Preparation and characterization of hemihydrate calcium sulfate-calcium hydroxide composite bone repair materials
Zheng-Dong Guo, Yang-Yang Bian, Xiao-Qian Liu, Dong Wang, Si-Yuan Zhang, Jian Yang, Lei Peng✉
1. Trauma medical center of the First Affiliated Hospital of Hainan Medical College, Haikou 570216, China
2. Key Laboratory of Emergency and Trauma Ministry of Education, Hainan Medical University, Haikou 570216, China
3. Key Laboratory of Hainan Trauma and Disaster Rescue, The First Affiliated Hospital of Hainan Medical University, Haikou 570216, China
Keywords:Calcium sulfate hemihydrate Calcium hydroxide Bone defect Bone repair material Compressive strength
ABSTRACT Objective: To prepare a bone repair material with certain mechanical strength and biological activity, this paper used calcium sulfate hemihydrate (CSH) powder compounded with calcium hydroxide (Ca(OH)2) powder to prepare a bone repair scaffold material for physicochemical property characterization and testing. Methods: The physical and chemical properties and characterization of the dried and cured bone repair materials were determined by Fourier infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and scanning electron microscopy;Universal material testing machine to determine the mechanical and mechanical strength of composite materials. Results: XRD showed that the structure of the composite material phase at 5% concentration was calcium sulfate hemihydrate and calcium hydroxide after hydration.The FT-IR and XRD analyses were consistent. Scanning electron microscopy (SEM) results showed that calcium hydroxide was uniformly dispersed in the hemihydrate calcium sulfate material. 0%, 1%, 5%, and 10% specimen groups had compressive strengths of 3.86±3.1,5.27±1.28, 8.22±0.96, and 14.4±3.28 MPa. 10% addition of calcium hydroxide significantly improved the mechanical strength of the composites, but also reduced the the porosity of the material. Conclusion: With the addition of calcium hydroxide, the CSH- Ca(OH)2 composite was improved in terms of mechanical material and is expected to be a new type of bone repair material.✉Corresponding author: PENG Lei.
1. Introduction
The number of patients with bone defects is increasing due to an aging population and an increase in accidents, and the demand for bone defect materials from patients has also increased [1].The treatment of large bone tissue defects is still a great challenge for orthopedic surgeons, and much effort has been put into developing new bone repair materials in recent years [2, 3]. Autologous bone grafting is the gold standard for bone tissue defect repair with excellent osteoinduction, but the limited amount of autologous bone and postoperative donor site pain lead to a more limited clinical application [4-6]. As a result, allogeneic, allogeneic bone and synthetic bone repair materials have been developed for the treatment of large segmental bone defects in orthopedics [7].Currently, the more common artificial bone materials include hydroxyapatite[8]、calcium phosphate[9]、calcium sulfate[10]、polylactic acid (PLA) [11]、poly(lactic-co-glycolic acid)(PLGA)[12] etc. Hydroxyapatite has good osteoinductive properties, but its defects such as greater brittleness, too slow degradation rate and poor mechanical properties limit its application in the field of bone repair. Calcium phosphate has good osteoconductivity, but its disadvantages such as relatively poor blood solubility, brittleness and poor mechanical properties limit its clinical application. PLA itself has poor hydrophilicity, difficult to control the degradation rate and low biological activity, which also limit its application in the field of bone. PLGA is widely used in the field of medicine and can be naturally degraded, but its metabolites in the body are acidic and easy to cause inflammatory reaction, which is not conducive to the repair of bone defects in patients.
Calcium sulfate hemihydrate can be divided into two types,α-calcium sulfate hemihydrate and β-calcium sulfate hemihydrate,with different physicochemical properties. The former is characterized by rod and prismatic crystals, while the latter is characterized by aggregation of crystals, irregular crystals and interstitial capillaries. Since α-calcium sulfate hemihydrate forms calcium sulfate dihydrate after hydration with higher strength,lower solubility and higher density, we chose the α-calcium sulfate hemihydrate prepared by our group for the test. Calcium sulfate hemihydrate has a long history of application in clinical orthopedics,and because of its better biocompatibility and biodegradability,the inflammatory response is relatively small and promotes the healing of bone tissue [13-15]. It has been shown that calcium sulfate hemihydrate also has a vascularization-promoting effect, facilitating vascular invasion into the osteoconductive matrix and peripheral mesenchyme, which is beneficial in promoting bone healing.
Calcium hydroxide is a white powder substance with the chemical formula Ca(OH)2. Calcium hydroxide has a self-coagulation effect (absorption of carbon dioxide in solution to generate calcium carbonate) and a role in regulating bone metabolism. The dissolution of calcium hydroxide produces Ca2+and OH-, which can effectively inhibit osteoclast activity, promote the activity of alkaline phosphatase, increase the concentration of phosphate ions in the local microenvironment, promote the deposition of minerals, and facilitate the healing of bone tissue. Ca(OH)2paste is widely used by dentists to treat dental diseases, and low doses of calcium hydroxide can effectively improve the mineralization of bone marrow mesenchymal stem cells and better facilitate the healing of alveolar bone[16-18]. Moreover, calcium hydroxide is less expensive and easy to obtain compared to other materials [19].
Since both CSH and calcium hydroxide have the ability to promote bone healing, there are few studies on the combination of both.Based on this, this experiment utilized deionized water as the curing solution to test the effect of Ca(OH)2at different ratios of 0% , 1% ,5% , and 10% on CSH bone cement and the characterization of the composite.
2. Materials and Methods
2.1 Test material
Calcium sulfate hemihydrate (provided by Key Laboratory of First Aid and Trauma Research Ministry of Education, Hainan Medical College), calcium hydroxide (Jiande Lianshun Calcification Co.)
Preparation of composite materials: Calcium hydroxide powder and calcium sulfate hemihydrate powder were weighed as the solid phase in a 50 ml centrifuge tube in the proportions of 0%, 1%, 5%and 10% by mass of calcium hydroxide, and mixed with full shaking using a vortex mixer. Using deionized water as the curing solution,the mixed bone cement powder and deionized water were mixed according to the solid-liquid ratio of 0.55 ml/g, stirred continuously to form a paste, and poured into the corresponding silicone molds for curing treatment. The cured material is placed in an electric blast dryer at 60℃ for drying.
2.2 FTIR of CSH-Ca(OH)2 composites
The infrared spectra of bone repair materials with calcium sulfate hemihydrate compounded with calcium hydroxide were measured by Fourier transform infrared spectroscopy (Nicolet iS10) with a scan range of 500-4000 cm-1.
2.3 XRD of CSH-Ca(OH)2 composites
The physical phase structure of bone repair materials with calcium sulfate hemihydrate compounded with calcium hydroxide was determined by X-ray spectrometry (D8) with a scan rate of 4 °/min and a scan interval of 10-80°.
2.4 SEM of CSH-Ca(OH)2 composites
Samples of different concentrations were glued onto copper sheets with an accelerating voltage of 3000 kV, and the composites were sprayed with gold, and the surface structure of the composites was observed by scanning electron microscopy (SU8020).
2.5 Mechanical properties of CSH-Ca(OH)2 composites
The mixed bone cement was filled into a mold with a diameter of 6 mm and a height of 12 mm according to the method of preparing bone repair materials described above. 3 samples of each group of materials were prepared, and after their final setting, they were placed in a constant temperature drying oven at 60℃ until they were completely dry. The compressive strength of each sample was tested by a multifunctional electronic universal material mechanical property testing machine (Instron 9657) with an indenter speed of 20 mm/min.
2.6 Statistical Methods
Data were statistically analyzed using Graphpad statistical software,and all data were expressed as±s. One-way ANOVA was used for data comparison, and differences were considered statistically significant at P < 0.05.
3. Results
3.1 FT-IR of CSH-Ca(OH)2 composites
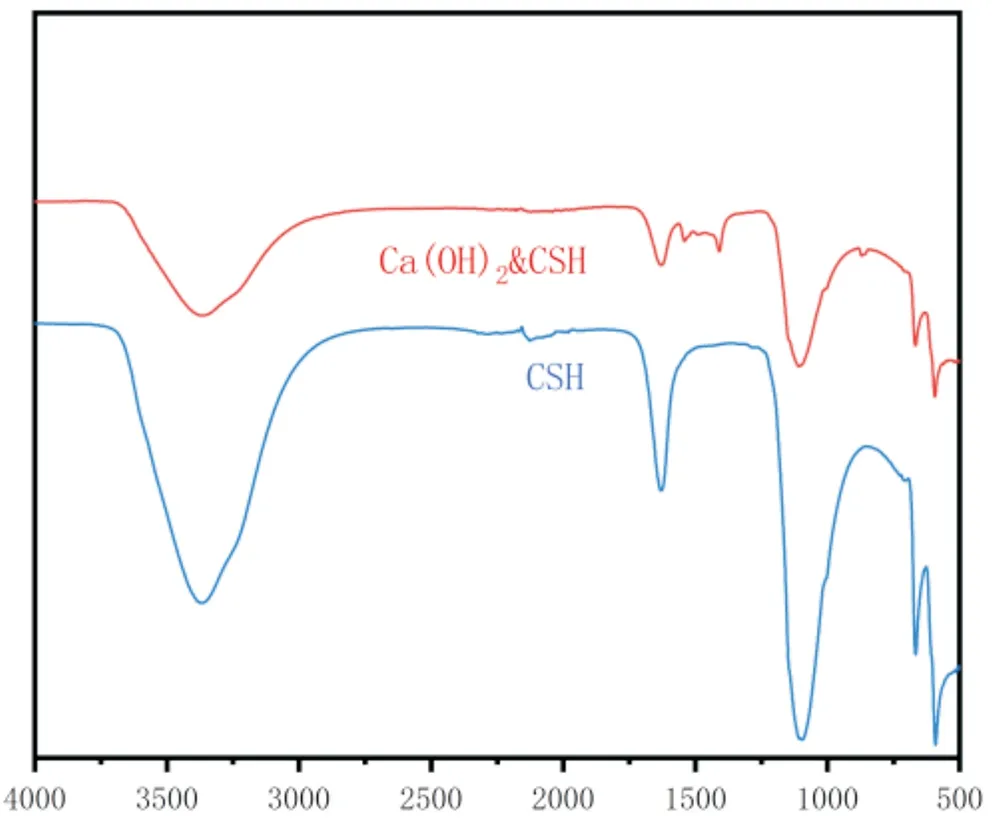
Figure1 FT-IR plots of CSH、CSH-Ca(OH)2
The figure shows the infrared spectra of the composite bone repair material. There are obvious absorption peaks at 860 cm-1 and 1400-1500 cm-1, which are characteristic wave peaks of Ca(OH)2, which shows the presence of calcium hydroxide in the composite product[20, 21]. The wave peaks at 598 cm-1, 670 cm-1, 1110 cm-1 and 1635 cm-1 are all characteristic wave peaks of post-hydration CSH, which shows the presence of post-hydration CSH in the composite product[22, 23]. With the addition of calcium hydroxide, the positions of the associated characteristic peaks were not significantly shifted and the characteristic wave peaks of the two materials overlapped.
3.2 XRD of CSH-Ca(OH)2 composites
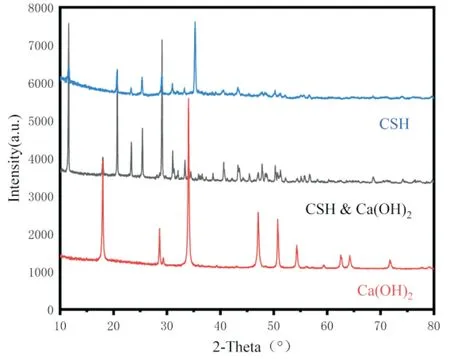
Figure2 XRD patterns of CSH、Ca(OH)2、CSH-Ca(OH)2
The XRD of the tested materials is shown in Fig. The characterization of the bone repair material properties is represented by the material with 5% concentration of calcium sulfate hemihydrate compounded with calcium hydroxide. The CSH diffraction peaks after hydration are more intense and crystalline,with characteristic peaks at 11.6°, 20.7°, 23.4°, 29.1°, 31.1°, 33.3°,43.3° and 47.9°, and there is a high agreement when comparing the characteristic diffraction peaks of the material (PDF#70-0982), and there is no generation of spurious peaks [24].The XRD pattern of Ca(OH)2shows that the peaks at 18.0°, 28.6°, 34.0°, 47.0° and 50.7°are in general agreement with the standard peaks, which proves that the substance is present in the compound. However, diffraction peaks appeared at 29.4° and 48.5°, and this peak is the diffraction peak of calcium carbonate (PDF#99-0022), indicating that a small amount of calcium carbonate was present during the synthesis. In the curve of XRD after mixing CSH and Ca(OH)2, the characteristic peaks of both substances appeared respectively, but the intensity of the peaks decreased, probably due to the interaction between both calcium sulfate and calcium hydroxide, resulting in a significant decrease in the intensity of their diffraction peaks, and the complex was more stable, and both did not lose their original crystal structures,indicating that the properties of both remained unchanged and would not affect their relevant properties.
3.3 SEM of CSH-Ca(OH)2 composites

Figure3 Scanning electron micrographs of composites with different concentrations
In Figure 3, A, B, C and D are the morphology of calcium sulfate porous scaffold materials with 0%, 1%, 5% and 10% calcium hydroxide, respectively. 0% group, under the field of view of scanning electron microscope at 500×, can be seen that the calcium sulfate is columnar and lumpy, and the crystals are in contact with each other, forming a wide gap structure, and the gap is relatively clear. 1%, 5% and 10% groups, under the field of view of scanning electron microscope at 500×, can be seen that The crystal structure of calcium sulfate gradually showed unclear, and the gap between the irregular flocculent structures also gradually became smaller, and the porosity of the composite material became smaller, and the field of view of the 10% group was almost occupied by the flocculent calcium hydroxide, and the crystals of calcium sulfate showed completely unclear.
3.4 Mechanical properties of CSH-Ca(OH)2 composites
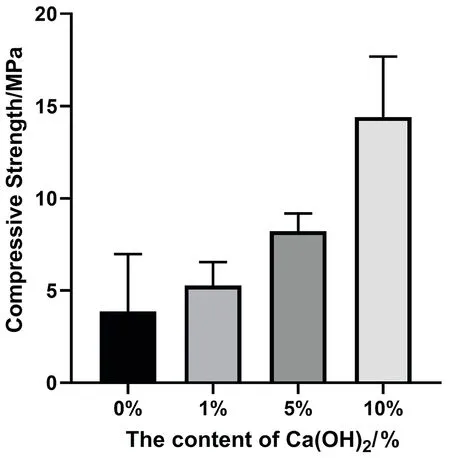
Figure4 Compressive strength of composites with different concentrations
The compressive strengths of the 0%, 1%, 5%, and 10% specimen groups were (3.86 ± 3.1), (5.27 ± 1.28), (8.22 ± 0.96), and (14.4± 3.28) MPa, respectively. as can be seen from Figure 4, the compressive strengths of the four groups of materials became larger as the percentage of calcium hydroxide mass increased. It is obvious that the addition of calcium hydroxide directly affected the mechanical properties of the composites. 1% and 5% groups were not statistically different (P > 0.05) compared to 0% blank control group, while 10% group was statistically different (P < 0.05)compared to blank control group.
4. Discussion
In the field of bone tissue engineering, a single material often has more or less disadvantages, and people often combine two or more bone materials to complement each other's strengths and give full play to their respective advantages. A relatively large number of domestic and international articles have investigated calcium sulfate hemihydrate and calcium hydroxide, both of which are known to promote bone healing, but few studies have combined the two materials and tested their related properties.
Different concentrations of CSH-Ca(OH)2composites were prepared, and from XRD and FT-IR analyses, the physical phases of the mixed composites were Ca(OH)2and CSH after hydration,and both did not lose their original crystal structures. The suitable size of pores and stable structure are more favorable for nutrient transport and facilitate cell migration and proliferation [25, 26].The bone repair material should have a certain compressive strength,which is shown by the fact that the higher the density, the higher the compressive strength of the material. Obviously, the size of the porosity of the composite material determines the compressive strength, and calcium hydroxide can directly affect the mechanical properties of the composite material. The addition of Ca(OH)2can significantly improve the mechanical properties of the composite scaffold material, and as the mass proportion of calcium hydroxide becomes larger, the compressive strength shows a trend of gradually increasing, which is sufficient to meet the mechanical requirements of human cancellous bone. The suitable compressive strength of the composite bone material can provide a stable structure for bone marrow mesenchymal stem cells, but the porosity of the scaffold material also decreases accordingly[27].
In summary, the group obtained a new bone repair material by mixing calcium sulfate hemihydrate and calcium hydroxide, which retain the properties of both calcium sulfate hemihydrate and calcium hydroxide while maintaining the porous structure. Due to the addition of calcium hydroxide, the compressive strength of the composite was enhanced and the physicochemical properties of the bone repair material should be possessed. In subsequent studies,we will conduct cytological and zoological tests on this composite material with the aim of making this material a new type of bone repair material.
Conflict of interest statement
The authors declare that there is no conflict of interest in the publication of this paper.
Author's contribution
First author: Zhengdong Guo, involved in the experiment, collecting and organizing data and writing the paper; corresponding author:Lei Peng, project design and paper proofreading; remaining authors:Yangyang Bian, Xiaoqian Liu, Siyuan Zhang, Dong Wang and Jian Yang, involved in the experiment and managing the accounts.
 Journal of Hainan Medical College2022年12期
Journal of Hainan Medical College2022年12期
- Journal of Hainan Medical College的其它文章
- Research progress of Cassytha filiformis L
- Research on anti-pancreatic cancer mechanism of Codonopsis codonopsis based on network pharmacology
- Risk factors for infection with multidrug-resistant organisms in diabetic foot ulcer patients: A systematic review and meta-analysis
- Correlation between NF-κB/TNF-αpathway and atrial fibrillation
- Effectiveness of Shengmai Injection on angina pectoris based on realworld propensity score method
- Construction and validation of prognostic model of hepatocellular carcinoma based on epigenetic factors
