.0.Then,the cells were cultured in induction medium containing 2% galactose until the OD600of the culture reached 0.4.Subsequently,the cells were set at 30°C in a shaking incubator at 280 r min-1to express the recombinant protein for 24 to 48 h.

Fig.2 The pYES2 vector map.
2.5.Enzymatic activity assays
The standard chemicals artemisinic acid (CAS no.80286-58-4),artemisinic alcohol (CAS no.125184-95-4)and artemisinic aldehyde (CAS no.125276-60-0) were purchased from Toronto Research Chemicals,Canada.Enzymatic catalysis by recombinant CYP was assayed in glass vials.The reaction systems included: (A) 5.0 mL of resuspended culture harboring yeast cells containing empty pYES2 plasmid+10.0 μL of 1.0 mmol L-1artemisinic aldehyde+5.0 μL of dimethylsulfoxide (DMSO);(B) 5.0 mL of resuspended culture harboring yeast cells containing empty pYES2 plasmid+10 μL of 1.0 mmol L-1artemisinic alcohol+5.0 μL of DMSO;(C) 5.0 mL of resuspended culture harboring recombinant CYP+10.0 μL of 1.0 mmol L-1artemisinic aldehyde+5.0 μL of DMSO;and (D)5.0 mL of resuspended culture harboring recombinant CYP+10.0 μL of 1.0 mmol L-1artemisinic alcohol+5.0 μL of DMSO.The reactions were incubated at 30°C for 4 h,and terminated by adding HCl to a final concentration of 0.05 mol L-1(Leeet al.2010).The SPME fiber was inserted into a glass vial to 1.0 cm from the top of the liquid level in order to collect volatile samples for 1 h at 30°C.SPME-GC-MS analysis was performed as described above.
2.6.EAG recordings
EAGs were used to record the electrophysiological responses of the antennae of male and femaleA.lucorumto artemisinic acid,artemisinic alcohol and artemisinic aldehyde.The three compounds were dissolved in Triton X-100 (Sigma-Aldrich,USA) at a concentration of 10 mg mL-1.cis-3-Hexenol was used as a reference chemical,and Triton X-100 was used as a control.Antennae from three-day-old male and female adult bugs were carefully removed at the base,and a few terminal segments at the distal end were removed.Then,the treated antennae were connected to electrode holders with electrode gel.Twenty microliters of odor solution was applied to a piece of folded filter paper (0.5 cm×5 cm) and placed into a glass Pasteur pipette.The antenna was exposed to a constant charcoal-filtered humid air flow (300 mL min-1)through a metal tube for 0.3 s.Eight replicates were carried out for each sex,and the sequence of exposures for each antenna was random.The interval between replications was at least 30 s to ensure antennal recovery.EAG signals were recorded and analyzed using Syntech Intelligent Data Acquisition Controller and EAGPro V.2(Syntech,Kirchzarten,Germany),respectively (Sunet al.2013).In the dose-response test,adult antennae were exposed to a series of ascending concentrations (0.01,0.1,1,10 and 100 mg mL-1) with an interval between exposures that allowed for antennal recovery.The EAG response was calculated as: EAG relative value=(EAG value of compound-EAG value of control)/(EAG value of reference-EAG value of control)×100
2.7.Indoor olfactometer assay
To investigate the behavioral effects of artemisinic acid,artemisinic alcohol and artemisinic aldehyde on adults ofA.lucorum,indoor behavioral assays were carried out in a three-cage olfactometer designed by our laboratory (Khashavehet al.2020).The olfactometer consists of three transparent plexiglass cages (each cage 25 cm×25 cm×25 cm) connectedviatwo 15 cm funnelshaped arms.The air,which is filtered by activated carbon,flows into the side cages of the treatment(T) and the control (C) at a rate of 0.5 L min-1,and discharges from the central or release cage (R).The three compounds were dissolved in Triton X-100 at a concentration of 10 mmol L-1,and Triton X-100 was also used as a blank control.One hundred microliters of test compound or Triton X-100 was applied to a cigarette filter dispenser,and then the dispenser was placed in the T or C cage.For each compound,one test was repeated three times for each sex,and 40 adults were released into cage R in each replicate.The number of insects moving from cage R to cage C or T was recorded for 4 h.The behavioral index value (BIV) was calculated based on the following formula: BIV=[(C-T)/(C+T)]×100,where C and T stand for the numbers of adult bugs in the C and T cages,respectively.According to the BIV,the behavioral activities of the tested components were categorized in four groups: no response,BIV<10%;weak,10≤BIV<30%;moderate,30%≤BIV<70% and strong,BIV≥70%.A compound with a negative BIV was considered to be an attractant.On the other hand,one with a positive BIV was regarded as a repellent.
2.8.Choice behavior in a net cage
In the greenhouse,a two-choice assay in nylon net cages (100 cm×50 cm×75 cm) was performed,imitating field conditions.A pot containing one cotton plant with 6-7 leaves was placed on each side of the cage.A cigarette filter dispenser was placed on each plant.The selected compound was diluted to a concentration of 10 mmol L-1in Triton X-100.One hundred microliters of chemical solution was dropped onto a cigarette filter which was then hung on the cotton plants at one end of a net cage.One dispenser containing 100 μL of Triton X-100 was hung on cotton plants at the other end of the net cages as a control.For each compound,one test was repeated three times for each sex,and 60 three-day-old adultA.lucorumwere placed in the center of the net cage at 8:00 a.m.The numbers of bugs on the cotton plants were counted every hour for 6 h.
2.9.Data analysis
SPSS STATISTICS 18.0 Software (SPSS Inc.,Chicago,IL,USA) was used for all statistical analyses.All data are presented as the mean±standard deviation (SD)and were transformed prior to analysis as needed.For comparisons between means,analysis of variance(ANOVA) was conducted,followed by Tukey’s honestly significant difference (HSD) test (P<0.05).Student’st-test (P<0.05) was used for comparisons of the recorded EAG values between males and females.In the net cage trial,at-test was performed to analyze the choice of bugs between treatments and controls.
3.Results
3.1.Emission of artemisinic acid from A.annua
GC-MS analysis showed that approximately 11.03±6.00 ng h-1artemisinic acid was detected in the HS-SPME samples fromA.annuaplants,whereas neither artemisinic alcohol nor artemisinic aldehyde was found in the volatile samples ofA.annuaplants (Fig.3;Table 1).When SPME was used to absorb the volatiles from the milledA.annualeaves,more artemisinic acid was detected,approximately 238.25±121.67 ng h-1.Similarly,neither artemisinic alcohol nor artemisinic aldehyde was found in the milledA.annualeaves (Fig.4;Table 1).

Fig.3 GC-MS analysis of artemisinic aldehyde and artemisinic alcohol in Artemisia annua samples.A,artemisinic aldehyde in volatile samples from total A.annua plants.I,gas chromatogram of authentic artemisinic aldehyde;II,gas chromatogram of volatile samples from A.annua;III,gas chromatogram of volatile samples from an empty jar (blank control).B,artemisinic alcohol in volatile samples from total A.annua plants.I,gas chromatogram of authentic artemisinic alcohol;II and III are the same as described in A.C,artemisinic aldehyde in volatile samples from milled A.annua leaves.I,gas chromatogram of authentic artemisinic aldehyde;II,gas chromatogram of volatile samples from milled A.annua leaves;III,gas chromatogram of volatile samples from an empty glass vial (blank control).D,artemisinic alcohol in volatile samples from milled A.annua leaves.I,gas chromatogram of authentic artemisinic alcohol;II and III are the same as described in C.①,artemisinic aldehyde;②,artemisinic alcohol.

Fig.4 GC-MS analysis of artemisinic acid in Artemisia annua samples.A,artemisinic acid in volatile samples from whole plants of A.annua.I,gas chromatogram of authentic artemisinic acid;II,gas chromatogram of volatile samples from A.annua;III,gas chromatogram of volatile samples from an empty jar (blank control).B,artemisinic acid in volatile samples from milled A.annua leaves.I and II are the same as described in A;III,gas chromatogram of volatile samples from an empty glass vial (blank control).C,mass spectrum of artemisinic acid.①,artemisinic acid.
3.2.Catalytic activities of CYP71AV1
SPME-GC-MS was employed to determine the enzymatic activities of the recombinant CYP71AV1 protein.When artemisinic aldehyde was added,CYP71AV1 catalyzed the conversion of the substrate to produce 56.16±1.53 ng h-1artemisinic acid (Fig.5;Table 1).When artemisinic alcohol was added,the recombinant CYP71AV1 also catalyzed the reaction to produce 47.54±7.77 ng h-1artemisinic acid (Fig.6;Table 1).However,when artemisinic aldehyde or artemisinic alcohol were added,only the culture harboring yeast cells containing the empty pYES2 plasmid failed to catalyze the conversion to artemisinic acid.

Fig.5 GC-MS analysis of artemisinic acid produced by recombinant CYP71AV in Saccharomyces cerevisiae with added artemisinic aldehyde.I,gas chromatogram of authentic artemisinic acid;II,gas chromatogram of volatiles produced by empty vector pYES2 with added artemisinic aldehyde;III,gas chromatogram of volatiles produced by recombinant CYP71AV1 with added artemisinic aldehyde.①,artemisinic aldehyde;②,artemisinic acid.

Fig.6 GC-MS analysis of artemisinic acid produced by recombinant CYP71AV in Saccharomyces cerevisiae with added artemisinic alcohol.I,gas chromatogram of authentic artemisinic acid;II,gas chromatogram of volatiles produced by empty vector pYES2 with added artemisinic alcohol;III,gas chromatogram of volatiles produced by recombinant CYP71AV1 with added artemisinic alcohol.①,artemisinic alcohol;②,artemisinic acid.
3.3.EAG recordings
The EAG results showed that all three selected compounds elicited electrophysiological responses in the antennae of males and females at different concentrations.As expected,the EAG values demonstrated dose-dependent effects of all three chemicals.There were no significant differences between males and females in the EAG responses to the selected chemicals at concentrations of 10 mg mL-1(Fig.7).

Fig.7 EAG values of female and male Apolygus lucorum exposed to artemisinic acid,artemisinic aldehyde and artemisinic alcohol.Bar indicates SD (n=8).ns,no significant difference.
3.4.Olfactometer assay
According to the behavioral index values (BIV),the three tested compounds were classified as attractants.Because the BIV response range observed in this study was 32.2-42.9%,all chemicals were considered to be only moderate attractants (Table 2).Statistical analysis indicated no significant differences between the BIVs of the different chemicals.

Table 1 Theamounts of art emisinic acid (ng h-1) emitted from different samples1)
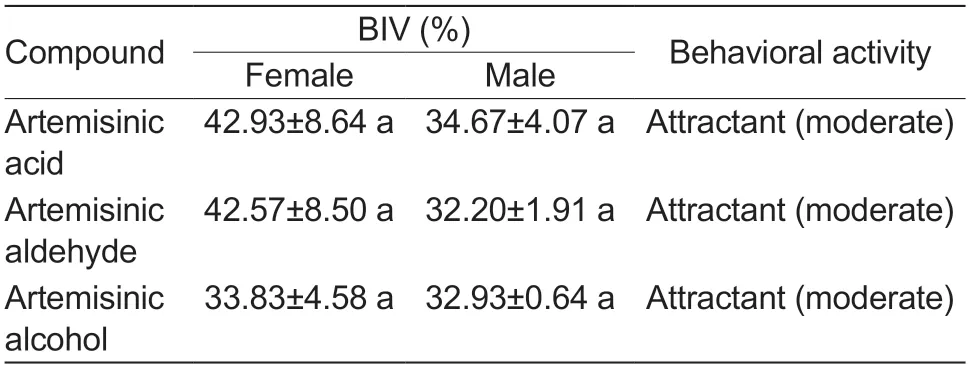
Table 2 Behavioral index values (BIVs) of male and female Apolygus lucorum adults exposed to different compounds at a concentration of 10 mmol L-1 in a 3-cage olfactometer
3.5.Behavioral trial in the net cage
In the net cage,both male and female adults ofA.lucorumshowed an obvious preference for artemisinic acid dispensers (male:P=0.026;female:P=0.024).In addition,maleA.lucorumhad a preference for artemisinic aldehyde (P=0.015),while females did not (P=0.075).Interestingly,femaleA.lucorumshowed a preference toward the artemisinic alcohol dispenser (P=0.002),while males did not (P=0.131) (Fig.8).

Fig.8 Behavioral responses of female (A) and male (B) Apolygus lucorum adults to the three compounds in net cages.Bar indicates SD (n=3).*,significant difference at the 0.05 level (P<0.05);ns,no significant difference.
4.Discussion
Due to the low volatility of artemisinic acid,we collected volatile samples using the SPME method.After SPME adsorption,artemisinic acid can be collected and analyzed.When the leaf samples were ground,more artemisinic acid was detected.Thus,sample grinding is conducive to the emission of low-volatility compounds,such as artemisinic acid.Artemisinic alcohol and artemisinic aldehyde were never found in any of the collected volatile samples.The possible reasons may be two-fold.(1) Artemisinic aldehyde is a joint intermediate that can follow two branch biosynthesis pathways: direct oxidization to artemisinic acid by aldehyde dehydrogenase or reduction to dihydroartemisinic aldehyde and then oxidation to dihydroartemisinic acid by aldehyde dehydrogenase (Zenget al.2020).The amounts of artemisinic alcohol and artemisinic aldehyde are almost negligible during their transit through these biosynthesis pathways (Shenet al.2012).(2) The catalytic reaction from artemisinic alcohol to artemisinic acid is accomplished in the glandular hairs on the leaves ofA.annua(Juddet al.2019).In this study,we collected volatiles fromA.annuaat the seedling stage.At this time,the glandular hairs ofA.annuahave not fully grown,which could have affected the collection of volatile samples.
CYP71AV1 catalyzes the three-step oxidation reaction of amorpha-4,11-diene to artemisinic alcohol,artemisinic aldehyde and artemisinic acid (Shibaet al.2007).Additionally,co-expression with CPR is essential for allowing CYP to perform its catalytic activities (Pomponet al.1996).InArabidopsis,CYP71A13 without CPR and NADPH could not convert indole-3-acetaldox-ime to indole-3-acetonitrile (Kleinet al.2013).In this study,a pYES2 vector and INVScI yeast-expressing cells were employed to co-express CYP71AV1 and CPR.Recombinant CYP71AV1 catalyzed the production of artemisinic acid from artemisinic alcohol or artemisinic aldehyde.Interestingly,the amounts of artemisinic alcohol and artemisinic aldehyde are almost negligible during this process,and even artemisinic aldehyde cannot be detected (Shenet al.2012).Consequently,when artemisinic alcohol was used as the substrate,artemisinic aldehyde was not detected in our enzymatic reaction system.The recombinant CYP71AV1 produced less artemisinic acidinvitrothan in the milled volatile samples.However,recombinant CYP71AV1 can produce artemisinic acid continuously when the substrate supply is sufficient.Thus,we need to improve the efficiency of the enzyme catalysis system.
Many plant volatiles have been found to affect behavior in insects (Bruceet al.2005;Schneeet al.2006).In the present study,both female and male adults ofA.lucorumdemonstrated stronger EAGs to artemisinic acid,artemisinic alcohol and artemisinic aldehyde.In addition,the indoor olfactometer assay and the choice behavior in the net cage trial further showed that artemisinic acid and its two precursors,artemisinic alcohol and artemisinic aldehyde,had moderate attractiveness toA.lucorum.These results were mutually verified with the previously reported finding thatA.lucorumobviously prefersA.annuain autumn.Artemisinic acid shows the highest content in glandular hairs ofA.annuaat the early flowering stage in autumn(Liet al.2021),andA.lucorumhas a habit of seasonal host transfer (Panet al.2021).
There were some differences between the behavioral preferences of male and female individuals toward the chemicals in the net cage trial.This phenomenon may indicate that different host plant volatiles have different signal clues to male and female bugs.Male bugs use phytochemical information to feed and find partners,while females use plant cues to feed,rest or lay eggs.Overall,artemisinic acid and its two precursors,artemisinic alcohol and artemisinic aldehyde,could be used to design potential attractants for the control ofA.lucorum.
5.Conclusion
In this work,artemisinic acid was detected inA.annuasamples.The pYES2 vector and INVScI yeast expression cells were successfully employed to express CYP71AV1.Recombinant CYP71AV1 catalyzed the production of artemisinic acid from either artemisinic alcohol or artemisinic aldehydeinvitro.Artemisinic acid and its two precursors showed attractiveness to the mirid bugA.lucorum.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31772176 and 31972338) and the National Key Research and Development Program of China (2019YFD0300100).This manuscript has been edited by the native English-speaking experts of Elsevier Language Editing Services.
Declaration of competing interest
The authors declare that they have no conflict of interest.
 Journal of Integrative Agriculture2022年10期
Journal of Integrative Agriculture2022年10期
- Journal of Integrative Agriculture的其它文章
- Responses of leaf stomatal and mesophyll conductance to abiotic stress factors
- Genetic dissection of the grain-filling rate and related traits through linkage analysis and genome-wide association study in bread wheat
- Expression profiling of transgenes (Cry1Ac and Cry2A) in cotton genotypes under different genetic backgrounds
- Linkage and association mapping of wild soybean (Glycine soja)seeds germinating under salt stress
- Genome-wide identification,expression and functional analysis of sugar transporters in sorghum (Sorghum bicolor L.)
- lnhibition of miR397 by STTM technology to increase sweetpotato resistance to SPVD
