Wumei pills attenuates 5-fluorouracil-induced intestinal mucositis through Toll-like receptor 4/myeloid differentiation factor 88/nuclear factor-κB pathway and microbiota regulation
Dong-Xue Lu, Feng Liu, Hua Wu, Hai-Xia Liu, Bing-Yu Chen, Jing Yan, Yin Lu, Zhi-Guang Sun
Abstract
Key Words: Wumei pills; Gut flora; Intestinal mucosal inflammation; Mucosal barrier; Short-chain fatty acids; Intestinal permeability
lNTRODUCTlON
Chemotherapy plays an important role in the comprehensive treatment of malignant tumors and can greatly improve the survival rate of patients with cancer. Although chemotherapy kills tumor cells, it also damages normal cells and induces serious gastrointestinal toxicity; this results in gastrointestinal mucositis, which has been associated with bone marrow suppression, nausea and vomiting, and diarrhea[1]. The gastrointestinal toxicity of chemotherapy seriously affects the treatment process and prognosis of cancer patients[2,3]. Chemotherapy-induced intestinal mucositis (CIM) is more common in patients receiving 5-fluorouracil (5-Fu), irinotecan, methotrexate, cisplatin, and cytarabine[4,5]. It has been reported that the incidence of mucositis is 40% after conventional chemotherapy and can increase up to 80% with high-dose or continuous chemotherapy. Villus atrophy, crypt goblet cell reduction,intestinal epithelial cell hypoplasia, and mucosal barrier dysfunction are the main pathological changes associated with intestinal mucositis[6,7]. Some studies have confirmed that an imbalance in the intestinal flora can be seen in CIM[8-10]. Therefore, regulating the intestinal flora may be a new therapeutic target for alleviating intestinal mucositis. The treatment of CIM in modern medicine is mainly through combating inflammation and oxidative stress,but the clinical effect of these strategies is not so satisfactory. Thence, there is an urgent need to identify drugs that can effectively treat intestinal mucositis. Increasing evidence shows that traditional Chinese medicine has a significant effect in improving the side effects of chemotherapy, especially with regard to gastrointestinal reactions[11-13].
Wumei pills (WMP) was originally documented in the Treatise on Exogenous Febrile Disease (200-210, AD), and it comprises ten herbs:FructusMume [Prunus mume(Sieb.) Sieb. et Zuce, WM],Angelicae sinensisRadix [Angelica sinensis(Oliv.) Diels, DG], Asari Radix Et Rhizoma (Asarum sieboldii Miq, XX),Aconiti Lateralis Radix Praeparata (Aconitum carmichaelii Debx, FZ), Phellodendri chinensis cortex (Phellodendron chinense Schneid., HB), Zanthoxyli Pericarpium (Zanthoxylum bungeanum Maxim, HJ),Cinnamomi Ramulus (Cinnamomum cassiaPresl, GZ), Radix Ginseng (Panax ginseng C. A. Meyer, RS),Rhizoma Coptidis (Coptis chinensis Franch., HL), and Rhizoma Zingiberis (Zingiber oj-jicinale Rosc., GJ). It is a classically prescribed traditional Chinese medicine for the treatment of a variety of digestive system diseases and significantly treats diarrhea, upper abdominal pain, and loss of appetite[14,15].Preliminary clinical studies have shown that WMP has a significant effect on chemotherapy-induced diarrhea[16,17]. It has been reported that WMP is a safe and effective formula. Moreover, WMP has shown significant clinical benefits for digestive diseases, such as ulcerative colitis[18], immune colitis,and irritable bowel syndrome[19-21]. It can also exert an anti-hyperglycemic effect by adjusting the intestinal flora, improving the inflammatory response, and increasing the levels of short-chain fatty acids (SCFA)[22]. However, there is little information on the effects of WMP on CIM. As inflammation and microbiota play significant roles in intestinal mucositis, we hypothesized that WMP might affect CIM in mice. Therefore, we examined the effect of WMP on intestinal mucositis, intestinal mucosal barrier, and intestinal flora in a CIM mouse model. The results of this study may provide new treatment strategies of using traditional Chinese preparations for the treatment of CIM.
MATERlALS AND METHODS
Materials
WMP (WM 24 g, DG 6 g, XX 9 g, FZ 9 g, HB 9 g, HJ 6 g, GZ 9 g, RS 15 g, HL 24 g, and GJ 15 g) was purchased from the Chinese Pharmacy of Jiangsu Provincial Hospital of Traditional Chinese Medicine(Nanjing, China). 5-Fu was obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (CAS: 51-21-8,Shanghai, China, Lot #: N09M10W82518) and FITC-dextran (FD4, 250 mg) was purchased from Sigma-Aldrich Co. LLC (Shanghai, China, Lot #: BCCC2153). Primary antibodies against rabbit Toll-like receptor 4 (TLR-4) (A5258), zonula occludens-1 (ZO-1) (21773-1-AP), mucin-2 (F-2) (sc-515032), GAPDH(AP0063), myeloid differentiation factor 88 (MyD88) (4283S), and nuclear factor-κB (NF-κB) p65 (8242S)were purchased from Abclonal (United States), Proteintech (United States), Santa Cruz Biotechnology(United States), and Bioworld (China). Primary antibodies against mouse claudin-1 (71-7800) and Ecadherin (14472s) were purchased from Invitrogen (United States) and Cell Signaling Technology(Danvers, MA/CST, United States). Goat anti-mouse immunoglobulin G (IgG) (H&L ab6708) and goat anti-rabbit IgG (H&L ab6717) were obtained from Shanghai Abcam Co., Ltd. (Shanghai, China). The primers used to amplify the interleukin-6 (IL-6) (forward: Lot No: 1922401831; reverse: Lot No:1922401832),IL-1β(forward: Lot No:1922401835; reverse: Lot No:1922401836), tumor necrosis factor-α (TNF-α) (forward: Lot No: 1922401833; reverse: Lot No: 1922401834), and myeloperoxidase (MPO)(forward: Lot No: 1922401837; reverse: Lot No: 1922401838) were purchased from Sangon Biotech Co.,Ltd. (Shanghai, China). The TRIzol@Reagent (No. 15596018) was obtained from Thermo Fisher Scientific Co., Ltd. (Shanghai, China); the HiScript II Q RT SuperMix for qPCR (+gDNA wiper) (No. R22) was purchased from Vazyme Biotech Co., Ltd. (Nanjing, China); and the iQTM RT SYBR@Green Supermix for qPCR (No. 170-8880AP) was purchased from BIO-RAD Co., Ltd. (Shanghai, China). All the reagents and chemicals were used for study only.
Animals
BABL/C mice (aged 6-8 wk, weighing 18 ± 2 g, male) were obtained from the Shanghai SLAC Laboratory Animal Co., Ltd. (Production License: SCXK: 2017-0005, Shanghai, China). Mice were kept under specific pathogen-free conditions (12 h light/12 h dark cycle, 45%-55% relative humidity, and temperature of 26-28 °C) with free access to food and clean water. The study protocols were approved by the Animal Experimental Ethics Committee of Nanjing University of Chinese Medicine (No.202006A022) and carried out on the basis of the animal ethics standards of the Nanjing University of Chinese Medicine Laboratory Animal Ethics Committee. The animal certificate number is 20170005027187. All reasonable efforts were made to minimize animal suffering.
Experimental design
After 7 d of adaptive feeding, the BALB/C mice were divided randomly into four groups (n= 10 per group): Control group, 5-Fu group (40 mg/kg), and WMP treatment groups (WMP-11.325, 11325 mg/kg; WMP-22.650, 22650 mg/kg). Except for the control mice, 5-Fu was administered to the mice through intraperitoneal injection once daily for the first 5 d. In addition, the vehicle or different doses of WMP were intragastrically administered to the mice during the experiment. After the treatment, the mice were fasted for 12 h, and they were anesthetized and sacrificedviacervical dislocation. The experimental process is illustrated in Figure 1.
Assessment of mucositis and sample collection
Indicators of disease severity, including body weight, food intake, and diarrhea severity, were evaluated daily for the assessment of mucositis. Diarrhea severity was classified into five grades according to stool consistency[23]: (1) 0, normal; (2) 1, slightly wet; (3) 2, moderate wet; (4) 3, loose; and (5) 4, watery stool.Jejunal and colonic tissues were removed quickly and douched with clean cold saline, and then the length of the colon was measured. The samples (1 cm × 1 cm) were immersed in 4 % paraformaldehyde for 24 h and embedded in paraffin for histopathological, immunohistochemical, and immunofluorescence analyses and Alcian blue staining. Blood samples were collected and centrifuged at 12000 rpm for 10 min at 4 °C. The residual intestinal tissue samples were stored at -80 °C and used for biochemical analysis. The feces were also collected from the colon/rectum of the mice to perform a microbiota analysis.
Histopathological assessment
The sections (3.0-μm thick) cut from paraffin blocks and stained with hematoxylin and eosin (H&E)were used for measuring the intestinal mucosal damage and inflammatory cell infiltration with a light microscope. Histopathological scores were calculated on the basis of a previous study[24].
Immunohistochemical analysis
The paraffin sections were dewaxed with xylene and absolute ethanol, washed with phosphate-buffered saline (PBS, pH 7.4), and blocked with 5% bovine serum albumin. Then, the paraffin sections were incubated with an anti-TLR4 antibody (1:600), anti-MyD88 antibody (1:600), or anti-NF-κB p65 antibody(1:600) overnight at -4 °C and incubated with an anti-rabbit secondary antibody the next day. The slides were stained with 3,3-diaminobenzidine (DAB) and counterstained with hematoxylin. The sections were observed with a light microscope.
Intestinal permeability
The toxic effects of chemotherapy may weaken the intestinal epithelial barrier, allowing the contents of the lumen to pass through the paracellular space into the blood. The animals were subjected to fasting from 9 pm on the first day and denied access to water for 12 h. On the second day, FITC-dextran (50 mg/mL) was orally administered to the animals according to their body weight (0.1 mL/10 g). After 3 h,isoflurane anesthesia was administered and the mice were investigated using IVIS Luminaseries II in Living Imaging (480 nm excitation wavelength, 520 nm emission wavelength). After the mice woke up,blood was obtained from the orbit and the serum was separated. A microplate reader was used to measure the serum fluorescence value (480 nm excitation wavelength, 520 nm emission wavelength)and FITC-dextran standards were used to prepare a standard curve for the leakage of FITC in the serum.
Alcian blue staining
The paraffin sections were dewaxed with xylene and absolute ethanol and oxidized with 0.5% periodate solution, followed by incubation with Alcian blue acidification solution for 3 min and staining solution for 30 min. Next, the sections were washed with running water for 5 min, and the slides were stained with nuclear fast red staining solution. The sections were observed under a light microscope and photographed.
Immunofluorescence
Paraffin sections dewaxed with xylene and absolute ethanol were used for antigen retrieval. They were incubated with anti-mucin-2 antibody (1:500), anti-ZO-1 antibody (1:500), anti-claudin-1 antibody(1:500), and anti-rabbit secondary antibody (FITC-labeled, 1:200). The slides were stained with 4’,6-diamidino-2-phenylindole in the dark for 10 min. The sections were observed under a light microscope.
Total RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted from the jejunum and colon/rectum and reverse transcribed into cDNA using the HiScript II Q RT SuperMix according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted by using the ChamQ Universal SYBR qPCR Master Mix in an Applied Biosystems Step-One Fast Real-Time PCR system. The reaction program was as follows: Pre-denaturation at 95 °C for 30 s, 40 cycles at 95 °C for 10 s and 60 °C for 30 s, followed by annealing at 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s. The primer sequences used for amplifying the genes encoding the target inflammatory factors IL-1β, IL-6, TNF-α, and MPO are listed in Table 1.The relative quantification of the target genes was carried out by the 2-ΔΔCtmethod, and GAPDH wasused as the internal control.

Table 1 Primer sequences

Figure 1 Flow chart of modeling and drug delivery. WMP: Wumei pills; 5-Fu: 5-fluorouracil.
Western blot analysis
The total proteins of the intestinal tissue were extracted with a protein extraction kit. Briefly, 100 mg of tissue was grinded with prepared protein lysis solution (RIPA lysis solution:Phosphatase inhibitor:1%PMSF = 100:1:1), and centrifuged at 12000 rmp for 10 min at 4 °C, and 10 μL of supernatant was used for protein quantification with the NanoDropTM One/OneC and the other used for western blot analysis.Protein samples were separated on 6% to 12% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes. The protein-loaded PVDF membranes were blocked for 2 h in 5% skim milk and incubated with primary antibodies overnight at 4 °C and then with secondary antibodies for 1.5 h according to the manufacturer’s instructions. Protein expression was detected using a western blot enhanced chemiluminescence substrate, and the results were compared using the ImageJ software. The working dilutions of the antibodies against the following proteins were prepared: TLR4, 1:600; MyD881:600; NF-kB, 1:1000; ZO-1, 1:1000; claudin-1, 1:1000; E-cadherin, 1:1000;and GAPDH, 1:20000. A GAPDH antibody was used for verifying the equal loading of the samples of different groups.
16S ribosomal DNA gene sequence analysis
To perform 16S rRNA sequencing, we pooled the feces from six mice in each group (control, 5-Fu, 5-Fu+ WMP 11325, and 5-Fu + WMP 22650 groups). Microbial DNA was extracted from 24 samples using the E.Z.N.A.®soil DNA Kit (Omega Bio-tek, Norcross, GA, United States) according to the manufacturer’s protocols. The final DNA concentration and purification were determined using a NanoDrop 2000 UV-VIS spectrophotometer (Thermo Scientific, Wilmington, United States), and DNA quality was checked through 1% agarose gel electrophoresis. The V3-V4 hypervariable regions of the bacterial 16S rRNA gene were amplified with the primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’)and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) using a thermocycler PCR system (GeneAmp 9700,ABI, United States). The resulting PCR products were extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States). The products were quantified using QuantiFluor™-ST (Promega, United States) according to the manufacturer’s protocol, and purified amplicons were pooled in equimolar amounts and subjected to paired-end sequencing (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, United States)according to the standard protocols of Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Quantification of SCFA
The fecal content (50 mg) of each mouse was placed into a 2-mL centrifuge tube, followed by the addition of 1.0 mL NaOH. The solution was homogenized for 10 min and centrifuged at 13000 rpm for 10 min. Next, 600 μL of supernatant was added to a centrifuge tube with 10 μL of caproic acid-d3, and the mixture was vortexed. The mixture (500 μL) was added to a 10-mL centrifuge tube, followed by the addition of 300 μL of ultrapure water; the mixture was then vortexed. Next, 500 μL of 1-propanol:Pyridine (3:2, v/v) and 100 μL of propyl chloroformate were added; the mixture was vortexed for 30 s and sonicated for 1 min. After derivatization, 300 μL ofn-hexane was added to extract the derivatized material, followed by vortexing for 1 min and centrifugation at 2000 rpm for 10 min. The upper layer of the solution (200 μL) was placed in a 1.5-mL centrifuge tube and 200 μL ofn-hexane was added to the centrifuge tube, vortexed for 1 min, and centrifuged at 2000 rpm for 10 min. The upper layer solution(200 μL) from the previous step was vortexed twice. After centrifugation at 18000 rpm for 10 min, the supernatant was filtered through a 0.22 μm membrane filter. SCFAs were quantified using gas chromatography-mass spectrometry (GCMS-QP2010, Shimadzu, Japan) equipped with an Rtx-5MS capillary column (30 m × 0.25 mm × 0.25 μm). For mass spectroscopy, an electron bombardment ionization source, a full scan, and a scanning mode were adopted. The electron energy was 70 eV.
Statistical analysis
Raw data related to intestinal flora were analyzed on the free online platform of Majorbio Cloud Platform (www.majorbio.com). Data are presented as the mean ± SDE of the mean. Differences between two groups were analyzed with GraphPad Prism 8.3. One-way analysis of variance was used for the comparison of more than two groups. The metabolites were filtered by variable influence on projection(VIP) selection from the PLS-DA and the filtering conditions were VIP > 1 andP< 0.05. In all cases,statistical significance was set atP< 0.05 (aP< 0.05,bP< 0.01,cP< 0.001 anddP< 0.0001, compared with the normal group;eP< 0.05,fP< 0.01,gP< 0.001, andhP< 0.0001, compared with the 5-Fu group).
RESULTS
Effect of 5-Fu
After intraperitoneal injections of 5-Fu, the mice exhibited mild to severe diarrheal symptoms including wet and watery stools, reduced movement, weight loss, and reduced appetite. The changes in colon length, spleen weight, body weight, and appetite, as well as the incidence of diarrhea were regarded as preliminary indices[25], and these changes are shown in Figures 2A-I. The colon length was significantly shortened, and the spleen weight was remarkedly decreased in the mouse models. At the beginning of the experiment, all the mice had similar body weights and appetites, with no diarrhea. In the experiment period, there was no diarrhea and the body weight and food intake gradually increased in the control group mice. However, for the mice that received 5-Fu, the colon length was significantly shortened, the spleen weight markedly decreased, the appetite reduced, and the body weight continuously declined. Serious diarrhea was observed in the mouse models after day 3. WMP increased the colon length of mice in the WMP-11325 mg/kg and WMP-22650 mg/kg groups in a dose-dependent manner. The mice showed increased appetites, and their body weights recovered after day 7. Notably,the diarrhea scores of the mice in the WMP-11325 mg/kg and WMP-22650 mg/kg groups began to decrease by day 8, although they still had severe diarrhea.
Histopathological assessment of the colon and jejunum
Morphological changes in the colon and jejunum were observed, as shown in Figures 2J-M. H&E staining showed that 5-Fu damaged the intestinal epithelium to different degrees, and the most significant epithelial damage was observed in the jejunal tissue (Figures 2L-M). There were no histological abnormalities in the control group, whereas huge ulcers with inflammatory cell infiltration,atrophied glands, and shortened villus length were observed in the jejunal epithelial tissues of the model group mice. The damage was milder in the colon mucosa (Figures 2J-K), which manifested as a small number of inflammatory cells infiltrating the mucosal layer, accompanied by shortened villus length. This condition was alleviated after WMP treatment. WMP effectively accelerated the recovery of injury in a dose-dependent manner. Model mice in the 22650 mg/kg WMP group presented wellordered glands, increased villus length, and a few impairments like those observed in the control mice.
Effect of WMP on inflammatory cytokines
To evaluate the effects of WMP on inflammation, the mRNA levels of the inflammatory cytokinesTNF-α, IL-1β, IL-6, andMPOin colonic and jejunal tissues were investigated using qRT-PCR (Figures 2N-O). In both tissues, the mRNA levels of the inflammatory cytokines were significantly elevated (P< 0.05) in the 5-Fu group. This condition was alleviated by WMP treatment. WMP effectively decreased the mRNA levels of inflammatory cytokines in a dose-dependent manner. Mice in the 22650 mg/kg WMP group showed lower mRNA levels of inflammatory cytokines (P< 0.05) than those in the 5-Fu group.


Figure 2 Effect of Wumei pills on routine observation, histopathological assessment (n = 10, 200 ×), and inflammatory cytokines on intestinal mucositis in mice. A and B: Colon length; C: Spleen weight; D and E: Body weight; F and G: Food intake; H and I: Diarrhea scores; J and K:Histopathological assessment of the jejunum; L and M: Histopathological assessment of the colon; N: Levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, IL-10, and myeloperoxidase (MPO) in the jejunum; O: Levels of TNF-α, IL-1β, IL-6, IL-10, and MPO in the colon. Values represent the mean ± SEM. aP <0.05, bP < 0.01 vs control group and eP < 0.05, fP < 0.01 vs 5-fluorouracil group. WMP: Wumei pills; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6; IL-1β:Interleukin-1β; MPO: Myeloperoxidase; 5-Fu: 5-fluorouracil.
Effect of WMP on inflammatory proteins
The western blot analysis of the changes in the TLR4/MyD88/NF-κB pathway proteins following WMP administration (Figures 3A-D) showed that 5-Fu administration significantly increased the expression of the TLR4, MyD88, and NF-κB proteins. Although 11325 mg/kg WMP administration had a mild effect,22650 mg/kg WMP markedly decreased the levels of TLR4, MyD88, and NF-κB proteins to almost normal levels. To further investigate the effect of WMP on the expression of TLR4/MyD88/NF-κB,immunohistochemical analysis was performed to detect protein expression (Figures 3E and 3F).Compared to that in the normal group, the 5-Fu group had significantly higher levels of TLR4/MyD88/NF-κB (P< 0.01). In contrast, the expression of TLR4/MyD88/NF-κB in the WMP group decreased in a dose-dependent manner.
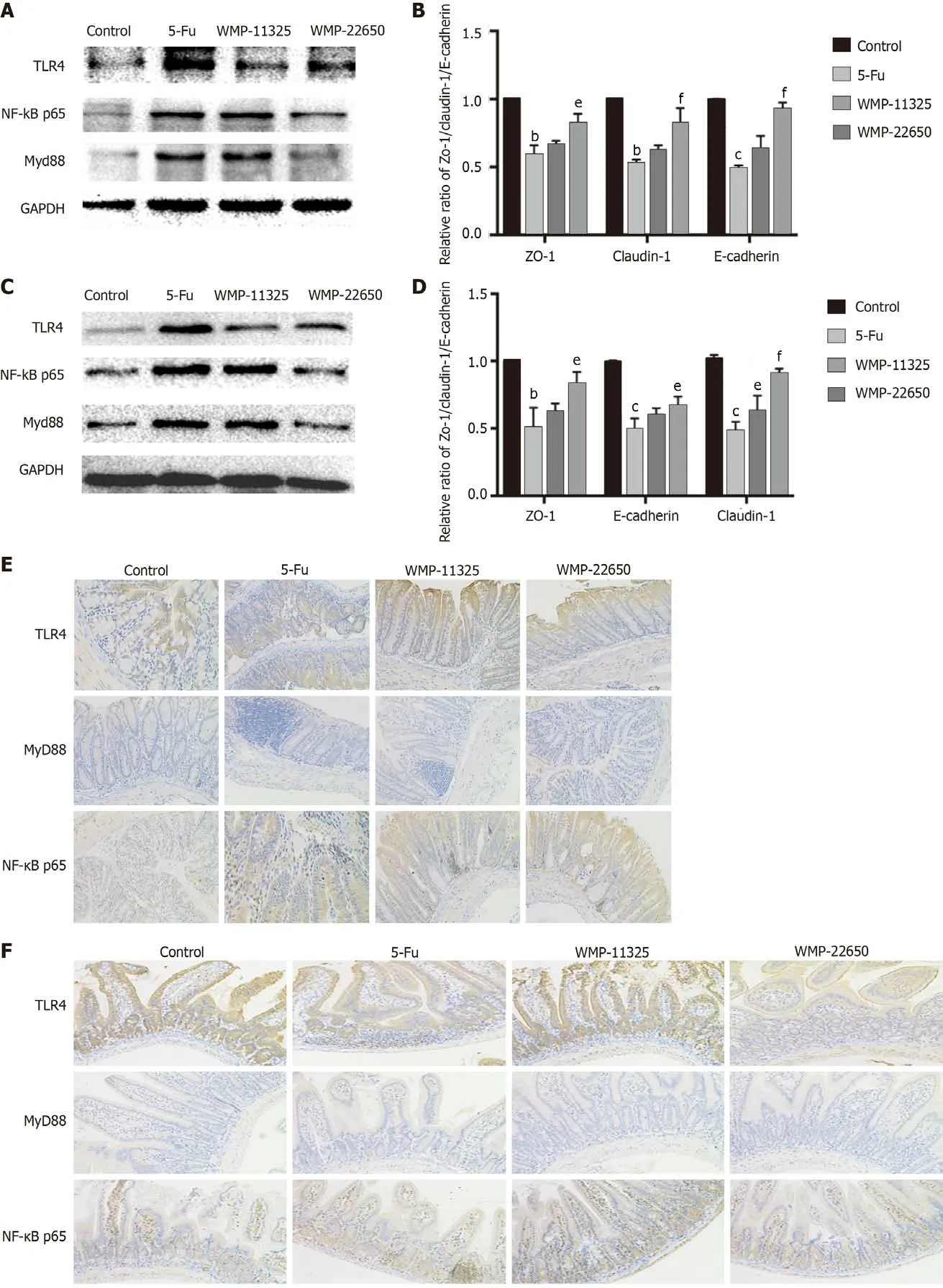
Figure 3 Effect of Wumei pills on expression of Toll-like receptor 4/myeloid differentiation factor 88/nuclear factor-κB pathway proteins as demonstrated by western blot and immunohistochemistry staining. A and B: Expression of Toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), and nuclear factor-κB (NF-κB) proteins in the jejunum; C and D: Expression of TLR4, MyD88, and NF-κB proteins in the colon; E: Expression of TLR4,MyD88, and NF-κB proteins in the colon; F: Expression of TLR4, MyD88, and NF-κB proteins in the jejunum. WMP: Wumei pills; TLR4: Toll-like receptor4; MyD88:Myeloid differentiation factor 88; NF-κB: Nuclear factor-κB; 5-Fu: 5-fluorouracil.
Effect of WMP on intestinal mucosal barrier
To evaluate the effects of WMP on the integrity of the intestinal mucosal barrier, we performed a FITCdextran leakage experiment (Figures 4A and 4B). Dextran leakage was significantly greater in the 5-Fu group than in the control group (P< 0.05). WMP effectively prevented dextran leakage. The serum FITC-dextran concentration decreased in the WMP group, while the model group had significantly higher FITC-dextran concentration than the control group.
Effect of WMP on intestinal mucosal barrier proteins
Western blot analysis of the changes in tight junction proteins (ZO-1, claudin-1, and E-cadherin) after WMP administration (Figures 4C-F) showed that 5-Fu administration significantly decreased the expression of the ZO-1, Claudin-1, and E-cadherin proteins (P< 0.05,vscontrol group), which was reversed by the administration of 11325 mg/kg and 22650 mg/kg WMP. To further estimate the effect of WMP on tight junction proteins, their expression levels were assessed using immunofluorescence(Figures 4G-J). The results showed that 5-Fu significantly reduced the expression of ZO-1, claudin-1,and E-cadherin; however, WMP treatment significantly increased the expression of these proteins to levels similar to those in the normal group.
Effect of WMP on the synthesis and secretion of mucin-2
To determine whether WMP affects the synthesis of mucin-2 in the model mice, Alcian blue staining and mucin fluorescent staining were conducted, and the number of goblet cells (Figures 4K-N) was determined. The thickness of the mucus layer (concentration of mucin-2) in the 5-Fu group was lower than that in the control mice (P< 0.01), suggesting that the goblet cells of the mice in the 5-Fu group were severely damaged. However, there was a significant increase in the mucus layer thickness (P<0.05,vs5-Fu group) after treatment with 11325 mg/kg WMP and 22650 mg/kg WMP.
Changes in microbial composition and diversity
We next assessed the richness and diversity of the microbiota by alpha diversity analysis (Figures 5A and 5B). Four indices were used to analyze microbial diversity, including the Shannon, Ace, Chao, and Simpson indices. The 5-Fu group mice had the lowest Ace, Chao, and Shannon indexes, but with a higher Simpson index; the results also revealed that the richness and diversity of the microbiota community in mice of the 5-Fu group were remarkably reduced compared with those in the control group. These adverse effects were ameliorated after WMP administration. The Ace, Chao, and Shannon indices increased while the Simpson index decreased in the WMP groups.
To evaluate the structure of the gut microbiota of each group, beta diversity analysis was performed and the results are presented in Figure 5C. Principal component analysis showed that compared to the other three groups, the structure of the gut microbial community in the 5-Fu group showed the highest diversity, whereas in the WMP groups, the diversity was reduced, and the values between those of the 5-Fu group and the 22650 mg/kg WMP group were much closer to those between the control group and the 11325 mg/kg group. These results indicate that WMP can increase microbial diversity and stabilize the microbial community.
Taxonomic analysis of microbiota community
The relative abundance of the classification units of the microbiota community is presented as a stacking histogram (Figures 5D-I). The Kruskal-Wallis test was used to analyze the difference in the microbial communities of the groups at different levels, and the results showed that at the phylum level, all samples containedBacteroidetes,Proteobacteria,Firmicutes,Verrucomicrobia,Campilobacterota,Patescibacteria,Desulfobacterota, andActinobacteria. The most abundant phyla wereProteobacteria,Bacteroidetes, andFirmicutes. 5-Fu led to a marked increase in the relative abundance ofBacteroidetesandCampilobacterota,but a decrease in that ofFirmicutes. The abundance ofFirmicutesincreased and that ofBacteroidetesdecreased in the 22650 mg/kg WMP group compared with the 5-Fu group (Figure 6A).
The data showed thatBacilli,Bacteroidia, andClostridiawere the major classes in the control mice,whereas the abundance ofBacilliwas significantly decreased and those ofBacteroidiaandClostridiawere increased in the 5-Fu group (Figure 6B). After WMP treatment, the number ofBacilliin the 11325 mg/kg WMP group was not significantly different, whereas in the 22650 mg/kg WMP group, a significant decrease was observed.BacteroidiaandClostridiawere decreased in the WMP group, but compared with the model group,Bacteroidiawere decreased more significantly in the 22650 mg/kg WMP group, andClostridiawere decreased more significantly in the 11325 mg/kg WMP group.
At the order level,Lactobacillales,Bacteroidales, andLachnospiraleswere the most abundant in the control mice, whileStaphylococciwere the most abundant in the 5-Fu mice (Figure 6C). At the family level,LactobacillaceaeandBacteroidaceaewere significantly decreased, andStaphylococcalesandMuribaculaceaewere increased after 5-Fu treatment. WMP administration effectively increased the abundance ofLactobacillaceaeandBacteroidaceaein the model mice and decreased the abundance ofStaphylococcalesandMuribaculaceae(Figure 6D). WMP increased the abundance ofLactobacillaceaein a dose-dependent manner. In the 11325 mg/kg WMP group, the abundance ofBacteroidaceaewas significantly increased. A total of 20 genera were detected in all the samples (Figure 6E). We found thatHelicobacterwas the characteristic bacterium in the 5-Fu group. In addition, mice in the 5-Fu group showed higher relative abundances ofStaphylococcales,Muribaculaceae, andLachnospiraceaethan the control mice (Figure 6F).

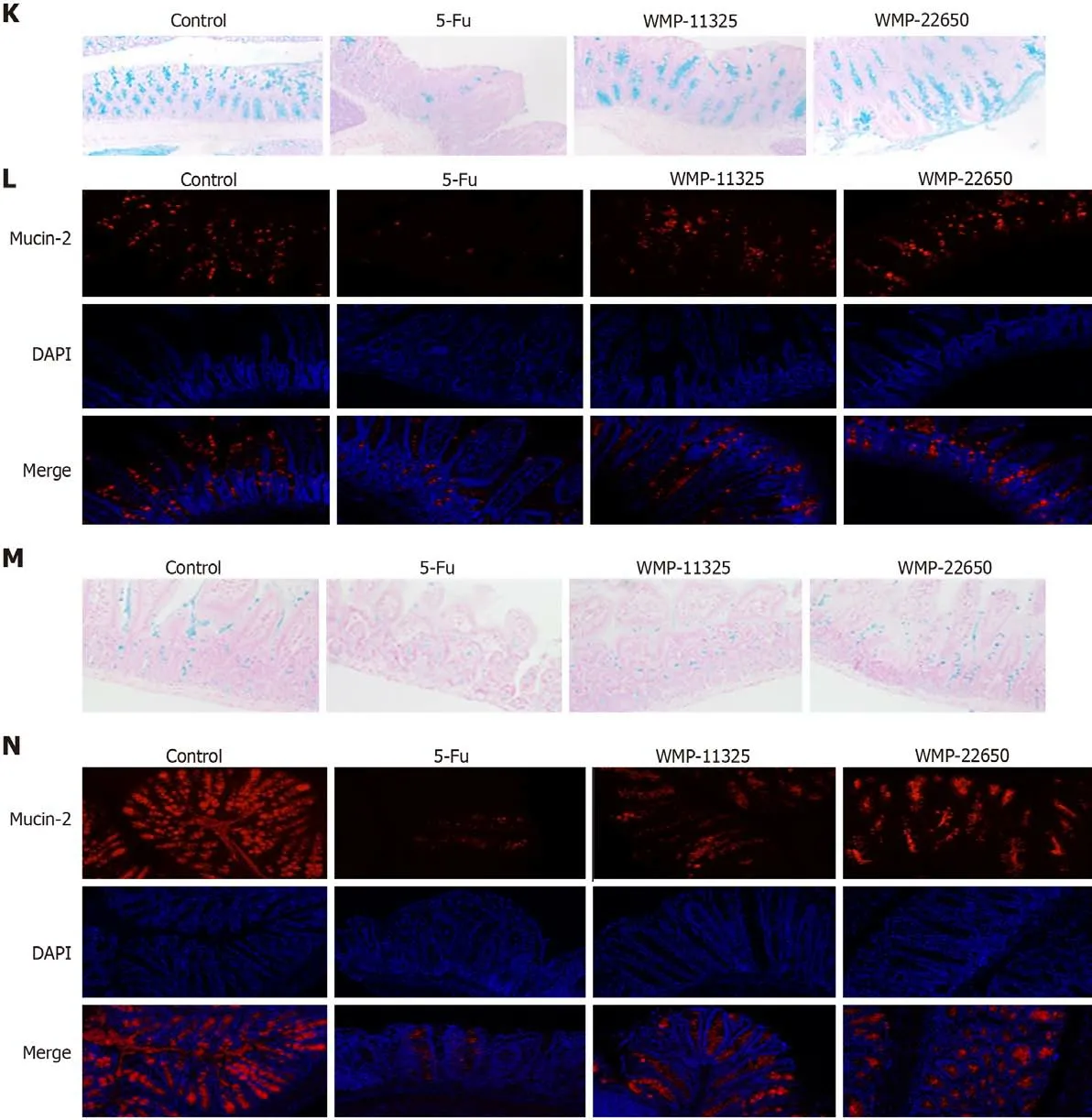
Figure 4 Effect of Wumei pills on intestinal leakage and expression of mucosal barrier proteins. A: Representative fluorescent images of mice assessed by IVIS. Excitation: 480 nm; emission: 520 nm; B: Concentration of dextran measured by mean fluorescence intensity of FITC in serum; C and D:Expression of zonula occludens-1 (ZO-1), E-cadherin, and claudin-1 proteins in the jejunum; E and F: Expression of ZO-1, E-cadherin, and claudin-1 proteins in the colon; G and H: Expression of ZO-1 and claudin-1 proteins in the jejunum; I and J: Expression of ZO-1 and claudin-1 proteins in the colon; K and L: Alcian blue staining and immunofluorescence staining for mucin-2 in the jejunum; M and N: Alcian blue staining and immunofluorescence staining for mucin-2 in the jejunum.Values represent the mean ± SEM. bP < 0.01, cP < 0.001, dP < 0.0001 vs control group and eP < 0.05, fP < 0.01, hP < 0.0001 vs 5-fluorouracil group. WMP: Wumei pills; ZO-1: Zonula occludens-1; 5-Fu: 5-fluorouracil.
LEfse and microbiota-cytokine correlation analysis
We used LEfse analysis to test the specific bacteria present in all groups. In view of the cladogram(Figure 6G), some specific bacteria were selected (Figure 6H) at the phylum level. Each group was tested for several specific bacteria. In general,Lactobacillus,Verrucomicrobiota, andAkkermansiawere the three major genera associated with the control group, whileHelicobacter,Muribaculaceae, andCampilobacterotawere the major genera associated with the 5-Fu group.Firmicuteswas significantly increased in the 22650 mg/kg WMP group due to the alleviation of intestinal mucositis in this group. However, in the 11325 mg/kg WMP group,Bacteroideteswas the main genus, which may be related to the LDA threshold or the concentration of WMP. In addition, Spearman analysis was used to analyze the association between microbiota and inflammatory cytokines (Figure 6I). The results indicated that the abundance ofLactobacillus,Akkermansia,Escherichia coli, andRumbutzianegatively correlated with the expression of inflammatory cytokines, whereasPrevotella,Isovaleribacter, andHelicobacter pyloripositively correlated with the expression of inflammatory cytokines.
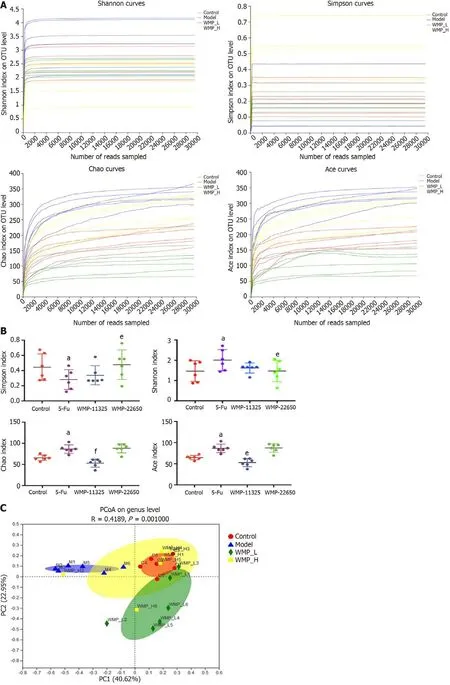

Figure 5 Microbial alpha, beta diversity, and taxonomy analysis. A: Dilution curve analysis; B: Alpha diversity (Shannon index, Simpson index, Ace index, and Chao index); C: Beta diversity; D-I: Taxonomy analysis of microbiota community. Values represent the mean ± SEM. aP < 0.05 vs control group and eP <0.05, fP < 0.01 vs 5-Fu group. WMP: Wumei pills; 5-Fu: 5-fluorouracil.
Taxonomic analysis was used to detect specific genera in all the samples (Figures 7A and 7B), which showed that the relative abundance of the probioticsLactobacilluswas reduced in the 5-Fu group compared with that in the control group. The abundance of pathogenic bacteria, such asMuribaculaceaeandStaphylococcales, increased following the 5-Fu injection. WMP administration both increased the relative abundance ofLactobacillusand decreased the relative abundance ofMuribaculaceaeandStaphylococcales.
SCFAs in fecal samples
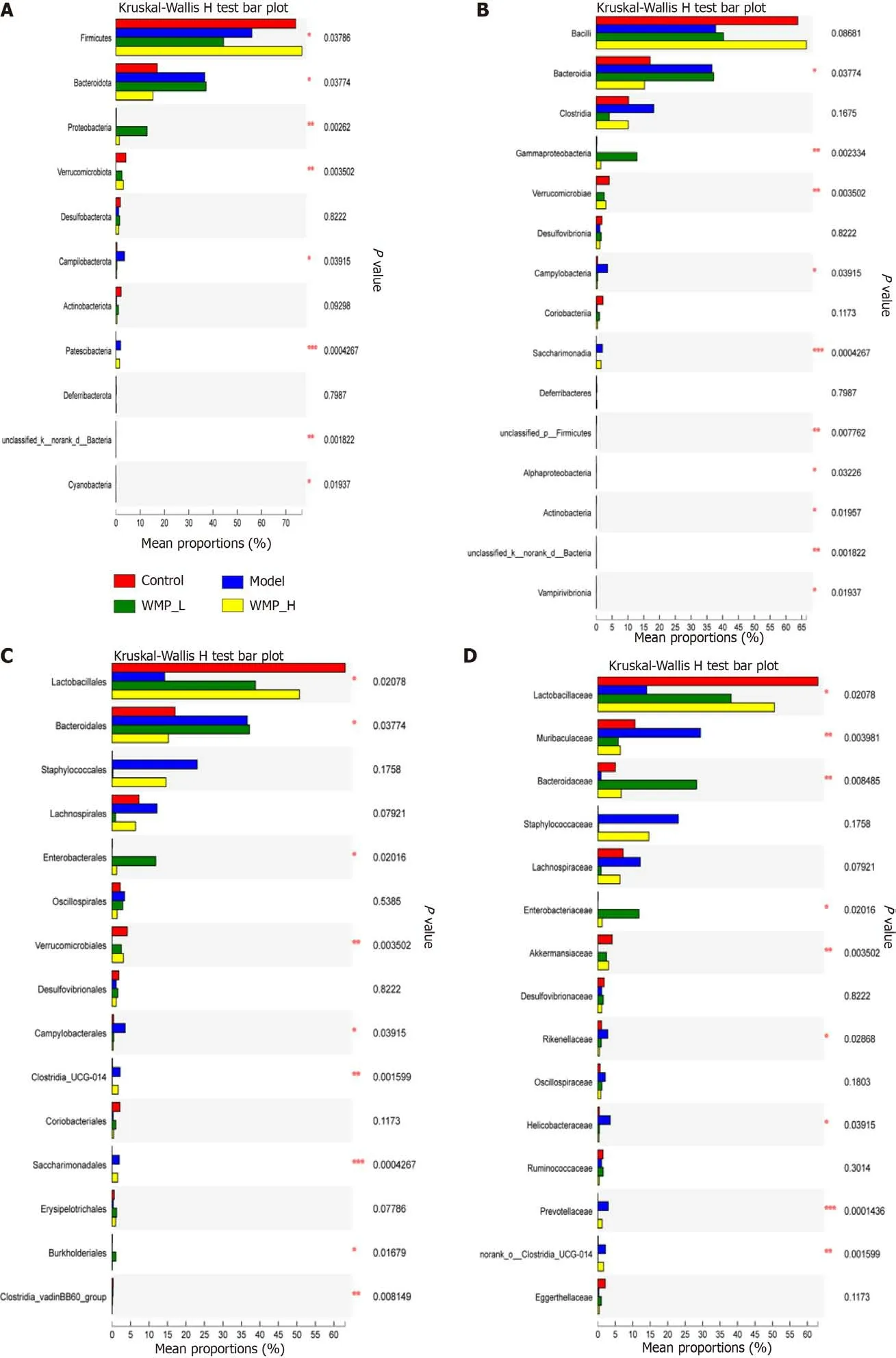


Figure 6 Kruskal-Wallis analysis of different bacterial groups, LEfse analysis, and microbiota-cytokine correlation. A: Phylum level; B: Class level; C: Order level; D: Family level; E: Genus level; F: Species level; G: Overall exhibition of LEfse analysis by cladogram; H: LDA scores of the specific enriched genera in each group; I: Spearman analysis of microbiota-cytokine correlation. WMP: Wumei pills; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6; IL-1β:Interleukin-1β; MPO: Myeloperoxidase.
The levels of SCFA are closely related to the barrier function of the intestinal epithelium. To assess the effect of 5-Fu and WMP treatments on microbial metabolites, the levels of SCFAs, such as acetate,propionate, butyrate, isobutyrate, isobutyrate, 2-methyl-butyrate, and valerate, in the feces were determined (Figure 7C). The levels of acetate, propionate, butyrate, 2-methyl-butyrate, and valerate markedly decreased in the feces of mice treated with 5-Fu, compared with those in the feces of the control mice (P< 0.05). WMP treatment significantly increased the levels of acetate, propionate, and butyrate, but had no significant effect on the levels of isobutyrate and isovalerate. In addition, the results showed that WMP restored the abundance ofLactobacillaceae, which is the main family that produces SCFA (Figure 7D).
DlSCUSSlON
Traditional Chinese medicine prescriptions are an important source of new drugs for the prevention and treatment of gastrointestinal toxicity caused by chemotherapy drugs[25]. Drug interaction is considered to play a vital role in the combination of traditional Chinese medicine and modern medicine[26]. WMP originated from the Treatise on Exogenous Febrile Disease (200-210 AD), comprising ten herbs: WM, DG, XX, GZ, FZ, HB, HJ, RS, GJ, and HL. According to previous reports, WMP has various pharmacological effects on gastrointestinal tract diseases, such as ulcerative colitis, immune enteritis,and irritable bowel syndrome[19-21]. In this study, we found that WMP effectively improved the weight, food intake, spleen weight, and diarrhea scores of mice with intestinal mucositis. In addition,WMP significantly reduced 5-Fu-induced morphological damage to the jejunum and colon, which manifested as the shortening of villi height, destruction of crypts, and inflammatory cell infiltration.This study explored the protective effect of WMP and its associated mechanism on intestinal mucosal damage caused by 5-Fu.
The mechanism of the pathogenesis of intestinal mucositis is complicated. Inflammation, intestinal barrier dysfunction, and imbalance of the intestinal flora can cause mucosal damage[27-29]. Inflammation is involved in intestinal mucositis, and TLRs play a key role in regulating the intestinal epithelial barrier and innate immunity. TLR4 induces NF-κB activation, thereby controlling the expression of inflammatory cytokine genes through the Toll/IL-1R domain with the specific adaptor protein, MyD88[30-32]. NF-κB is a key regulator of innate and adaptive immune responses, and is involved in the regulation of inflammation and cell cycle[33]. After the treatment with 5-Fu, the NF-κB pathway was activated, leading to the release of inflammatory factors. Moreover, the secretion of pro-inflammatory cytokines (such as TNF-α) increased and caused the infiltration of granulocytes and promoted intestinal barrier damage. In addition, it has been demonstrated that MPO, a marker of neutrophil infiltration,participates in the inflammatory process by causing oxidation in the inflammatory area[34]. Changes in the levels of inflammatory cytokines have been reported to be regulated by NF-κB p65[35]. NF-κB is a universal transcription factor involved in the inflammatory response, and studies have confirmed that it plays an important role in the pathological mechanism of CIM[36,37]. Therefore, the inhibition of the NF-κB signaling pathway may be an important factor in reducing intestinal mucosal damage. IL-1β, IL-6, and TNF-α are vital pro-inflammatory cytokines that widely affect the inflammatory process[38-40].IL-1β and TNF-α have been reported to exhibit a synergistic effect on the activation of the NF-κB pathway and inflammation[41].IL-6 exhibits a range of biological effects,especially those related to the pathogenesis of inflammatory diseases[42]. In addition, under inflammatory conditions, the protein level and activity of MPO increase[43]. The results of this study also revealed that 5-Fu induced an increase in the expression of proteins associated with the TLR4-MyD88 signaling pathway and inflammatory cytokines, which was significantly inhibited by WMP. WMP significantly decreased the expression levels of TNF-α, IL-1β, IL-6, and MPO in mice with intestinal mucositis and inhibited the increase in TLR4 levels induced by 5-Fu. The expression of the downstream proteins, including MyD88 and NF-κB, was also significantly downregulated. The inhibitory effect of WMP on the TLR4-MyD88 signaling pathway suggests that this pathway may be involved in the alleviation of intestinal mucositis by WMP.
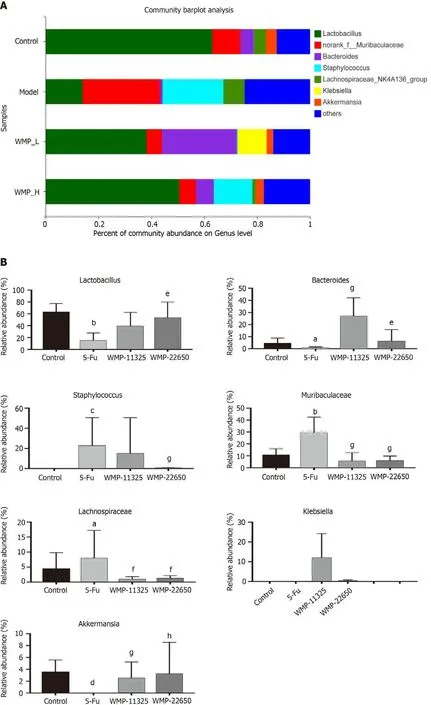

Figure 7 Effect of Wumei pills on taxonomy analysis of specific genera, the contents of short-chain fatty acids in feces, and heatmap of gut microbiota. A: Relative abundance of specific genera at each sample; B: Relative abundance of Lactobacillus, Bacteroides, Staphylococcus, Muribaculaceae,Lachnospiraceae, Klebslella, and Akkermansla in each group; C: Effect of Wumei pills on the contents of short-chain fatty acids in feces; D: Heatmap of gut microbiota (family level). Values represent the mean ± SEM. aP < 0.05, bP < 0.01, cP < 0.001 and dP < 0.0001, compared with the normal group; eP < 0.05, fP < 0.01, g P < 0.001, and hP < 0.0001, compared with the 5-Fu group. WMP: Wumei pills; 5-Fu: 5-fluorouracil.
In addition to inflammation, damage to the intestinal epithelial barrier is one of the mechanisms of CIM. The dysfunction of the integrity of the intestinal mucosal barrier (mainly the tight junctions,mucus layer, and intestinal epithelial cells) plays an significant role in the pathogenesis of chemotherapy-induced intestinal toxicity and related diarrhea[44]. Tight junctions maintain the structure and function of the intestinal mucosal barrier by sealing the paracellular space of the intestinal epithelium; this is destroyed under inflammatory conditions[45,46]. Tight junctions comprise core proteins, which are the most adhesive junction complexes in mammalian epithelial cells. The lack of tight junction increases intestinal permeability and contributes to the development of intestinal inflammation[47,48]. Therefore, through the FITC-dextran leakage experiment, we found that WMP reduced intestinal penetration in mice treated with 5-Fu. Furthermore, through western blot and immunofluorescence, we found that the tight junction protein (ZO-1, claudin-1, and E-cadherin) expression levels in the 5-Fu group were significantly reduced and that WMP was able to reverse these changes and increase the expression levels of the tight junction proteins. This effect was more significant in the 22650 mg/kg WMP group than in the 11325 mg/kg group.
In addition to the epithelial barrier, a mucus barrier is also present. The mucus barrier is composed of a hydrated gel covering the surface of the epithelial mucosa, which is formed by the mucin synthesized and secreted by special epithelial cells (such as intestinal goblet cells). This forms a barrier that prevents the direct contact between the large particles (including most bacteria) and the epithelial cell layer[49].For instance, unlike normal mice, mucin-2-knockout mice developed severe colitis following exposure to harmful stimuli[49,50]. In addition, these mice developed colitis at the same time, and the microbiota was detected in the deep sterile intestinal crypts. By using Alcian blue staining and immunofluorescence to analyze the synthesis and secretion of mucin-2 in goblet cells, we found that the level of mucin-2 synthesis or secretion in goblet cells in the 5-Fu group was lower, indicating that 5-Fu had no serious damage to goblet cells. After the intragastric administration of WMP, we observed a gradual recovery of the biological function of goblet cells, an increase in the synthesis of mucin-2, and an improvement of the intestinal mucosal barrier.
An increasing number of studies have shown that the intestinal flora plays a key role in intestinal inflammation-related diseases, such as antibiotic-related diarrhea, irritable bowel syndrome,radiotherapy, and chemotherapy-related diarrhea[51-54]. The dynamic balance of the intestinal flora is a key factor affecting gastrointestinal health, and gastrointestinal diseases disrupt the dynamic balance of the intestinal flora. Some studies have shown that WMP or its active ingredients can affect the inflammatory response by regulating the intestinal flora[3,22,55]. However, there is no direct evidence proving that WMP can relieve 5-Fu-induced intestinal mucositis by influencing the intestinal flora. Therefore, we used 16S rDNA amplification sequencing to analyze the composition and abundance of the intestinal flora in stool samples. According to the analysis of microbial diversity, the intestinal flora of mice in the 5-Fu group changed significantly. After WMP treatment, the intestinal flora of mice in the 5-Fu group gradually tended towards healthy intestinal microbes, and the abundance of lactobacilli was significantly increased. The 22650 mg/kg WMP group had a better effect. In addition, the intestinal flora can regulate the changes in inflammatory cytokines[56], which is consistent with our results. The composition of the intestinal flora in the WMP group negatively correlated with inflammatory factors,while that in the 5-Fu group showed the opposite trend.
Bacteroides,Lactobacillus,Staphylococcus,Muribculaceae,Helicobacter pylori, andParabacteriaplay an important role in the development of intestinal mucositis.Lactobacilluscan enhance TJ protein expression, inhibit NF-κB activation, and reduce intestinal permeability[57-60].Bacteroides,Staphylococcus,Muribculaceae,Helicobacter pylori, andParabacteriaare generally considered pathogenic or conditional pathogens. For instance, elevated levels ofParabacteriaandBacteroidescan induce colitis in mice[61], andHelicobacter pyloriinfection can cause peptic ulcer disease and gastric cancer[62].Methicillin-resistantStaphylococcus aureusoften colonizes the rectum and is associated with necrotizing enterocolitis[63,64]. Bacteroides S24-7 (Muribaculaceae) is the dominant bacterial family of Bacteroides,and its increase is closely related to inflammatory enteritis[65]. In this study, 5-Fu administration activated NF-κB, inhibited tight junction protein expression, and increased inflammatory cytokines and intestinal permeability. The relative abundance ofLactobacilliin the 5-Fu group markedly dropped.However, 22650 mg/kg WMP exerted a stronger effect than 11325 mg/kg WMP on the relative abundance ofLactobacilli. SCFA-producing bacteria have beneficial effects on the host by inhibiting inflammation, providing nutrition for colon cells, and protecting the intestinal mucosa[66]. As an SCFAproducing bacterium,Lactobacilluscan lower the pH of the host colonic cells and inhibit the growth of pathogenic bacteria[67]. Therefore, this study preliminarily confirmed that WMP can change the composition and abundance of the intestinal flora and exert anti-inflammatory effects.
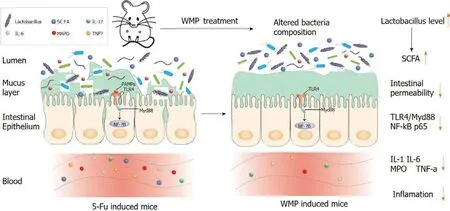
Figure 8 Schematic illustration of mechanisms for Wumei pills treatment in 5-fluorouracil-induced intestinal mucositis. WMP: Wumei pills;TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6; IL-1β: Interleukin-1β; MPO: Myeloperoxidase; SCFAs: Short-chain fatty acids; TLR4: Toll-like receptor 4; MyD88:Myeloid differentiation factor 88; NF-κB: Nuclear factor-κB; 5-Fu: 5-fluorouracil.
The functions of the intestinal microflora, including immune regulation, nutritional support of the colonic epithelium, and maintenance of stable barrier function, are closely related to SCFA. A previous study reported the involvement of metabolites in the development of intestinal mucositis[68]. It has been reported that SCFA protect the intestinal mucosa by reducing the level of inflammation[69]. SCFA can be absorbed by fluids that cause sodium decomposition through a process independent of circulating AMP. Therefore, the reduction in SCFA is related to diarrhea[70]. Butyrate reduces diarrhea by enhancing intestinal permeability[71]. In 5-Fu-induced diarrhea, the production of SCFAs was significantly reduced. Previous studies have found that WMP can significantly increase the abundance of butyric acid-producing bacteria (Firmicutes) in 5-Fu treated mice. Therefore, the results of this study were consistent with those of previous studies. WMP significantly changed the fecal SCFA content in 5-Fu treated mice. It increased the concentrations of acetic acid, propionic acid, and butyric acid,indicating that WMP can improve the production of SCFA and inhibit the progression of chemotherapyinduced intestinal mucosal inflammation.
CONCLUSlON
In summary, our results demonstrate the beneficial effects and the associated mechanisms of WMP against CIM in mice (Figure 8). WMP activates the NF-κB pathway by restraining p65 translocation from the cytoplasm to the nucleus and inhibiting the expression of TLR4/MyD88/NF-κB proteins,resulting in the downregulation of expression of the inflammatory factors IL-1β, IL-6, MPO, and TNF-α,and increased expression of tight junction proteins (ZO-1, claudin-1, E-cadherin, and mucin-2).Moreover, WMP restores the diversity and abundance of the intestinal flora. WMP increases the relative abundance ofLactobacillibut decreases that ofBacteroides,Helicobacter, andParabacteroides. Although further fecal bacterial transplantation or the downstream-mediated potential mechanisms of specific bacteria are worth exploring, WMP has potential therapeutic effects against CIM.
ARTlCLE HlGHLlGHTS
Research background
Radiotherapy and chemotherapy can kill tumor cells and improve the survival rate of cancer patients.However, they can also damage normal cells and cause serious intestinal toxicity, leading to gastrointestinal mucositis. Traditional Chinese medicine is effective in improving the side effects of chemotherapy. Wumei pills (WMP) is a classic prescription in Treatise on Febrile Diseases. It has a significant effect on chronic diarrhea and other gastrointestinal diseases, but it is not clear if it has an effect on chemotherapy-induced intestinal mucositis (CIM).
Research motivation
To explore the potential mechanism of WMP in the treatment of CIM using experimental research.
Research objectives
Exploring the mechanism of WMP to alleviate chemotherapy-induced intestinal mucosal inflammation may be to regulate the intestinal flora, thereby inhibiting the activation of downstream inflammatory signaling pathways and exerting anti-inflammatory effects.
Research methods
We used an intraperitoneal injection of 5-fluorouracil (5-Fu) to establish a mouse model of CIM and oral gavage of WMP decoction (11325 and 22650 mg/kg) to evaluate the efficacy of WMP in CIM. We evaluated the effect of WMP on CIM by observing the general conditions of mice (body weight, food intake, spleen weight, diarrhea score, and hematoxylin and eosin staining). To explore the potential mechanism of WMP in CIM, the expression of the inflammatory factors tumor necrosis factor-α (TNF-α),interleukin- 6 (IL-6), IL-1β, and myeloperoxidase (MPO), Toll-like receptor 4/myeloid differentiationfactor 88/nuclear factor-κB (TLR4/MyD88/NF-κB) signaling pathway proteins, and tight junction proteins [zonula occludens-1 (ZO-1), claudin-1, E-cadherin, and mucin-2] was determined. Furthermore,intestinal permeability, intestinal flora, and the levels of short-chain fatty acids (SCFA) were also assessed in the mice.
Research results
WMP effectively improved the body weight, spleen weight, food intake, diarrhea score, and inflammatory pathological status of mice with intestinal mucositis, which preliminarily confirmed the efficacy of WMP in CIM. Further experiments showed that WMP did not only reduce the levels of TNF-α, IL-1β,IL-6, and MPO, and inhibited the expression of TLR4/MyD88/NF-κB, but also repaired the integrity of the mucosal barrier of mice, regulated the intestinal flora, and increased the levels of SCFA (such as butyric acid).
Research conclusions
In summary, our results demonstrate the beneficial effects of WMP against CIM in mice and the associated mechanisms. WMP activates the NF-κB pathway by restraining p65 translocation from the cytoplasm to the nucleus and inhibiting the expression of TLR4/MyD88/NF-κB proteins, resulting in the downregulated expression of inflammatory factors (IL-1β, IL-6, MPO, and TNF-α) and increased expression of tight junction proteins (ZO-1, claudin-1, E-cadherin, and mucin-2). Moreover, WMP restores the diversity and abundance of the intestinal flora. In particular, WMP increases the relative abundance ofLactobacillibut decreases the relative abundance ofBacteroides,Helicobacter,andParabacteroides.
Research perspectives
WMP has potential therapeutic effects against CIM, and further fecal bacterial transplantation or the downstream-mediated potential mechanisms of specific bacteria are worth exploring.
ACKNOWLEDGEMENTS
We would like to thank all the team members for their assistance during data collection and compiling the manuscript. We are also grateful to all the members of the faculty and staff of the College of Foreign Languages who have supported and guided us throughout this study.
FOOTNOTES
Author contributions:Lu DX and Liu F contributed to the study design, data analysis and interpretation, and writing of the manuscript; Yan J contributed to the study design, data analysis, and drafting of the manuscript; Lu Y, Chen BY, Liu HX, and Wu H were responsible for data collection and analysis.
Supported bythe National Natural Science Foundation of China, No. 81673795.
lnstitutional animal care and use committee statement:All animal experiments were approved by Animal Experimental Ethics Committee of Nanjing University of Chinese Medicine (No. 202006A022).
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
ARRlVE guidelines statement:The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Dong-Xue Lu 0000-0002-8206-2509; Feng Liu 0000-0003-0331-4067; Hua Wu 0000-0002-6377-5810; Hai-Xia Liu 0000-0003-4541-3469; Bing-Yu Chen 0000-0002-0105-3978; Jing Yan 0000-0003-3616-072X; Yin Lu 0000-0003-2603-8485; Zhi-Guang Sun 0000-0002-1641-8888.
S-Editor:Wang JJ
L-Editor:Wang TQ
P-Editor:Guo X
 World Journal of Gastroenterology2022年32期
World Journal of Gastroenterology2022年32期
- World Journal of Gastroenterology的其它文章
- The mechanism of Yinchenhao decoction in treating obstructivejaundice-induced liver injury based on Nrf2 signaling pathway
- Anoctamin 5 regulates the cell cycle and affects prognosis in gastric cancer
- Effects of Granule Dendrobii on chronic atrophic gastritis induced by N-methyl-N'-nitro-N-nitrosoguanidine in rats
- Machine learning predicts portal vein thrombosis after splenectomy in patients with portal hypertension: Comparative analysis of three practical models
- Sirolimus increases the anti-cancer effect of Huai Er by regulating hypoxia inducible factor-1α-mediated glycolysis in hepatocellular carcinoma
- lnternational patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis
