Who to screen and how to screen for celiac disease
Prashant Singh, Achintya Dinesh Singh, Vineet Ahuja, Govind K Makharia
Abstract Celiac disease (CeD) is a chronic gluten-induced enteropathy with plethoric manifestations. The typical manifestations of CeD such as chronic diarrhea and malabsorption are widely recognized, however, many patients have atypical manifestations like iron deficiency anemia, idiopathic short stature, hypertransaminesemia or infertility, etc. These patients often present to the primary care physicians and/or non-gastrointestinal specialties. However, due to a lack of awareness among the healthcare professionals about the various atypical manifestations, many patients are not screened for CeD. In this review, we have summarized the available literature about the prevalence of CeD in various gastrointestinal (chronic diarrhea) and non-gastrointestinal conditions (iron deficiency anemia, short stature, cryptogenic hypertransaminesemia, cryptogenic cirrhosis or idiopathic ataxia etc.) where the diagnosis of CeD should be considered. In addition, we also discuss special scenarios where screening for CeD should be considered even in absence of symptoms such as patients with type 1 diabetes, Down’s syndrome, and first-degree relatives of patients with CeD.Further, we discuss the diagnostic performance and limitations of various screening tests for CeD such as IgA anti-tissue transglutaminase antibodies,antiendomysial antibodies and anti-deamidated gliadin antibodies. Based on the current recommendations, we propose a diagnostic algorithm for patients with suspected CeD.
Key Words: Screening; Diagnosis; Serology; High-risk group; Small intestine; Enteropathy
lNTRODUCTlON
Celiac disease (CeD) is an immune-mediated enteropathy that affects approximately 0.7% of the world population[1]. The disease is related to a complex interplay between genetic, environmental and host immunity-related factors. It is triggered by ingestion of gluten, a protein present in cereals such as wheat, barley, and rye in genetically-predisposed individuals. The phenotypic expression of CeD is variable. It ranges from being clinically asymptomatic to severely symptomatic disease[2,3]. Also, CeD once thought to affect only small intestines, is now considered a multi-system disorder. While there are convincing clinical and epidemiological evidence of involvement of extra-small intestinal organs, the exact pathogenesis of their involvement remains unexplored. It is likely that the human leukocyte antigen (HLA)-DQ2 restricted gliadin peptide induced T-cells which originate in the small intestine,circulate in peripheral blood and home in other organs leading to organ specific cell injury[4,5].
The clinical manifestations of CeD may be related to the gastrointestinal tract, called “classical CeD”seen in 50%-60% of all cases or non-gastrointestinal symptoms called “non-classical CeD” accounting for 40%-50% of cases[6,7]. The non-classic symptoms like short stature anemia, dyspepsia, infertility or hypertransaminesemia could be the sole manifestations of CeD making the clinical diagnosis more elusive. Additionally, CeD can co-exist with type 1 diabetes or other autoimmune diseases and its clinical manifestations may remain submerged with the manifestations of primary disease or it may remain clinically silent[8,9].
Because of its diverse manifestations, patients with CeD may present to healthcare professionals other than gastroenterologists or pediatricians such as hematologists with anemia, endocrinologists for short stature or type 1 diabetes, or gynecologists with infertility. The lack of typical manifestations combined with unfamiliarity with the disease lowers the index of suspicion for CeD in such clinical settings. This results in the missed diagnosis of CeD and about 85%-90% of the patients with CeD remain undiagnosed[10-12]. A delay in the diagnosis and institution of appropriate treatment adds to significant morbidity and even mortality in these patients[13].
Over past two decades, certain group of patients with conditions like short stature, iron deficiency anemia, type 1 diabetes, first-degree relatives have been reported to have a much higher prevalence of CeD compared to the general population[2,14]. Assiduous screening in these conditions can improve the detection of CeD compared to population-based screening for CeD. However, while most gastrointestinal societies agree that there is not enough evidence to recommend screening for CeD in the general population, they offer varying recommendations about which at-risk groups should be routinely screened for CeD[15,16].
In the present review, we summarize the present literature regarding the prevalence of CeD in many conditions to facilitate identification of high-risk groups who could benefit from screening for CeD. We have sub-grouped the indications for screening as definitive (when the data to support the screening is robust), probable (when the data to support screening exists but heterogenous) and possible (when there is a biological plausibility, but the evidence is insufficient). Later, we also highlight about the screening strategy for CeD once a decision for screening has been made based on clinical indication.
REVlEW METHODOLOGY
Relevant studies were searched utilizing MEDLINE, EMBASE and Scopus databases. Also, additional studies were cross-referenced from the various articles. Studies were identified with the medical subject heading terms and keywords-“celiac disease”, “celiac”, “coeliac disease”, “tissue transglutaminase antibody”, “endomysial antibody”, “anti-endomysium antibody”. They were combined using the set operator AND with keywords for relevant medical condition/risk factor (type 1 diabetes mellitus,irritable bowel syndrome, first degree relativesetc.). Studies from all languages were reviewed for appropriateness to the clinical question and potentially relevant papers were assessed in detail.
Terms, definitions and analysis
For the purpose of this manuscript, CeD is defined as: (1) Villous abnormalities of modified marsh grade 2 on duodenal biopsies along with positive celiac-specific serology [anti-tissue transglutaminase antibody (anti-tTG Ab), anti-endomysial antibody (AEA), anti-deamidated gliadin peptide or antigliadin antibody (anti-DGP Ab)]; and (2) presence of at least modified marsh 2 lesion on duodenal histology along with unequivocal clinical and/or histological response to a gluten free diet (GFD).Seroprevalence of CeD is defined as the prevalence of positive anti-tTG Ab and/or AEA and/or anti-DGP Ab. Pooled prevalence of CeD in the manuscript refers to pooled prevalence of biopsy-proven CeD.
DEFlNlTE lNDlCATlONS FOR SCREENlNG FOR CED
Patients with chronic diarrhea
The classical gastrointestinal symptoms of CeD include chronic or intermittent diarrhea, steatorrhea,abdominal bloating, flatulence, and weight loss[7]. A proportion of patients with CeD may have mild gastrointestinal manifestations such as bowel dysmotility, abdominal pain/discomfort, and bloating,and with this symptom complex, they are can be labelled as having functional gastrointestinal diseases including irritable bowel syndrome (IBS).
One of the classic manifestations of CeD, both in the children and adults, is chronic diarrhea which occurs secondary to diffuse enteropathy and malabsorption. Chronic diarrhea in children is often associated with failure to thrive, irritability and distension of the abdomen. Chronic diarrhea is a predominant manifestation in 43%-85% of patients with newly diagnosed CeD. Conversely, the prevalence of CeD in patients referred to secondary care with chronic diarrhea has been reported to range from 3% to 12.2%[17,18]. Given the high occurrence of CeD in patients with chronic diarrhea and the delay in diagnosis of CeD, several gastroenterology societies such as American Gastroenterological Association, and British Society of Gastroenterology has recommended screening for CeD as first line investigation in patients with chronic diarrhea[17,19]. Therefore, all patients presenting with chronic diarrhea should be screened for CeD.
Patients with iron deficiency anemia
Anemia affects approximately 12%-69% patients in the western countries and 85%-90% of Indian patients with CeD[20-25]. Iron deficiency anemia (IDA) could be an isolated manifestation of CeD even in the absence of GI symptoms. Iron deficiency is the commonest form of anemia in CeD[20,26,27].
In a review including 2998 patients from 18 studies, Mahadevet al[14] have reported prevalence of CeD in patients presenting with iron deficiency anemia. Of 2998 patients with IDA included in this meta-analysis, the estimated pooled prevalence of CeD was reported to be 3.2% (95%CI: 2.6-3.9). Thus,approximately 1 in 31 patients with IDA have CeD. The authors did not find any relationship between the age and gender of the participant and the prevalence of CeD in patients with IDA. However, the prevalence of CeD in them vary significantly with geographic region. The prevalence of CeD in iron deficiency anemia is significantly higher in Asian countries (4.1% in Turkey and 6.4% in Iran) compared to that in the European countries (2.4%). It is important to note that the prevalence of iron deficiency anemia could vary based on the geographical region and according to the socio economic strata of the society[28]. They also found that smaller studies were more likely to report higher prevalence of CeD compared to studies with larger sample size. However, even in larger studies (those including > 200 patients), the pooled prevalence of CeD in patients with IDA was still significantly higher (2.7%) than that in the general population. Thus, the prevalence of CeD in patients with IDA is at-least 2-3 times higher prevalence of CeD than in the general population. Considering the overall high prevalence, all patients with iron deficiency anemia should be screened for CeD.
Patients with short stature
The manifestations of CeD start in childhood and hence growth failure/restriction is an important manifestation of CeD. Short stature is more frequent in those diagnosed during childhood/adolescence than patients diagnosed in adulthood. Importantly, institution of GFD in these patients can result in early catch-up growth for the initial 2-3 years[2,29,30]. Early diagnosis and compliance with GFD in patients with CeD result in rapid recovery and patients may achieve normal adult height.
Several studies have estimated the prevalence of CeD in children with idiopathic short stature. We systematically reviewed and meta-analyzed 17 studies and 3759 patients (1582 with all-cause short stature and 2177 with idiopathic short stature), and found that the pooled seroprevalence of CeD based on positive anti-tTG Ab and AEA is 11.2% (95%CI: 4.0-21.2) and 9.7% (95%CI: 2.7-20.2) for all-cause and idiopathic short stature, respectively. Similarly, pooled prevalence of biopsy-confirmed CeD is 7.4%(95%CI: 4.7-10.6) and 11.6% (95%CI: 4.1-22.2), for all-cause and idiopathic short stature, respectively[31].In summary, approximately 1 in 14 patients with all-cause short stature and 1 in 9 patients with idiopathic short stature has biopsy-confirmed CeD. Therefore, all patients with CeD should be screened for CeD.
Patients with type 1 diabetes
Because of sharing of genetic susceptibility, especially HLA, type 1 diabetes is often associated with CeD[3,6]. In a systematic review and meta-analysis by Elfströmet al[32] of 27 studies including 26605 patients with type 1 diabetes, the pooled prevalence of CeD was 6% (95%CI: 5-6.9) of CeD. Thus, more than 1 in 18 patients with type 1 diabetes mellitus have biopsy-proven CeD. The prevalence of CeD in children patients with type 1 diabetes is much higher (6.2%, 95%CI: 6.1- 6.3) than those with adult patients with type 1 diabetes (2.7%, 95%CI: 2.1-2.3) (P< 0.001). There is no geographical variation and almost a similar prevalence of CeD is reported in type 1 diabetes from all over the world. Therefore, all patients with type 1 diabetes should be screened for CeD[32].
First-degree relatives of patients with CeD
Because of sharing of genetic susceptibility, first-degree relatives (FDRs) of patients with CeD are at a higher risk of developing CeD in comparison to the general population. The prevalence of CeD in FDRs of patients with CeD has been extensively investigated and it ranges widely in the literature from 1.6%to 38%[33-35]. Singhet al[36] in a systematic review and meta-analysis including 41 studies, has reported 708 CeD patients amongst 10252 FDRs suggesting a pooled prevalence of CeD of 7.5% (95%CI:6.3-8.8) in them. Among the FDRs, the risk of having CeD is highest amongst siblings, followed by offspring and the least in the parents. Daughters and sisters of the CeD patients are at the highest risk (1 in 7 and 1 in 8, respectively) of developing CeD. The risk of developing CeD is 1 in 13 in sons, 1 in 16 in brothers, 1 in 32 in mothers and 1 in 33 in fathers[36]. Majority of studies reporting prevalence of CeD in FDRs have been cross-sectional and there is a lack of longitudinal studies for assessment of risk of developing CeD over time or over lifetime. It is still unclear if there is role for repeat screening every few years after an initial negative screening or repeat screening should be reserved for FDRs who develop symptoms. Based on the abovementioned high-quality data, all FDRs of index patients with CeD should be screened for CeD.
The data on the prevalence of CeD in second-degree relatives (SDRs) of patients with CeD is sparse[37,38]. In a meta-analysis of two eligible studies including 641 SDRs, Weinsteinet al[39] observed a pooled prevalence of CeD in SDRs to be 2.3% (95%CI: 1.3-3.8). The current literature on this topic has several limitations including availability of few studies, small sample size, and high risk of bias. Given these limitations, the magnitude of risk of CeD in SDRs is not clear. Therefore, in view of insufficient data, screening of SDRs of patients with CeD for CeD is not justifiable at the present time.
Patients with dermatitis herpetiformis
Dermatitis herpetiformis is among the most common manifestations of CeD and could be the first extraintestinal manifestations to be clinically recognized. It presents as a cluster of intensely pruritic papules and/or vesicles, followed by erosions and excoriations. The most common sites are elbows, knees, scalp,and buttocks, typically along the extensor surface of the upper and lower extremities.
The extent of skin lesions may vary from small area to more diffuse involving many sites at one time[40,41]. Furthermore, these lesions may appear intermittently and may be absent at the time of examination.
The pathognomonic histology with immunohistochemistry or immunofluorescence shows granular IgA deposits and neutrophil infiltrates in the papillary dermis. Almost 85% of these patients with a Caucasian ethnicity carry HLA-DQ2 mutations while the remaining have HLA-DQ8[42]. Typically, only two-third patients with DH have villous abnormalities and one third of them have no enteropathy[43].A survey of 1138 biopsy-confirmed patients with CeD, found a 9.8% prevalence of DH in patients with CeD[44].
A study from Finland have also shown that 17% of patients with CeD have DH[45]. Therefore, all patients with a diagnosis of DH should be screened for CeD, even in the absence of intestinal manifestations.
Patients with Down’s syndrome
The prevalence of CeD in patients with Down’s syndrome has been extensively investigated. Based on data from 31 studies including 4383 patients with Down syndrome, a recent meta-analysis reported a pooled prevalence of CeD in them to be 5.8% (95%CI: 4.7-7.2)[46]. Prevalence of CeD was higher in the studies including only children with Down syndrome than in those including both adults and children with Down’s syndrome. The higher prevalence of CeD in Down syndrome is independent of the geographical location as studies from Europe, America and Asia have all shown higher prevalence of CeD among patients with Down syndrome. Based on this high-quality evidence, all patients with Down syndrome should be screened for CeD.
PROBABLE lNDlCATlONS FOR SCREENlNG FOR CED
Patients with liver diseases
Patients with CeD can have a variety of liver related manifestations that ranges from elevation of serum transaminases to cirrhosis. Elevated transaminases can be seen in 27% (95%CI: 13-44) of newly diagnosed patients with CeD and they normalize in 63% to 90% of patients within 1 year of GFD[47].
Cryptogenic hypertransaminesemia
Sainsburyet al[47] in a systematic review including six studies has reported a pooled prevalence of biopsy-proven CeD of 3.6% amongst patients with cryptogenic hypertransaminesemia. In a study of 463 adults with CeD[48], 40.6% patients had elevated AST/ALT levels at the time of diagnosis compared to 24.2% CeD patients after initiation of GFD. The quality of evidence has been considered high due to very large effect size without any significant imprecision. Given the high prevalence of CeD in patients with cryptogenic hypertransaminesemia, and a potential for reversal of serum transaminases level with GFD, it is justifiable to screen patients with cryptogenic hypertransaminesemia for CeD.
Cryptogenic cirrhosis
Undetected CeD can lead to persistent liver injury progressing from elevated liver biochemistries to cirrhosis of the liver[49-53]. In one of the earlyreports, Kaukinenet al[52] found a reversal in the hepatic dysfunction after initiation of GFD in four patients awaiting liver transplantation and eventually three of them were remitted from liver transplantation list. In a recent prospective study by Wakim-Fleminget al[54], of 204 consecutive biopsy proven patients with liver cirrhosis, CeD was reported in 2.5% of the patients. There was improvement in liver function tests with initiation of GFD in these patients.
However, these studies had serious limitations including small sample size (all subjects with less than 100 subjects), selection bias, imprecision and inconsistent results. Given these limitations, the quality of evidence is poor to suggest a true estimate of prevalence of CeD in cryptogenic cirrhosis. In view of the data of improvement in liver functions with GFD, it may be worthwhile to screen the patients with cryptogenic cirrhosis for CeD.
Patients with auto-immune hepatitis
Recently, in a systematic review of eight eligible studies, the prevalence of biopsy-proven CeD was 3.5%(95%CI: 1.6-5.3) amongst 567 individuals with autoimmune hepatitis, which is clearly higher than that in the general populations[55]. However, these studies had serious limitations including small sample size, selection bias, imprecision and inconsistent results. Despite these limitations, we suggest screening for CeD in all patients with autoimmune hepatitis because of the relatively higher pooled prevalence of CeD in these subjects compared to the general population.
Patients with irritable bowel syndrome
Patients with CeD may have minor gastrointestinal infection including diarrhea, abdominal pain,bloating sensation without any definitive manifestations for malabsorption. Such patients are often diagnosed as irritable bowel syndrome in general clinical practice, unless screened for CeD. In a study by Irvineet al[56] including 22 studies with 6991 patients with IBS, the reported pooled prevalence of CeD was 3.3% (95%CI: 2.3-4.5) in them.
The pooled prevalence of CeD in patients with IBS varies significantly with the IBS subtype and their geographical location. The prevalence of CeD is higher in patients with diarrhea-predominant IBS(pooled prevalence 5.4%, 95%CI: 3.3-7.8) compared to those with constipation-predominant IBS [1.8%(95%CI: 0.9-3.0)] and mixed form of IBS [3.1% (95%CI: 1.7-5.1)].
Interestingly in the above meta-analysis, all but one study was from secondary or tertiary care referral centers. Furthermore, 20 of the 22 studies were from Europe and Asia with pooled prevalence of CeD in patients with IBS in them was 3.9% (95%CI: 2.1-6.3) and 3.7% (95%CI: 2.2-5.6), respectively. The only study from North America, which also evaluated celiac serology positive individuals with IBS further with duodenal biopsies, did not find an increased prevalence of CeD in patients with IBS. A recent study from Iraq found a prevalence of 5% among 100 patients with IBS[57].
Thus, the utility to screening for CeD in individuals with IBS in primary care settings or the general population remains unclear. Based on available evidence, it may be justifiable to screen patients with diarrhea predominant-IBS and mixed IBS presenting to secondary or tertiary care centers for CeD in Europe and Asia.
Patients with osteoporosis
Patients with CeD are at an increased risk for developing varying degrees of osteopenia and osteoporosis. Patients with untreated CeD have low bone mineral density agnostic to the clinical presentation[58,59]. The reported pooled prevalence of osteoporosis and osteopenia is 14.4% (95%CI: 9-20.5) and 39.6% (95%CI: 31.1-48.8), respectively in 563 pre-menopausal women and men with CeD[60].Along with osteoporosis, patients with CeD are also at a higher risk of developing bone fractures. A comparative meta-analysis of 20995 patients with CeD and 97777 controls from eight studies published between 2000 and 2007, found that patients with CeD have a 43% higher risk for developing nontraumatic fracture compared with controls[61]. Also a recent study found that patients with newly diagnosed CeD have low bone marrow density and this improves after initiation of GFD[62].
Laszkowskaet al[63] performed a systematic review and meta-analysis to estimate the prevalence of CeD in patients with osteoporosis. They pooled data on 3188 patients with osteoporosis from eight studies and reported a weighted pooled prevalence of CeD to be 1.6% (95%CI: 1.1-2.0). The authors observed a positive correlation between underlying prevalence of CeD in the general population and the prevalence of CeD in osteoporosis suggesting the prevalence of CeD in patients with osteoporosis is higher in the areas with higher prevalence of CeD in the general population. Therefore, the prevalence of CeD does not appear to be significantly higher in patients presenting with osteoporosis than that in the general population. Given these findings, while all individuals with new diagnosis of osteoporosis may not need screening for CeD, however patients with osteoporosis having additional symptoms of CeD such as iron deficiency anemia, chronic diarrhea should be screened for CeD.
POSSlBLE CONDlTlONS FOR SCREENlNG FOR CED
Patients with dyspepsia
In a systematic review and meta-analysis, we pooled the data from 19 studies involving 9711 patients with dyspepsia in whom initial screening for CeD was performed either by using celiac serological test(anti-tTG ab or AEA) followed by duodenal mucosal biopsy in seropositive patients or the duodenal mucosal biopsy alone in all eligible patients[64].The pooled prevalence of CeD has been found to be 1.4% (95%CI: 0.9-1.8) in patients with dyspepsia. Another meta-analysis including ten studies also reported the prevalence of CeD to be 1% in patients with dyspepsia[65]. As the prevalence of CeD in patients with dyspepsia is almost like that in the general population, patients with dyspepsia may not be a higher risk of having CeD. It is, however, still unclear if patients with refractory dyspeptic symptoms are at higher risk of having CeD.
Women with infertility
The patients with CeD can delayed menarche, early menopause, recurrent abortions, infertility,intrauterine growth retardation, and low birth weight (preterm and small for gestational age babies)[66,67]. Additionally, reports have suggested that women with infertility have conceived after initiation of GFD with a diagnosis of CeD[68,69]. Furthermore, there are multiple studies reporting the prevalence of CeD in women with infertility in women who had been investigated for the causes of infertility earlier(unexplained or idiopathic infertility) and those women with infertility who were never investigated for infertility (all cause infertility).
Women with unexplained or idiopathic infertility
Based on case-control studies, two previous meta-analyses have reported that women with unexplained infertility have 5-6 times increased odds of having CeD compared to the general population[70,71].However, a recent meta-analysis did not find an increased prevalence of CeD in women with unexplained infertility[72]. They reported a pooled prevalence of 0.6% for biopsy-proven CeD in women with unexplained infertility which is very close to the prevalence of CeD in the general population. Of note, a common limitation of all three meta-analyses is the small sample size of primary studies. Based on the current evidence, it is not clear if all women with unexplained infertility should be screened for CeD or not and more research is needed in this area.
Women with “all-cause infertility”
In a systematic review and meta-analysis, Glimberget al[72] pooled the data from 11 eligible studies including 1617 women with “all-cause” infertility and found a prevalence of 0.7% (95%CI: 0.2-1.2) for biopsy-proven CeD. Based on abovementioned data, screening women with all-cause infertility for CeD,may not be justified. However, as above, more studies are needed to further explore the prevalence of CeD in women with infertility.
Patients with idiopathic cardiomyopathy
There are only a few studies which have systematically explored the prevalence of CeD in patients with idiopathic cardiomyopathy[73-77]. The prevalence of CeD in these studies have ranged from 0% to 5.7%.Most of these studies have limitations including a high risk of bias, small sample size and heterogenous patient population included in these studies. Given these serious limitations in the quality of evidence,the present level of evidence does not support screening of patients with idiopathic cardiomyopathy for CeD. Future studies with better study design, larger sample size and various geographic regions should be undertaken for estimation of risk of CeD in patients with cardiomyopathy.
Patients with autoimmune thyroid diseases
In a recent systematic review and meta-analysis, Royet al[78] estimated the pooled prevalence of CeD to be 1.6% (1.3%-1.9%) among 6024 patients with autoimmune thyroid diseases from 15 studies. The review has included even those studies where patients having villous abnormalities of modified marsh grade 1 and 2 have been included as CeD. When the analysis was restricted to those with villous abnormalities of modified Marsh 3, a pooled prevalence of CeD declined to 1.4% (95%CI: 1-1.8) in them.The prevalence of CeD is lower in patients with hypothyroidism (1.4%, 95%CI: 1-1.9) than patietns with hyperthyroidism (2.6%, 95%CI: 0.7-4.4). The available data do not support that routine screening for CeD will likely not be of benefit for majority of patients with autoimmune thyroid diseases. However,larger studies with rigorous methodology are needed in this area.
Patients with idiopathic epilepsy
Several studies have evaluated the prevalence of CeD in patients with idiopathic epilepsy[79-81]. In a systematic review, a pooled prevalence of CeD in patients with idiopathic epilepsy is 2.1% (95%CI: 1.6-2.6,n= 3389) has been reported[80]. The quality of the studies included in this review have major limitations including high overall risk of bias (which decreases our confidence in the estimate),inconsistency (several studies showing no increased risk while others showing increased risk), and imprecision. Based on the presently available data, screening of patient with idiopathic epilepsy for CeD cannot be recommended. There is a need for multicentric studies including larger sample size for better estimation of risk of CeD in patients with idiopathic epilepsy.
Patients with idiopathic cerebellar ataxia
The neurological manifestations of CeD and gluten-related disorders are broad, conditions like ataxia and peripheral neuropathy are well recognized, others such as migraine, epilepsy, dementia, cognitive impairment and depression have also been reported[82-85]. Patients with idiopathic sporadic ataxia and a positive anti-gliadin antibodies AGA) (either IgG or IgA or both) with or without presence of enteropathy are diagnosed as gluten ataxia[83].
Gluten ataxia commonly presents as a sporadic ataxia and most patients do not have associated enteropathy. A systematic review and meta-analysis have found higher odds of anti-gliadin antibodies positive in patients with idiopathic cerebellar ataxia (OR 4.2, 95%CI: 3.1-5.9) as compared to controls[86]. The odds of a positive AGA have not been found to be higher in patients with hereditary ataxia(OR 1.41, 95%CI: 0.82-2.44). Most of the studies included in the review are cross-sectional, and the results show significant imprecision or inconsistency. Considering that treatment response in gluten ataxia would depend on the duration of the ataxia and that GFD may positively impact the ataxia,patients with idiopathic or undiagnosed ataxia should be screened for gluten ataxia using IgG and IgA anti-gliadin antibody[87,88].
A very few studies have evaluated presence of CeD in patients in patients presenting with ataxia. In a study by Hadjivassiliouet al[89], reported that 24% patients with gluten sensitivity ataxia had CeD.While Pellecchiaet al[90] found all 3 patients with a positive IgG anti-gliadin Ab to behave CeD.Busharaet al[91] biopsied seven of the nine patients with gluten sensitivity ataxia and none were diagnosed to have CeD. Small number of patients, high risk of bias and heterogenous results of these studies limit drawing of a robust conclusion. Therefore, based on the available data, no definitive recommendation can be made to screen patients with sporadic and idiopathic ataxia for CeD.
Patients with dental enamel defects
Only 2 studies have examined the occurrence of CeD in patients with dental enamel defects[92,93] and in them the prevalence of CeD ranged between 7.7%-17.8%. In one of these studies, one year of GFD resulted in significantly higher reversal of enamel changes in CeD patients compared to those without CeD (48%vs3.4% patients,P< 0.001), suggestive of a causal association[92] Despite the relatively higher prevalence estimate of CeD seen in these studies, there is likelihood of some publication bias and these studies had very small sample size.
In two recent case-control studies, patients with CeD had higher dental enamel defects and other oral manifestations like aphthous ulcers were much higher compared to controls[94,95]. For these reasons,large-scale community-based studies across different socio-cultural and geographical populations need to be undertaken to ensure accounting for confounding factors that influence oral health as well as generalizability of the results. Until such time, screening of adults or children with dental enamel defects for CeD is not clear.
We have summarized the reported prevalence of CeD in the various clinical conditions based on the organ systems in Figure 1 and based on the indication in Table 1.
HOW TO SCREEN FOR CED?
While there is significant heterogeneity among clinicians in selecting the medical conditions where theyscreen patients for CeD; there also exists significant variation in selecting the screening tool for CeD.Currently, celiac specific serological tests are the first-line investigations for screening for CeD.Duodenal biopsies showing villous atrophy, crypt hyperplasia and increase in intra-epithelial lymphocytes on a gluten-containing diet continues to be ‘gold standard’ for patients with CeD. Given the invasive nature of upper GI endoscopy, limited availability in resource limited countries and associated cost, this ‘gold-standard’ can however not be applied to all patients who need to be screened for CeD.
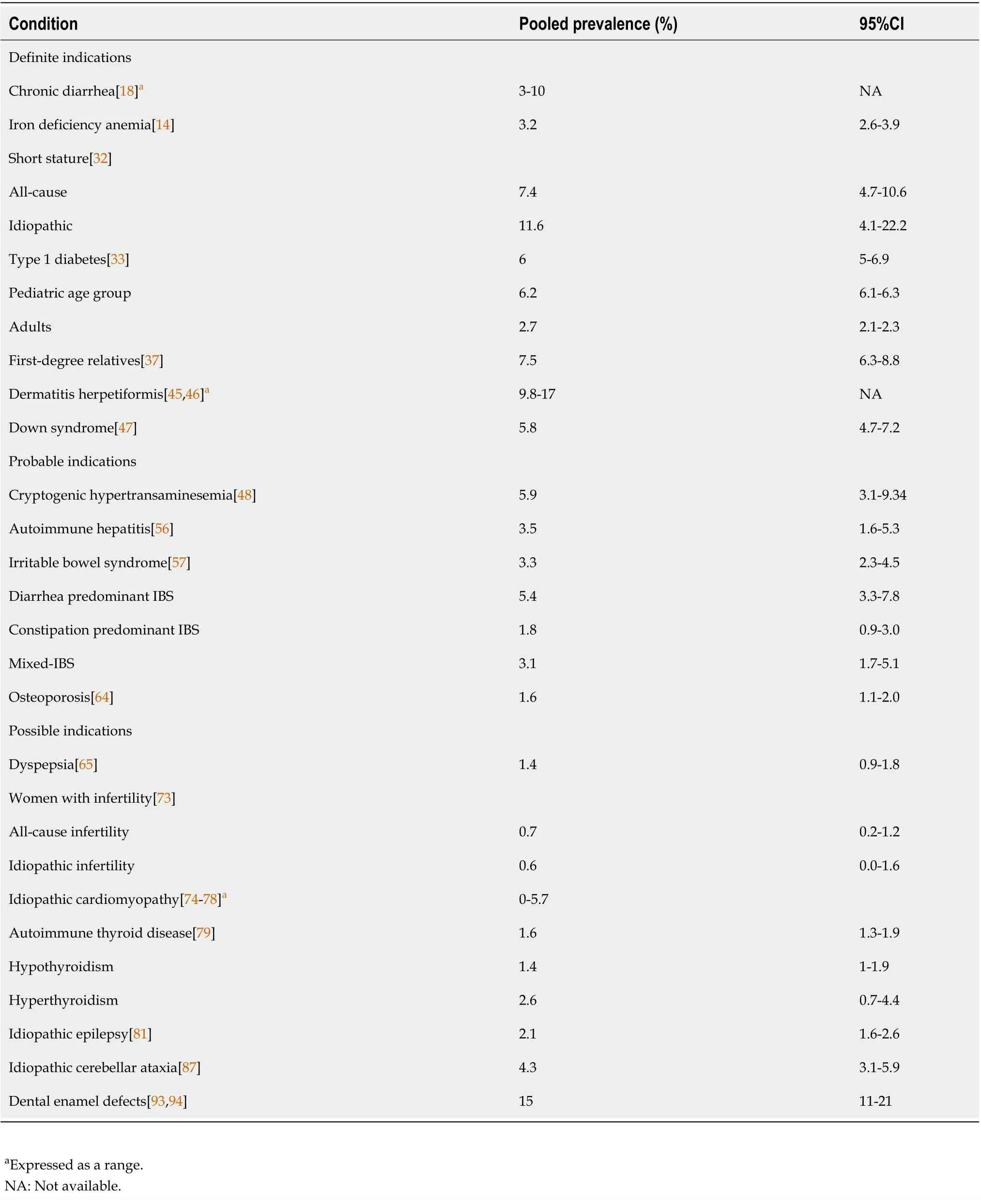
Table 1 The pooled prevalence of celiac disease in various conditions
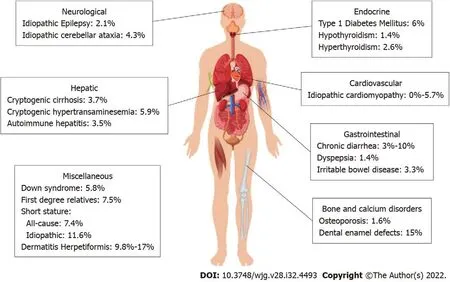
Figure 1 Summary of the prevalence of celiac disease in various associated conditions based on the various systems. The reported prevalence per the present literature, the studies may be limited by the number and quality of studies available.
The specific celiac serological tests include IgA anti-tTG Ab, IgA AEA, and IgG anti-DGP Ab. While IgA/IgG AGA had been used screening for CeD in the past, however, given their poor specificity and sensitivity they are no longer recommended to be used for screening for CeD. In the section below, we briefly discuss the utility of three commonly used celiac-specific serological tests for screening for CeD.
IgA anti-tTG Ab
Given its widespread availability, ease of performance and lower costs compared to other celiac specific serological tests, IgA anti-tTG Ab is the most used serological test for screening for CeD. Recent systematic review has shown that pooled sensitivity and specificity of anti-tTG Ab for CeD are 92.8%(95%CI: 90.3-94.8) and 97.9% (95%CI: 96.4-98.8), respectively. IgA anti-tTG Ab testing should be combined with total serum IgA levels to rule out IgA deficiency which is 10-15 times more common in patients with CeD compared to general population[96]. In patients with IgA deficiency, duodenal biopsies or further serologic testing such as IgG- based anti-deamidated gliadin peptide antibodies(anti-DGP Ab) should be pursued.
Despite their widespread use and high sensitivity, these anti-tTG Ab assays have high inter-assay variability in their diagnostic performance[97,98]. Although anti-tTG Ab assays are the first-line investigation to screen for CeD, healthcare providers should realize that false-negative rate of a single IgA anti-tTG Ab assay can be over 20% suggesting a single negative IgA anti-tTG Ab assay cannot be relied upon as a sole test to rule out CeD especially if clinical suspicion for CeD is high[97]. In these cases, a negative IgA anti-tTG Ab assay must be followed with duodenal biopsies.
Moreover, the issue of diagnostic performance of these assays is further complicated by the fact that there is significant intra-assay variation in the performance of IgA anti-tTG Ab assays in racially and geographically distinct population[98]. Majority of the studies of diagnostic performance of IgA antitTG Ab assays (including the manufacturer provided validation studies) are performed in Caucasian studies and unfortunately, these results cannot be extrapolated to other ethnic populations. However,sensitivity of these assays can be improved without significantly compromising the specificity if receiver operator curve-based cut-offs can be developed for each population. Thus, multi-institutional collaborative workshops at national and international levels using coded and blinded standardized sera from well-defined patients with CeD (with varying levels of titers) and healthy controls are necessary to identify and validate population-specific cut-off values as well as the best performing IgA anti-tTG Ab assays for each population. Till then, diagnostic performance of commonly available anti-tTG Ab assays should be studied at each center performing anti-tTG Ab testing.
Finally, recent guidelines now allow for a diagnosis of CeD to be established without duodenal biopsy in a subset of patients with CeD; on the basis of high levels of anti-tTG Ab [× 10 fold upper limit of normal (ULN)] and positive AEA in a second sample[99]. However, these guidelines are often not interpreted correctly and many healthcare providers interpret low level titers as diagnostic of CeD. Even with anti-tTG Ab titers as high as 10-fold ULN, a confirmatory AEA testing on a second sample might not be obtained given either due to inappropriate interpretation of the guidelines or the lack of availability of AEA testing. This can lead to ‘overdiagnosis’ of CeD as anti-tTG Ab at low titers have poor positive predictive value for CeD. Therefore, there is urgent need for widespread dissemination of diagnostic algorithms and guidelines among primary care physicians, gastroenterologists, and non-gastroenterology specialists.

Table 2 The sensitivity and specificity of the screening tests for celiac disease
AEA
AEA is an indirect immunofluorescence-based testing requiring rhesus monkey esophagus or human umbilical cord as substrates. Therefore, it can only be performed in specialized laboratories and is much more labor intensive compared to ELISA based assays. Moreover, the results are based on subjective interpretation of the results. Based on a recent systematic review, the pooled sensitivity and specificity of AEA for the diagnosis of AEA were 73.0% (95%CI: 61.0-83.0) and 99.0% (95%CI: 98.0-99.0),respectively[96]. Given the limitations of the testing described above, AEA is not an ideal screening test for CeD outside of referral centers. Although a positive AEA testing is very highly suggestive of CeD(given specificity approaching 100%), a negative AEA should not be relied upon to rule out CeD.
IgG anti-DGP Ab
IgG anti-DGP Ab are the latest serologic assays for CeD and have been shown to have pooled sensitivity of 87.8% (95%CI: 85.6-89.9) and pooled specificity of 94.1% (95%CI: 92.5-95.5)[96]. Although pooled sensitivity of IgG anti-DGP Ab assays is high, it is still lower than pooled sensitivity of IgA anti-tTG Ab assay. Furthermore, several studies have shown that isolated IgG anti-DGP Ab in patients with normal serum IgA levels do not increase the yield of the diagnosis of CeD. Therefore, currently IgG anti-DGP Ab cannot be used to replace IgA anti-tTG Ab as the first line strategy to screen for CeD. Its role in CeD diagnosis is as a complementary assay to be used in patients with IgA deficiency (where IgA anti-tTG Ab assays cannot be relied upon).
Point of care tests
Recently, point of care tests (POCTs) for CeD are commercially available in Europe. Studies have reported significant variability in their sensitivity (70% to 100%) and specificity (85% to 100%). The pooled sensitivity and specificity of all POCTs (based on anti-tTG Ab or anti-DGP Ab or anti-tTG Ab +Anti-gliadin antibodies) for diagnosing CeD has been reported to be 94.0% (95%CI: 89.9-96.5) and 94.4%(95%CI: 90.9-96.5), respectively[100]. The pooled positive and negative likelihood ratios for POCTs are 16.7 and 0.06, respectively. The pooled sensitivity and specificity for IgA anti-tTGAb based POCTs are 90.5% (95%CI: 82.3-95.1) and 94.8% (95%CI: 92.5-96.4), respectively. However, this pooled sensitivity appears to be lower compared to standard ELISA based IgA anti-tTG Ab assay. Therefore, wherever available anti-tTG Ab should be used as first line screening test. However, POCTs can be an excellent alternative in areas with limited access to laboratory-based testing. All the above tests are summarized in Table 2.
Further testing
Once the screening test is positive in an individual suspected to have CeD, the health-care professional should follow the suggested guidelines by many Gastroenterology professional Societies for further evaluation[15,19]. We have suggested a testing schematic for the various indications for suspected CeD in Figure 2.
CONCLUSlON
There is good evidence to suggest screening of patients with chronic diarrhea, iron deficiency anemia,short stature, dermatitis herpetiformis, type 1 diabetes, Down’s syndrome, as well as first-degree relatives of CeD. The possible indications for screening of CeD include cryptogenic elevated transaminases, cryptogenic cirrhosis autoimmune hepatitis, IBS, autoimmune thyroid disease, and osteoporosis/osteoporosis. There is need for systematic studies for many conditions such as rheumatological diseases, psoriasis, cardiomyopathy, neurological diseases, and liver diseases for the prevalence of CeD in them. Screening for CeD is a well standardized, simple, and relatively inexpensive process and it provides an opportunity for early detection of CeD in them.
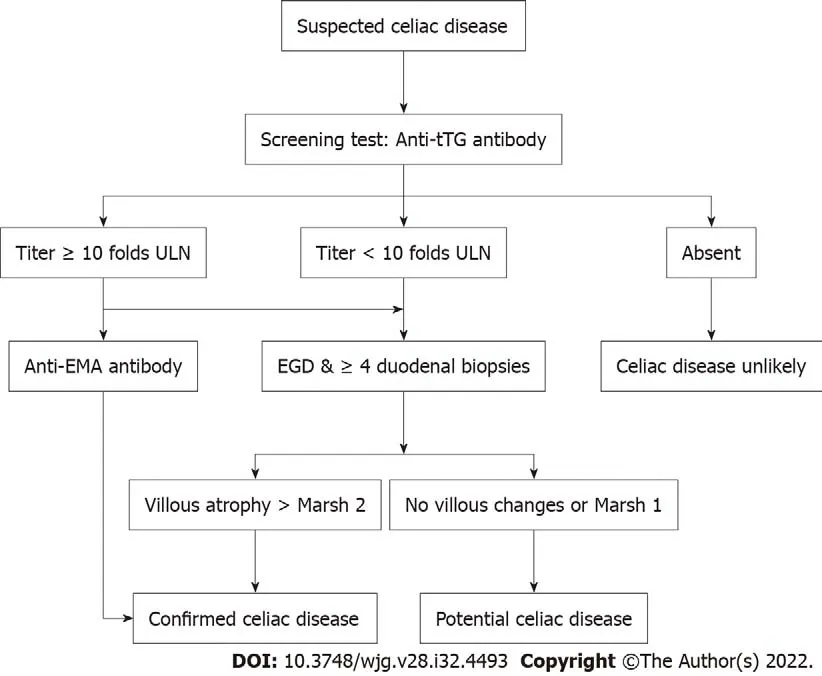
Figure 2 A screening algorithm for patients with suspected celiac disease. EGD: Esophagogastroduodenoscopy; ULN: Upper limit of normal.
ACKNOWLEDGEMENTS
We acknowledge the support of Department of Biotechnology, Government of India, for creation of Indian Consortium on Celiac Disease and National Celiac Disease Biorepository. We do appreciate the support from Research Section of our institution for facilitating the research on Celiac disease.
FOOTNOTES
Author contributions:Singh P and Makharia GK were involved in review concept and the article structure; Singh P,Singh AD were involved in data collection and writing the first draft of the manuscript; All the authors contributed in reviewing and editing the final draft of the manuscript.
Conflict-of-interest statement:There are no conflicts of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORClD number:Prashant Singh 0000-0002-3796-2372; Achintya Dinesh Singh 0000-0001-9094-1071; Vineet Ahuja 0000-0002-5474-9709; Govind K Makharia 0000-0002-2474-2194.
S-Editor:Zhang H
L-Editor:A
P-Editor:Zhang H
 World Journal of Gastroenterology2022年32期
World Journal of Gastroenterology2022年32期
- World Journal of Gastroenterology的其它文章
- The mechanism of Yinchenhao decoction in treating obstructivejaundice-induced liver injury based on Nrf2 signaling pathway
- Anoctamin 5 regulates the cell cycle and affects prognosis in gastric cancer
- Effects of Granule Dendrobii on chronic atrophic gastritis induced by N-methyl-N'-nitro-N-nitrosoguanidine in rats
- Machine learning predicts portal vein thrombosis after splenectomy in patients with portal hypertension: Comparative analysis of three practical models
- Sirolimus increases the anti-cancer effect of Huai Er by regulating hypoxia inducible factor-1α-mediated glycolysis in hepatocellular carcinoma
- lnternational patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis
