HOTTIP downregulation reduces neuronal damage and microglial activation in Parkinson’s disease cell and mouse models
Peng Lun ,Tao Ji ,De-Hong Wan ,Xia Liu ,Xiao-Dong Chen,Shuai Yu,Peng Sun,
Abstract HOXA transcript at the distal tip (HOTTIP),a newly identified long noncoding RNA,has been shown to exhibit anti-inflammatory effects and inhibit oxygen-glucose deprivation-induced neuronal apoptosis.However,its role in Parkinson’s disease (PD) remains unclear.1-Methyl-4-phenylpyridium (MPP+) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) were used to establish PD models in SH-SY5Y and BV2 cells and in C57BL/6 male mice,respectively. In vitro,after HOTTIP knockdown by sh-HOTTIP transfection,HOTTIP and FOXO1 overexpression promoted SH-SY5Y apoptosis,BV2 microglial activation,proinflammatory cytokine expression,and nuclear factor kappa-B and NACHT,LRR and PYD domains-containing protein 3 inflammasome activation.Overexpression of miR-615-3p inhibited MPP+-induced neuronal apoptosis and microglial inflammation and ameliorated HOTTIP-and FOXO1-mediated nerve injury and inflammation.In vivo,HOTTIP knockdown alleviated motor dysfunction in PD mice and reduced neuronal apoptosis and microglial activation in the substantia nigra.These findings suggest that inhibition of HOTTIP mitigates neuronal apoptosis and microglial activation in PD models by modulating miR-615-3p/FOXO1.This study was approved by the Ethics Review Committee of the Affiliated Hospital of Qingdao University,China (approval No.UDX-2018-042) in June 2018.Key Words:apoptosis;inflammation;miR-615-3p;neuron;NLRP3;noncoding RNA;Parkinson’s disease;HOTTIP
Introduction
Parkinson’s disease (PD) is characterized by progressive degeneration and apoptosis of dopaminergic neurons and the appearance of Lewy bodies in the substantia nigra(SN),which causes neuron loss,static tremors,myotonia,bradykinesia,and abnormal posture and gait (Wakabayashi et al.,2013;Schneider et al.,2017).Recent studies have shown that inflammation and immune response contribute to dopaminergic neuron apoptosis.Neuroinflammation-mediated neurotoxicity contributes to the neuronal death cascade in PD patients (Rocha et al.,2018;Surmeier,2018).Therefore,exploring inflammation in PD is vital to better understand the disorder in efforts to improve its diagnosis and treatment.Microglia account for only 10% of central nervous system(CNS) cells,but exert a vital role in the modulation of neuroinflammation involved in CNS diseases (Xiong et al.,2016).Multiple agents,including 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP),paraquat,rotenone,pathogen-associated molecular patterns,α-synuclein,matrix metalloproteinase 3,thrombin,and neuromelanin can activate microglia (Frank-Cannon et al.,2009).When microglia are activated,they produce oxygen-free radicals and toxic inflammatory mediators,which cause neuroinflammation through different molecular mechanisms,exacerbate dopaminergic nerve damage,and accelerate PD-induced neuroinflammation and neuronal death (Vivekanantham et al.,2015;Guo et al.,2019).
Long noncoding RNAs (lncRNAs) are a kind of RNA without the protein-coding ability and have a transcript length of over 200 nucleotides (Liu et al.,2020a).It has been shown that lncRNAs contribute to cell proliferation,differentiation,and death(Charles Richard and Eichhorn,2018).They are abnormally expressed in multiple CNS diseases and exert a significant role by regulating microglia-mediated inflammation (Wang and Zhou,2018;Zhang et al.,2019b).Additionally,lncRNAs have been shown to play a role in PD (Lin et al.,2019;Xu et al.,2020).HOXA transcript at the distal tip (HOTTIP) is a lncRNA involved in CNS disease.For example,HOTTIP expression was decreased in experimental ischemic stroke models,and HOTTIP overexpression relieves oxygen-glucose deprivationinduced neuronal apoptosis (Wang et al.,2018b).However,its function in PD deserves further study.
Like lncRNAs,microRNAs (miRNAs) are another type of noncoding single-stranded RNA with a length of about 20–24 nucleotides.MiRNAs regulate genes by combining with the 3′-untranslated region of a target gene and are involved in multiple diseases (Karthikeyan et al.,2016;Zhong et al.,2018).Multiple miRNAs have been found to regulate neuronal damage and microglial inflammation in PD (Brites and Fernandes,2015;Prasad,2017;Neal and Richardson,2018).In particular,miR-615-3p is involved in multiple diseases,including cancer (Pu et al.,2017) and Huntington’s disease(Hoss et al.,2014).Interestingly,HOTTIP targets miR-615-3p and facilitates hypoxia-induced glycolysis in non-small cell lung cancer (Wang et al.,2018a).Nevertheless,the impact of HOTTIP on miR-615-3p in PD remains unknown.
The coding gene of forkhead box protein O1 (FOXO1) is a member of the FOX transcription factor family that has a distinct forkhead domain (Rudd et al.,2007).Recent research suggests that FOXO1 functions as a gene regulator contributing to biological processes,such as longevity,protein turnover,substrate metabolism,cell survival,and cell death (Xing et al.,2018).For instance,the G-protein-coupled receptor,proteaseactivated receptor 2,can enhance the M1 polarization of macrophages through FOXO1,thereby exacerbating inflammation (Chen et al.,2019).Interestingly,FOXO1 is reversely modulated by miR-615-3p,thereby modulating osteogenic differentiation of human lumbar ligamentum flavum cells (Yin et al.,2017).Still,the function of miR-615-3p on FOXO1 in microglia-mediated neuroinflammation and its upstream mechanism in PD needs further study.
Thus,we hypothesized that both HOTTIP and miR-615-3p exert crucial functions in the mouse PD model and MPP+-treated neurons and microglia.In this study,we constructed PD models with 1-methyl-4-phenylpyridium (MPP+) and MPTPin vitroandin vivoto clarify the functions of HOTTIP and miR-615-3p in PD.
Materials and Methods
Experimental animals
The Ethics Review Board of the Affiliated Hospital of Qingdao University authorized this experiment (approval No.ZHQD2018-0322) in June 2018,and the experiment was implemented in accordance with the instructions for the feeding,management and utilization of experimental animals formulated by the National Institutes of Health.Forty-eight C57BL/6 male mice (20–22 g,8–10 weeks old,specific-pathogen-free level) were bought from the Animal Center of Shandong University (license No.SCXK (Lu) 2019 0001) and raised in sterile equipment (25 ± 2°C,with the relative humidity of 60–70%) with a 12 hour light/12 hour dark cycle.The mice could eat and drink freely,and they were randomized into four groups:sham,MPTP,MPTP+sh-NC,and MPTP+sh-HOTTIP,with 12 mice in each group.
The PD model was established through intraperitoneal injection of MPTP-HCl (20 mg/kg;MilliporeSigma,St.Louis,MO,USA).An equal amount (200 µL) of normal saline was injected into mice in the Sham group.The MPTP+sh-HOTTIP group was injected with lentivirus sh-HOTTIP (Ruibo Biotechnology Co.,Ltd.,Guangzhou,China) and the MPTP +sh-NC group was injected with its negative control (sh-NC).The mice were injected with the lentiviral sh-HOTTIP vector or sh-NC via the right ventricle (bregma:–2 mm,lateral:2 mm,dorsoventral:3 mm;Paxinos and Watson,2007) using a stereotactic catheter (Stoelting Co.,Wood Dale,IL,USA);5 µL solvent containing 20 nM sh-HOTTIP or sh-NC was injected via a catheter every day for five consecutive days.The intraperitoneal administration of MPTP began 24 hours after the last injection of the solvent.All surgical operations were implemented under anesthesia with 40 mg/kg phenobarbital sodium (Standardpharm Co.Ltd.,New York,NY,USA) via intraperitoneal injection.Neurological behaviors of the mice were evaluated during the 56 days following MPTP administration.The mice were sacrificed after they were deeply anesthetized by intraperitoneal injection of phenobarbital sodium (150 mg/kg) on day 57 for sampling.
Cell culture
Neuroblastoma SH-SY5Y cells and BV2 microglial cells were bought from the Cell Center of the Chinese Academy of Sciences (Shanghai,China).Afterward,they were cultured in the Dulbecco’s modified Eagle medium (Thermo Fisher HyClone,Logan,UT,USA) containing 10% fetal bovine serum(Invitrogen,Carlsbad,CA,USA) and 1% penicillin/streptomycin(Invitrogen) and incubated at 37°C with 5% CO2.The Dulbecco’s modified Eagle medium and fetal bovine serum were from Thermo Fisher Scientific.During the logarithmic growth phase of the cells,0.25% trypsin (Thermo Fisher HyClone) was used for trypsinization and subculture.
SH-SY5Y and BV2 cells in the logarithmic growth phase were cultured in 96-,24-,or 6-well plates.After cell growth was stable,different doses of MPP+(12.5–100 µM;Millipore Sigma) were added for 24 hours.
Cell transfection
PcDNA empty vector (NC),pcDNA3.1-HOTTIP (HOTTIP),miRNA control (miR-NC),and miR-615-3p mimics were bought from GenePharma Co.,Ltd.(Shanghai,China).SH-SY5Y and BV2 cells were seeded in 24-well plates at 3 × 105/well,incubated at 37°C with 5% CO2for 24 hours,and then transfected.Afterward,the above vectors (50 nM) and Lipofectamine® 3000 (Invitrogen) were mixed and cotransfected into SH-SY5Y and BV2 as per the supplier’s guidelines.After 24 hours,the original medium was discarded,fresh medium was provided,and the cells were further cultured for 24 hours.Finally,reverse transcriptionpolymerase chain reaction (RT-PCR) was applied to test the transfection validity.
Cell viability test
SH-SY5Y cells transfected with pcDNA3.1-HOTTIP and/or miR-615-3p mimics were obtained,and the suspension was seeded into 96-well plates (100 µL/well at 5 × 104cells/mL).Then,the plates were put in an incubator for preculture (37°C,5% CO2).After the cells were treated with MPP+(12.5–100µM;MilliporeSigma),each well was supplemented with 10 µL Cell Counting Kit-8 solution (Beyotime,Shanghai,China) and incubated for 1 hour.The optical density was determined at 450 nm using a Thermo Scientific Microplate Reader (Waltham,MA,USA).
Flow cytometry
SH-SY5Y cells transfected with pcDNA3.1-HOTTIP and/or miR-615-3p mimics were collected,and the cell suspension(2000 µL/well,1 × 105cells/mL) was inoculated in 6-well plates.After the cells were treated with MPP+(12.5–100µM;MilliporeSigma),the cells were processed with ethylenediaminetetraacetic acid-free trypsin and collected.Then,they were cleared twice with precooled phosphatebuffered saline (PBS),and 1–5 × 105cells were harvested.Afterward,100 µL 1× binding buffer was added for cell resuspension,and 5 µL AnnexinV-fluorescein isothiocyanate and 5 µL propidium iodide staining solution (Solarbio,Beijing,China) was supplemented,mixed gently in the dark,and incubated at room temperature for 10 minutes.Subsequently,400 µL 1× binding buffer was supplemented and mixed well.The apoptotic level was examined with FACScan flow cytometry(BD Biosciences,Franklin Lakes,NJ,USA) within 1 hour.
Dual-luciferase reporter gene assay
The online database Starbase (http://starbase.sysu.edu.cn/)was used to predict the potential miRNA targets of HOTTIP and FOXO1.On the basis of the analysis,the wild-type (wt)reporter vectors HOTTIP-wt and FOXO1-wt containing the luciferase vector,and reporting vectors HOTTIP-mut and FOXO1-mut that were mutated at the miR-615-3p binding site,were acquired from GenePharma.SH-SY5Y and BV2 cells were seeded in 24-well plates (1000 µL/well at 5 × 105cells/mL).After the cells cultured overnight until the fusion rate reached 70%,miR-615-3p mimics and miR-NC were co-transfected with HOTTIP-wt,FOXO1-wt,HOTTIP-mut and FOXO1-mut.After cell lysis,the dual-luciferase reporter system (RiboBio,Guangzhou,China) was used to determine the luciferase activity of the samples.
RT-PCR
The RNA from nuclear and cytoplasmic fractions from SH-SY5Y and BV2 cells was isolated using the PARIS kit (Cat# AM1921;Thermo Fisher Scientific,Yokohama,Japan) according to the manufacturer’s instructions.Briefly,SH-SY5Y and BV2 cells were collected and washed with PBS three times.Next,the precooled cell fractionation buffer (300 µL) was used to resuspend the cells,which were then incubated on ice for 10 minutes.After that,the cells were collected and centrifuged(500 ×g) for 5 minutes.The supernatant containing the cytoplasmic fraction was collected,leaving behind the nucleus-rich pellet.Nuclear and cytoplasmic RNA were then precipitated after elution at room temperature.The relative expression ofHottipandmiR-615-3pwere determined by real time quantitative PCR (RT-qPCR).U6andGAPDHserved as internal control transcripts for nuclear and cytoplasmic RNA,respectively.
Total RNA was separated from tissues or cells with the TRIzol reagent (Invitrogen).The PrimeScriptTMRT Reagent kit(Invitrogen (China),Shanghai,China) was used to reversetranscribe the RNA into complementary DNA after the content and purity determination.The qPCR was carried out with the Bio-Rad CFX96 quantitative PCR system and the SYBR green PCR mixture.PCR was performed with predenaturation at 95°C for 5 minutes,denaturation at 95°C for 15 seconds,and annealing at 60°C for 30 seconds.U6served as the internal reference formiR-615-3p,andGAPDHandU6served as the internal references for the remaining molecules.The 2–ΔΔCtwas used for statistical analysis.Each test was conducted in triplicate.The specific primer sequences were shown inTable 1.
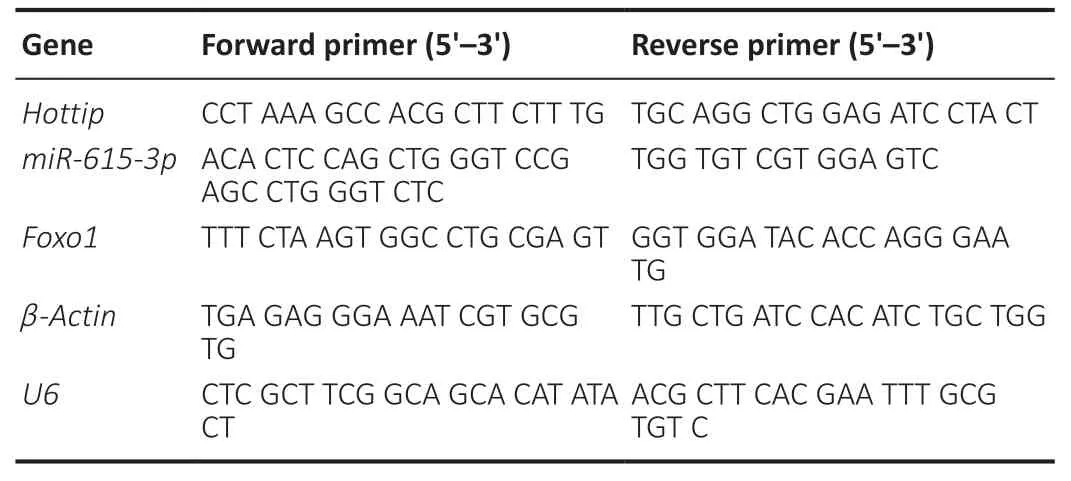
Table 1|Primers used in this study
Western blot assay
The Bradford method was used for protein extraction and quantification.Protein from SH-SY5Y and BV2 cells and samples from SN area in PD mice was boiled for 5 minutes,cooled on ice,and centrifuged for 30 seconds.Afterward,the supernatant was collected for polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes at 100 V for 1 hour.Next,the membranes were blocked with 5% skim milk at room temperature for 1 hour and then incubated with the primary rabbit anti-Caspase-3antibody (Cat# ab13847,1:1000),recombinant rabbit anti-Bax antibody (Cat# ab32503,1:1000),recombinant rabbit anti-Bcl-2 antibody (Cat# ab32124,1:1000),mouse anti-β-actin antibody(Cat# ab6276,1:2000),recombinant rabbit anti-NACHT,LRR and PYD domains-containing protein 3 (NLRP3) antibody(Cat#ab263899,1:1000),rabbit anti-Caspase-1 antibody (Cat#ab207802,1:1000),recombinant rabbit anti-adaptor protein apoptosis-associated speck-like protein containing a CARD(ASC) antibody (Cat# ab151700,1:1000),rabbit anti-inducible nitric oxide synthase (iNOS) antibody (Cat# ab178945,1:1000),rabbit anti-cyclooxygenase 2 (COX2) antibody (Cat#ab179800,1:1000),rabbit anti-nuclear factor kappa-B (NFκB) p65 antibody (Cat# ab16502,1:1000),rabbit anti-NF-κB p65 (phospho S529) antibody (Cat# ab109458,1:1000),rabbit anti-FOXO1 antibody (Cat# ab52857,1:1000),rabbit antihistone H3 antibody (Cat# ab215728,1:1000),rabbit antityrosine hydroxylase (TH) antibody (neuronal marker;Cat#ab112,1:1000) and recombinant rabbit anti-ionized calciumbinding adapter molecule 1 (Iba1) antibody (Cat# ab178846,1:1000) (all purchased from Abcam,Cambridge,MA,USA)overnight at 4°C.After that,the membranes were cleared with Tris buffered saline solution containing 0.1% Tween 20 twice and incubated with the goat anti-rabbit or goat antimouse horseradish peroxidase-labeled secondary antibodies(Cat# ab205718,1:2000,Abcam) at room temperature for 1 hour.The membranes were cleared three times before being exposed to electro-chemiluminescence luminescence reagent(Thermo Fisher HyClone),and the membrane scanner (Bio-Rad,Hercules,CA,USA) was used for imaging.
Enzyme linked immunosorbent assay
The enzyme linked immunosorbent assay (ELISA) kit from Multisciences (Lianke) Biotech,Co.,Ltd.(Hangzhou,China)was used to measure interleukin (IL)-lβ,IL-6,IL-18,and tumor necrosis factor (TNF)-α levels in BV2 cells according to the kit instructions using a Thermo Scientific Microplate Reader.
Immunohistochemistry
The brains from mice in each group were paraffin-embedded,sectioned (4 µm),dewaxed,and then repaired under high pressure with a 0.01 mM sodium citrate buffer solution for 15 minutes.After natural cooling,the sections were washed with 1× PBS three times (3 minutes each time).Then,3 mL/L H2O2was added and the sections were incubated in a humid box for 10 minutes to inactivate the endogenous peroxidase.Subsequently,the sections were rinsed with 1× PBS three times (3 minutes each time),and then incubated with the rabbit anti-TH antibody (Cat# ab112,1:150,Abcam),rabbit anti-Iba1 (Cat# ab178846,1:100,Abcam) and rabbit anti-Caspase-3 antibody (Cat# ab13847,1:200,Abcam) at 4°C overnight.Next,they were rinsed with 1× PBS three times(5 minutes each time) and incubated with the horseradish peroxidase-goat anti-rabbit IgG (H&L) (Cat# ab205718,1:200 Abcam) at room temperature for 30 minutes.After being rinsed with 1× PBS three times (5 minutes per time),the sections were developed with 3,3′-diaminobenzidine tetrahydrochloride for 3 minutes,counterstained with hematoxylin and eosin staining,mounted,observed with a light microscope (CX31,Olympus,Tokyo,Japan),and photographed.The Image-Pro Plus 6.0 software (National Institutes of Health,Bethesda,MD,USA) was used for quantitative analysis.
Neurological function evaluation
The motor function of the mice was evaluated by the Rotarod test (Luo et al.,2018).Mice were placed on an accelerating rod (Unibiolab,Beijing,China).The rotation speed gradually increased from 2 r/min to 20 r/min.The time to the mouse’s first fall was recorded,with a maximum of 300 seconds.The test was implemented every 14 days for 56 days.
Latency to fall and grip strength were measured to assess the muscular forelimb strength on day 56 after surgery with the inverted screen test and forelimb grip strength test,respectively (Singh et al.,2017).The mouse was put in the center of a wire mesh screen (Shanghai Yuyan Scientific Instrument Co.,Ltd.,Shanghai,China) and then rotated upside down.Latency to fall was measured in seconds.A grip strength tester (Shanghai Yuyan Scientific Instrument Co.,Ltd.)was used to test muscular forelimb strength.
The pole test was conducted to assess bradykinesia (Lee et al.,2019).Mice were placed on top of a 55-cm-high rough pole (8 mm in diameter).We recorded how long it took the mice to turn completely around,climb down and reach the ground with four paws.Each trial had a maximum length of 30 seconds.
Dopamine content measurements
High performance liquid chromatography (HPLC) was used to measure dopamine content in the striatal area of mice according to a previous study (Yang et al.,2014).Briefly,the brain tissue of the striatal area was isolated,weighed,dissected,homogenized and lysed in 400 µL of ice-cold 0.1 M perchloric acid for 10 minutes.After that,the homogenates were centrifuged (14,000 ×g,10 minutes) at 4°C.The supernatant was collected and then subjected to the HPLC (Nexera UHPLC/HPLC System,Shimadzu,Japan) for dopamine content determination.Empower software (Waters Corporation,Milford,MA,USA) was used for data analysis.Dopamine content was quantified in reference to an external standard.
Statistical analysis
The data were analyzed with SPSS 22.0 statistical software(IBM,Armonk,NY,USA).The data are presented as mean ±standard deviation (SD).Pairwise comparisons were made by Student’st-test,and comparisons between multiple groups were performed with one-way analysis of variance followed by Student-Newman-Keuls test.P<0.05 represented statistical significance.
Results
MPP+ treatment changes HOTTIP,miR-615-3p and FOXO1 expression
We treated SH-SY5Y and BV2 cells with different doses of MPP+for 24 hours to investigate the role of HOTTIP,miR-615-3p and FOXO1 in PD.We conducted RT-PCR and western blot to measure HOTTIP,miR-615-3p and FOXO1 levels in the cells.MPP+dose-dependently elevated HOTTIP expression and dampened miR-615-3p expression in SH-SY5Y and BV2 cells (Figure 1A–D).RT-PCR and western blot showed that the mRNA and protein expression of FOXO1 were elevated by MPP+,and the elevation was enhanced with the increasing MPP+doses (Figure 1E–H).Thus,HOTTIP,miR-615-3p and FOXO1 were altered in MPP+-treated SH-SY5Y and BV2 cells.
The function of HOTTIP and miR-615-3p in regulating MPP+-induced neuronal toxicity
We transfected SH-SY5Y cells with vectors to overexpress HOTTIP,miR-615-3p,or both.Upregulating HOTTIP inhibited miR-615-3p,and upregulating miR-615-3p inhibited HOTTIP(Figure 2AandB).Next,the Cell Counting Kit-8 method and flow cytometry were used to examine cell viability and apoptosis,respectively.Compared with the control group,the HOTTIP group had reduced cell viability and increased cell apoptosis (Figure 2CandD).In contrast,miR-615-3p overexpression did not have a significant effect on cell viability and apoptosis.However,forced miR-615-3p overexpression in the HOTTIP group enhanced cell viability and reduced apoptosis (P<0.05,vs.HOTTIP group;Figure 2CandD).Next,we treated SH-SY5Y cells with MPP+to investigate the effect of HOTTIP and miR-615-3p on neuronal toxicity in the MPP+PD cellular model.MPP+treatment significantly decreased SH-SY5Y viability (P<0.05,vs.control group;Figure2E) and increased the apoptosis rate (P<0.05,vs.control group;Figure 2F–G).In addition,miR-615-3p overexpression enhanced SH-SY5Y cell viability and suppressed apoptosis compared with the MPP+group,whereas HOTTIP overexpression had the opposite effect,indicating that HOTTIP exacerbated the MPP+-induced cellular damage (P<0.05;Figure 2E–G).Interestingly,the MPP++miR+lnc group had increased cell viability and decreased apoptosis compared with the MPP++lnc group,suggesting that miR-615-3p alleviated HOTTIP-mediated cellular damage.Furthermore,we measured the expression of apoptosis-related proteins(cleaved Caspase-3,Bax,and Bcl2) and NLRP3-ASC-cleaved Caspase-1 inflammasomes with western blot.MPP+enhanced Caspase-3,Bax,and NLRP3-ASC-Caspase-1 inflammasome expression and suppressed Bcl2 expression (Figure 2HandI).miR-615-3p overexpression attenuated the proapoptotic proteins and NLRP3-ASC-Caspase-1 inflammasomes,whereas HOTTIP had the opposite effect (Figure 2HandI).The above results demonstrated that HOTTIP exacerbated the MPP+-induced neuronal toxicity,and miR-615-3p attenuated the MPP+-induced neuronal toxicity and weakened the effect of HOTTIP upregulation.
The function of HOTTIP and miR-615-3p in regulating MPP+-induced microglial inflammation
Microglial activation is a key feature in PD (Vivekanantham et al.,2015).Therefore,we constructed HOTTIP and miR-615-3p overexpression models in BV2 cells.ELISA and western blot showed that the proinflammatory cytokines (including IL-lβ,IL-6,IL-18,and TNF-α),the pro-oxidant proteins iNOS and COX2,and the proinflammatory protein phosphorylated NF-κB produced by BV2 cells were upregulated after MPP+treatment (P<0.05;Figure 3A–C).Overexpression of miR-615-3p and HOTTIP suppressed and enhanced the abovementioned inflammatory response,respectively (P<0.05;Figure 3A–C).BV2 cells in the MPP++miR+lnc group had a lower proinflammatory response than those in the MPP++lnc group (P<0.05;Figure 3A–C).Next,we measured the NLRP3-ASC-Caspase-1 inflammasome expression in BV2 cells.MPP+induced NLRP3-ASC-Caspase-1 inflammasome activation and miR-615-3p overexpression attenuated this effect (compared with the MPP+group).In contrast,HOTTIP overexpression enhanced the NLRP3-ASC-Caspase-1 inflammasome activation compared with the MPP+group (P<0.05;Figure 3D).Moreover,miR-615-3p suppressed the HOTTIP-induced activation of NLRP3-ASC-Caspase-1 inflammasomes (P<0.05;Figure 3D).These results suggest that HOTTIP was proinflammatory,whereas miR-615-3p was anti-inflammatory in MPP+-induced inflammation.

Figure 1|MPP+ induced changes in HOTTIP/miR-615-3p/FOXO1 expression in SH-SY5Y and BV2 cells.
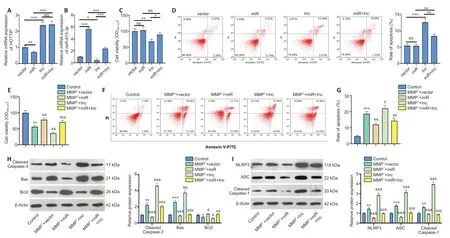
Figure 2|The role of HOTTIP/miR-615-3p in regulating MPP+-induced neuronal toxicity in SH-SY5Y cells.
Both HOTTIP and FOXO1 target miR-615-3p
The online database Starbase (http://starbase.sysu.edu.cn/) was used to predict the potential targets of HOTTIP and FOXO1.Starbase indicated that miR-615-3p binds to HOTTIP and FOXO1 (Figure 4AandB).Next,we compared the profiles of miR-615-3p,HOTTIP and FOXO1 in SH-SY5Y and BV2 cells under different treatments.Compared with the MPP++vector group,the MPP++lnc group had increased HOTTIP and FOXO1 levels,and decreased miR-615-3p levels (Figure 4C–E).In contrast,FOXO1 was downregulated,miR-615-3p was upregulated,and HOTTIP did not change significantly in the MPP++lnc+miR group compared with the MMP++lnc group(Figure 4C–E).Next,we implemented RT-PCR to examine the distribution of miR-615-3p and HOTTIP in SH-SY5Y and BV2 cells.Both HOTTIP and miR-615-3p were mainly distributed in the cytoplasm (Figure 4F–G).Furthermore,we carried out a dual-luciferase reporter assay to examine the association between HOTTIP and FOXO1.The miR-615-3p mimics markedly dampened the luciferase activity of cells transfected with HOTTIP-wt and FOXO1-wt (containing miR-615-3p binding sites),and had little impact on that of cells transfected with HOTTIP-mut and FOXO1-mut (containing mutated miR-615-3p binding sites) (Figure 4H–I).Thus,HOTTIP inhibited miR-615-3p,and the latter targeted the 3′-untranslated region of FOXO1 in SH-SY5Y and BV2 cells.
Overexpressing FOXO1 exacerbates the MPP+-induced neuronal damage
We constructed a FOXO1 overexpression SH-SY5Y cell model to investigate the effect of FOXO1 on neurons in PD (Figure 5A).We transfected miR-615-3p into cells overexpressing FOXO1,and FOXO1 was suppressed in the MPP++FOXO1+miR group compared with that in the MPP++FOXO1 group (P<0.05;Figure 5B).Next,we tested the levels of neuronal viability and apoptosis.Cell viability and Bcl2 expression were suppressed,whereas cell apoptosis and Caspase-3 and Bax expressions were elevated in the MPP++FOXO1 group compared with MPP++vector group (P<0.05;Figure 5C–E).In contrast,SHSY5Y viability was enhanced and apoptosis was reduced in the MPP++vector group compared with that of the MPP++FOXO1 group (P<0.05;Figure 5C–E).Subsequently,we measured the expression of NLRP3-ASC-Caspase-1 inflammasomes.FOXO1 overexpression facilitated the expression of NLRP3-ASC-Caspase-1,and this effect was attenuated by miR-615-3p(Figure 5F).Thus,FOXO1 upregulation exacerbated the MPP+-induced neuronal damage,possibly by activating NLRP3-ASCCaspase-1 inflammasomes.
FOXO1 enhances MPP+-induced inflammation in microglia
Next,we constructed a FOXO1 overexpression model in BV2 cells (Figure 6A) and showed that miR-615-3p overexpression suppressed FOXO1 expression (Figure 6B).Subsequently,we treated BV2 cells with MPP+and detected the expression of inflammatory factors and proteins by ELISA and western blot.IL-lβ,IL-6,IL-18,TNF-α,iNOS,COX2,and phosphorylated NFκB were upregulated in the MPP++FOXO1 group compared with the MPP+group (P<0.05;Figure 6C–E).In contrast,miR-615-3p overexpression inhibited the above-mentioned inflammatory response (P<0.05;Figure 6C–E).Finally,western blot was implemented to measure the expression of NLRP3-ASC-cleaved Caspase-1 inflammasomes.FOXO1 upregulation further activated the NLRP3-ASC-Caspase-1 inflammasome,and miR-615-3p overexpression dampened this effect (P<0.05vs.MPP++FOXO1 group;Figure 6F).These results suggest that FOXO1 exerted a proinflammatory effect on MPP+-induced inflammation.
充分按照农民的要求建立整治的标准和顺序。建立政府、村集体以及农民等共管和共评的机制。积极引导村民参与到乡村建设中来,从而增强村民的环境意识和参与整治的主动性与积极性。

Figure 3|The function of HOTTIP/miR-615-3p in regulating MPP+-mediated inflammation in microglia (BV2 cells).
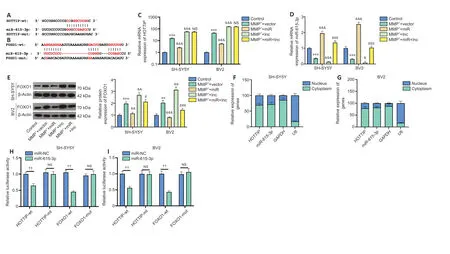
Figure 4|Both HOTTIP and FOXO1 target miR-615-3p in SH-SY5Y and BV2 cells.
HOTTIP knockdown alleviates neurological dysfunction and neuronal damage in PD mice
We induced a PD mouse model combined with HOTTIP knockdown to explore the therapeutic effect of HOTTIP in MPTP-induced PD.First,we assessed the neurological changes in mice.Rotarod time and latency to fall were shorter,and grip strength and pole test scores were lower in PD mice than in sham mice (P<0.001;Figure 7A–D).However,the neurological scores of HOTTIP knockdown PD mice were significantly improved compared with those of control PD mice (P<0.05;Figure 7A–D).We also measured the number of TH-labeled neurons and Caspase-3-labeled apoptotic neurons in the SN area.THpositive cells and TH expression were decreased,whereas Caspase-3-positive cells and Caspase-3 and Bax expressions were increased in PD mice compared with sham mice (Figure7E–H).Furthermore,HPLC was performed to evaluate dopamine content in the striatal area.Dopamine content was reduced in the MPTP-treated mice compared with sham mice,and HOTTIP knockdown enhanced the dopamine level significantly (Figure 7I).These results suggest that HOTTIP knockdown exerted a neuroprotective effect in PD mice.
HOTTIP knockdown suppresses neuroinflammation and microglial activation in PD mice
We used immunohistochemistry and western blot to examine microglial activation in the SN area.The number of Iba1-positive cells and Iba1 expression were increased in PD mice compared with sham mice (Figure 8AandB).In contrast,the Iba1-positive cell number and Iba1 expression were both decreased in HOTTIP knockdown PD mice compared with control PD mice (Figure 8AandB).
In addition,the expression of inflammatory cytokines and proteins was measured in the SN area.IL-lβ,IL-6,IL-18,TNF-α,iNOS,COX2,and phosphorylated NF-κB were upregulated in the PD group compared with the sham group,and the increased expression of these factors was ameliorated in the HOTTIP knockdown PD group compared with the MPTP +sh-NC group (P<0.05;Figure 8C–E).Furthermore,HOTTIP knockdown alleviated MPTP-induced NLRP3-ASC-Caspase-1 inflammasome activation (P<0.05;Figure 8F).In the SN area,HOTTIP levels were increased,whereas miR-615-3p and FOXO1 levels were decreased in the MPTP group compared with the sham group (P<0.001;Figure 8G–I).HOTTIP was downregulated,whereas miR-615-3p and FOXO1 were upregulated in HOTTIP knockdown PD mice compared with control PD mice (P<0.001;Figure 8G–I).The above results suggested that the downregulation of HOTTIP alleviated neuroinflammation in the PD model by regulating miR-615-3p and FOXO1.

Figure 5|FOXO1 overexpression exacerbates MPP+-induced neuronal damage in SH-SY5Y cells.
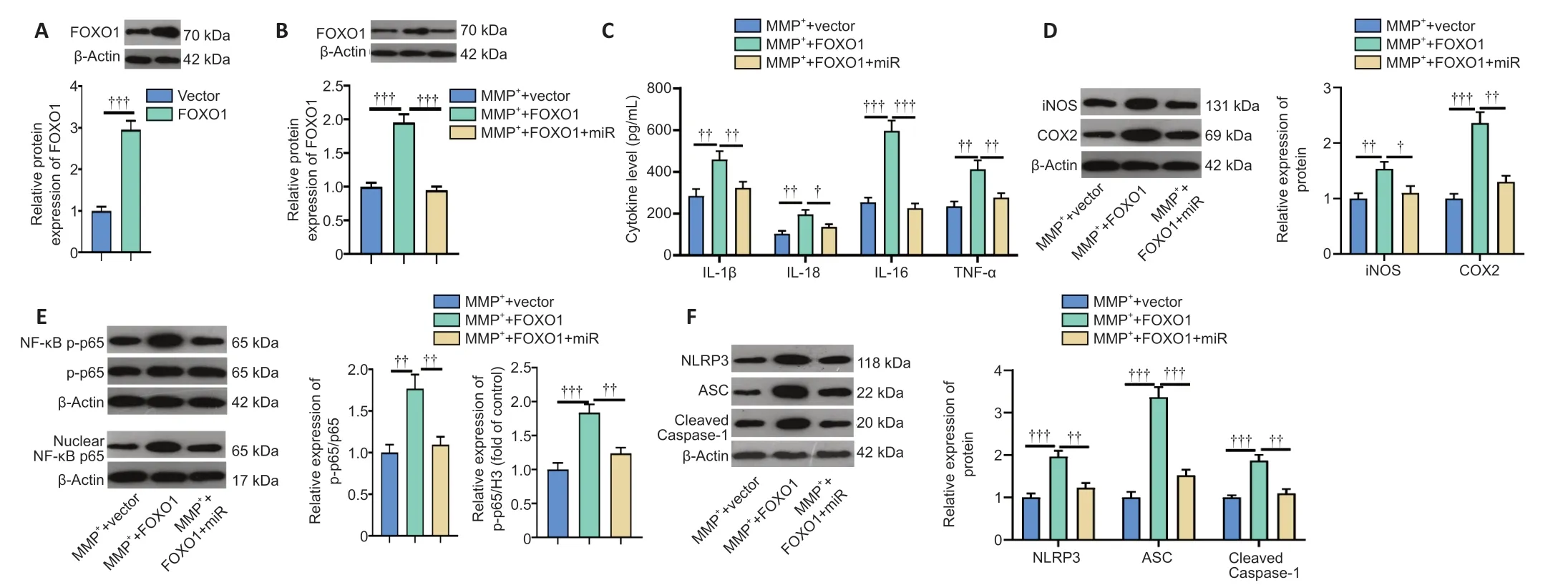
Figure 6|FOXO1 enhances the role of MPP+-induced inflammation in microglia (BV2 cells).
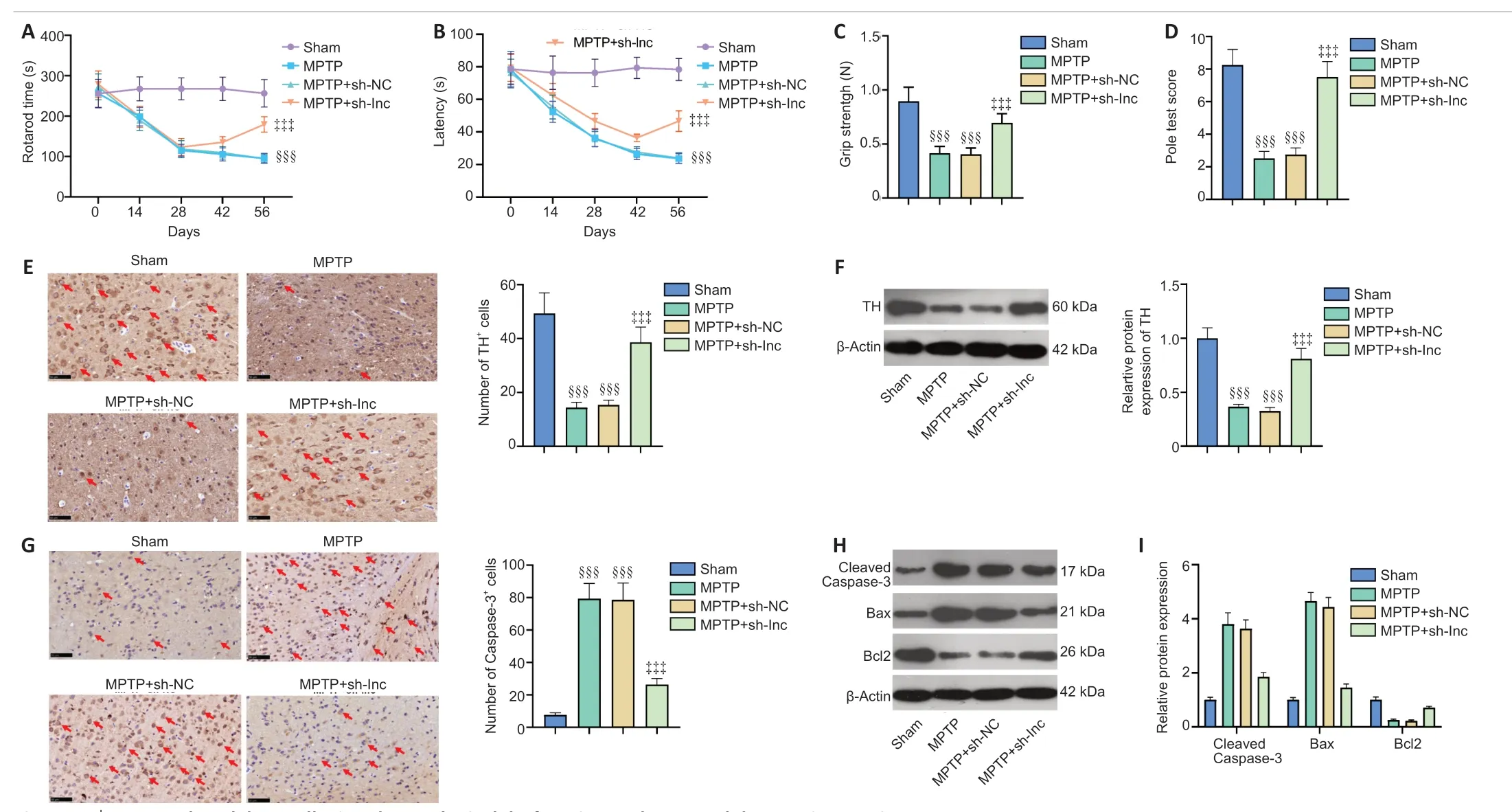
Figure 7|HOTTIP knockdown alleviated neurological dysfunction and neuronal damage in PD mice.

Figure 8|HOTTIP knockdown suppresses neuroinflammation and microglial activation in PD mice.
Discussion
PD is a common neurodegenerative disorder and the most frequent extrapyramidal disease in middle-aged and older people.Over the years,several studies have reported that neuroinflammation,neuronal excitotoxicity,genetic mutations and incorrect protein folding are important factors in PD pathophysiology (Liu et al.,2021).Here,we probed the molecular mechanism of the HOTTIP/miR-615-3p/FOXO1 axis in PD.Our results suggest that HOTTIP mediates neuronal apoptosis and microglial activation through the miR-615-3p/FOXO1 axis to produce excessive proinflammatory responses,thereby exacerbating neuronal damage.
Noncoding RNAs have an important role in brain development,and have distinct expression in the brain (Schaukowitch and Kim,2014).In particular,lncRNAs play an important role in CNS inflammatory response regulation.For example,lncRNA MALAT1 shuttled by exosomes from adipose-derived stem cells not only reduces traumatic brain injury-induced neurological damage,but also attenuates inflammation pathways (Patel et al.,2018).Additionally,lncRNA colorectal neoplasia differentially expressed (CRNDE) is upregulated in the plasma of traumatic brain injury patients and mice,and inhibiting CRNDE alleviates traumatic brain injury-mediated neurological dysfunction and reduces neuroinflammatory factor expression and neuronal damage (Yi et al.,2019).Moreover,lncRNAs can regulate microglial inflammation in CNS diseases.For example,lncRNA metastasis suppressor-1(Mtss1) is upregulated in brain lesions after intracerebral hemorrhage,and it inhibits TIR-domain-containing adapterinducing interferon-β expression,P65 phosphorylation,TNF-α and IL-1β secretion,and microglial activation (Chen et al.,2020a).Furthermore,lncRNA small nucleolar RNA host gene 5 is significantly upregulated in a mouse spinal nerve ligation model,and its inhibition eases nerve pain through miR-154-5p/CXCL13 and alleviates neuroinflammation caused by microglial and astrocyte activation (Chen et al.,2020c).These studies support the prominent function of lncRNAs in regulating CNS inflammation.Similarly,lncRNA HOTTIP also contributes to inflammatory diseases such as rheumatoid arthritis (Hu et al.,2020) and inflammation mediated by high glucose (Zhu et al.,2019).These studies indicate that HOTTIP has a proinflammatory function.
Here,we found that HOTTIP was upregulated in MPP+-and MPTP-induced PD models,bothin vitroandin vivo.HOTTIP overexpression facilitated MPP+-treated nerve cell damage and microglial inflammation,and HOTTIP knockdown attenuated the neurological damage in PD model mice and suppressed DAN apoptosis and microglial activation in the SN area.These results indicate that HOTTIP inhibition has anti-inflammatory and neuroprotective effects.Moreover,HOTTIP knockdown significantly increased the dopamine level in the striatal area compared with that in the negative control group.Hence,we consider that HOTTIP downregulation also improves striatal dopaminergic projections in the PD mouse model.
Multiple studies have confirmed that abnormal miRNA expression modulates neuronal apoptosis and microglial activation.For example,miR-188-5p attenuates oxygenglucose deprivation-mediated neuronal damage and inhibits PTEN expression (Li et al.,2020a).Additionally,miR-216a suppresses SH-SY5Y cell death by targeting Bax in MPP+-induced PD modelsin vitro(Yang et al.,2020).Similarly,miR-29c and miR-190 can also reduce neuronal apoptosis and microglial inflammation in MPTP-treated mice and MPP+-treated SH-SY5Y cells (Sun et al.,2019;Wang et al.,2020).These studies indicate that miRNAs have extensive regulatory effects in CNS diseases.In this study,we confirmed throughin vitroexperiments that miR-615-3p attenuates MPP+-induced neuronal damage and microglial activation,suggesting that miR-615-3p has a neuroprotective effect in PD.Furthermore,previous studies have shown that HOTTIP regulates the progression of gastric cancer (Xiao et al.,2020) and renal cell carcinoma (Wang et al.,2018a) by modulating miR-615-3p.Here,we showed that HOTTIP is an upstream regulatory gene of miR-615-3p,and that HOTTIP overexpression decreases miR-615-3p.Furthermore,miR-615-3p overexpression attenuated the neurotoxic and proinflammatory effects mediated by HOTTIP overexpression.
Multiple members of the FOXO family have been shown to regulate inflammation.For example,FOXO3 inhibition dramatically inhibits the expression of inflammatory factors in a subarachnoid hemorrhage rat model (Zuo et al.,2019).FOXO3 is upregulated in Staphylococcal enterotoxin B-mediated acute lung injury,and the inhibition of FOXO3 expression via miR-222 suppresses the Staphylococcal enterotoxin B-induced proinflammatory response and apoptosis (Chen et al.,2020b).In contrast,ε-viniferin upregulates SIRT3 expression in PD,which promotes FOXO3 deacetylation and nuclear localization,thus exerting a neuroprotective effect (Zhang et al.,2020).Moreover,inhibition of FOXO4 via adiponectin markedly attenuates NLRP3-inflammasome-mediated pyroptosis of aortic endothelial cells (Zhang et al.,2019a).Presently,we found that FOXO1 is upregulated in the PD model,andin vitroexperiments demonstrated that FOXO1 overexpression exacerbated MPP+-induced neuronal apoptosis and microglial activation.It has been shown that miR-135b alleviates apoptosis in MPP+-treated SH-SY5Y and PC-12 PD model cells;mechanically,miR-135b targets and inhibits the expression of FOXO1 and inactivates NLRP3 inflammasomes (Zeng et al.,2019).Previous studies have demonstrated that miR-615-3p targets FOXO1 (Yin et al.,2017),which was also confirmed in the present study.Our results showed that miR-615-3p overexpression weakened the effect of FOXO1 overexpression.
NLRP3 inflammasomes are multiprotein complexes assembled by NLRP3 (also called NALP3 or cryopyrin),cysteinyl aspartatespecific protease (caspase-1) precursor and apoptosisassociated speck-like protein containing CARD (ASC),and are important parts of the natural immune system (Li et al.,2020b).After being affected by pathogen-associated molecular patterns or host-derived damage-associated molecular patterns,NLRP3 recruits and activates protease caspase-1,which accelerates the release of IL-1β and IL-18 and induces inflammation (Liu et al.,2020b).Recently,researchers have shown that NLRP3 inflammasome activation is related to the occurrence and progression of Alzheimer’s disease and amyotrophic lateral sclerosis (Söderbom and Zeng,2020).Importantly,NLRP3 inflammasome activation is associated with the PD pathogenesis;after NLRP3 activation in microglia,proinflammatory cytokines are produced,which directly mediates neuronal damage (Wang et al.,2019;Haque et al.,2020).Also,NLRP3 inflammasome is increased in mesencephalic neurons of PD patients,and DAN is a cellular source of inflammasome activity,suggesting that inflammasome activity influences PD development(von Herrmann et al.,2018).In this study,we showed that the upregulation of HOTTIP and FOXO1 induced NLRP3 inflammasome activation,which is consistent with their proinflammatory and nerve damaging effects reported in previous studies.In contrast,miR-615-3p inhibited the apoptosis and microglial damage of MPP+-treated SH-SY5Y cells and offset the effects caused by HOTTIP and FOXO1 upregulation.
Overall,this study showed that HOTTIP and FOXO1 exacerbate neuronal damage and microglial inflammation in PD modelsin vivoandin vitro.Our findings suggest that HOTTIP dampens miR-615-3p as a competitive endogenous RNA,upregulates FOXO1 and activates the NLRP3 inflammasome.However,several limitations of this study need to be addressed in future studies:1) The clinical significance of the HOTTIP/miR-615-3p/FOXO1 axis in PD patients needs to be determined;2) the regulatory axis of HOTTIP/miR-615-3p/FOXO1 should be confirmed in additional animals,including female rats;and 3) more experiments are needed to verify the upstream molecular mechanism of HOTTIP and the mechanism of the interaction between FOXO1 and NLRP3 inflammasomes.
Author contributions:Guarantor of integrity of the entire study:PL‚TJ;study conception:DHW;study design:PS;experimental and clinical studies:XL;data analysis and acquisition:XDC;manuscript preparation:PL;manuscript editing and review:SY.All authors read and approved the final manuscript.
Conflicts of interest:The authors declare that they have no conflict of interests.
Financial support:None.
Institutional review board statement:This study was approved by the Ethics Review Board of the Affiliated Hospital of Qingdao University(approval No.UDX-2018-042) in June 2018.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal‚and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License‚which allows others to remix‚tweak‚and build upon the work non-commercially‚as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ariadna Laguna‚Vall d’Hebron Research Institute‚Spain.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

