Superb VOCs capture engineering carbon adsorbent derived from shaddock peel owning uncompromising thermal-stability andadsorption property
Fu Yang,Wenhao Li,Rui Ou,Yutong Lu,Xuexue Dong,Wenlong Tu,Wenjian Zhu,Xuyu Wang,Lulu Li,Aihua Yuan,,Jianming Pan
1 School of Environmental and Chemical Engineering,Jiangsu University of Science and Technology,Zhenjiang 212003,China
2 School of Chemistry and Chemical Engineering,Jiangsu University,Zhenjiang 212013,China
3 Jiangsu Agricultural Hormone Engineering Technology Research Center Co.LTD,Changzhou 213022,China
Keywords:Thermal-stability Carbon absorbent VOCs Shaddock peel Capture
ABSTRACT High applied thermal-stability and superior structural property for activated carbon adsorbent are extremely promising,which also is the determining short slab in volatile organic compounds (VOCs) adsorption applications.Herein,we develop the outstanding engineering carbon adsorbents from waste shaddock peel which affords greatly-enhanced thermal-stability and super structural property(SLang=4962.6 m2·g-1, Vmicro=1.67 cm3·g-1).Such character endows the obtained adsorbent with ultrahigh adsorption capture performance of VOCs specific to benzene (16.58 mmol·g-1) and toluene(15.50 mmol·g-1),far beyond traditional zeolite and activated carbon even MOFs materials.The structural expression characters were accurately correlated with excellent adsorption efficiency of VOCs by studying synthetic factor-controlling comparative samples.Ulteriorly,adsorption selectivity prediction at different relative humidity was demonstrated through DIH (difference of the isosteric heats),exceedingly highlighting great superiority (nearly sixfold) in selective adsorption of toluene compared to volatile benzene.Our findings provide the possibility for practical industrial application and fabrication of waste biomass-derived outstanding biochar adsorbent in the environmental treatment of threatening VOCs pollutants.
1.Introduction
The rapid development of the petrochemical industry has caused serious damage to global environment.In particular,the emission of VOCs,mainly benzene homologs such as benzene,toluene,and xylene,even severely threatens human health [1-4].To achieve energy saving and emission reduction,green manufacturing not only needs the formulation of national and local atmospheric emission standards but also requires efficient environmental engineering treatment technology [5-7].
In terms of VOCs terminal treatment,the activated carbon adsorption method attracts most wide attention thanks to advantages of high-efficiency and low-cost compared to catalysis oxidation [8-10],plasma technology [11-13],etc.,but also dominantly relies on the utilization of high-performance adsorbents [14].In general,two key factors including structural properties and applied thermal stability for the activated carbon adsorbents are the core consideration in the practical application.The employed adsorbents require high specific surface area,large pore volume and suitable pore size suitable for gas molecules to accommodate enough adsorbates [15].To our known,the adsorbents for VOCs adsorption treatment include zeolite[16-21],aerogel[22,23],activated carbon [24,25],carbon fiber [26],and MOFs [27,28] were used most widely,but among of which still suffer from several obvious disadvantages such as inadequate surface area,complicated preparation process,high cost,and low yield which are unbearable in real practical applications [29,30].
At present,activated carbon has been widely used as an adsorbent in enterprises,and how to enable activated carbon with large capacity is the focus of current research [31].To be delight,at present,the developed activated carbon adsorbent has afforded relatively appreciable structural performance especially surface area and porous volume,outperforming most commercially available adsorbent [32].But another consequent bothering problem that the obtained activated carbon possibly suffers from low VOCs adsorption selectivity when undergoing the competitive adsorption along with water vapor under the complex environment[33].This might ascribe to the defined microscale structure and special surface character such as hydrophilic and hydrophobic property of activated carbon,which affords different affinity for VOCs molecules [34].In fact,the endowed feature also depends on the carbonized process of activated carbon by exploiting the controlled activated temperature and activated agent such as KOH.This certainly will consider the implanting difference of activated agents such as KOH in the original internal pore of precursor,which might be determined by selected used precursor.
To our knowledge,activated carbon in the market generally derived from some special raw materials such as petroleum coke,coal [35],coconut shell [17],etc.Also,some mineral carbides and wastes containing carbon were also involved in the preparation of activated carbon.One widespread suffered issue for the activated carbon use in gas adsorption is the undesirable thermal stability during the desorption process.Because hot air desorption for the VOCs demands high thermal stability of adsorbent to avoid ignition of activated carbon.In recent years,researchers have been looking for suitable carbon sources that can produce high quality activated carbon possessing high thermal stability and outstanding structural property [34].Some successful cases using waste biomass-based precursors have been explored,for instance,coconut shell-derived activated carbon under the static adsorption test of VOCs(benzene,dichloromethane,chloroform,carbon tetrachloride)[31,33].Shaddock peel as one waste possesses wide resources in Southeast Asia region,and well-developed porous structure for itself,which normally used for adsorption of special smell in daily life.Kang and others used shaddock peel to prepare activated carbon for supercapacitor [36].Taoet al.used shaddock peel to prepare activated carbon for adsorption of Cr(VI) in sewage and found that the removal efficiency was more than 20% [37].Donget al.used shaddock peel-activated carbon as a high-performance electrode for deionized capacitors.So far,to our knowledge,the specific surface area of shaddock peel-derived activated carbon prepared was just up to 2726 m2·g-1[38].From the current research situation,the preparation of outstanding activated carbon from shaddock peel for VOCs remains blank,and the relevant structural properties including specific surface area of activated carbon prepared from shaddock peel have no advantage compared with activated carbon prepared from other carbon sources.This inspires us the outstanding carbon adsorbent derived from shaddock peel still can be further improved through rational synthesis process.
In this contribution,one high-performance engineering carbon adsorbent with ultrahigh structural property was developed from shaddock peel based on a comprehensive optimized synthesis process through controlling different activator ratios and activated temperatures.The obtained C4-800 activated carbon adsorbent affords the best low-pressure adsorption performance for benzene and toluene.Apart from the obtained outstanding structural properties,the resultant activated carbon adsorbent C4-900 was still endowed with uncompromising thermal stability (603°C) based on the TG test under air condition.The synergetic effect derived from the above-controlled synthesis factors was comprehensively identifiedviastudying the correlation between VOCs adsorption performance and matched structural properties includingSBET,SLangmuir,Vtotal,andVmicro,which demonstratesVtotaldominantly determined adsorption effect for the VOCs capture.Finally,the competitive adsorption of benzene and toluene to water was evaluated according to the DIH(difference of the isosteric heats)equation calculation and claimed the remarkable superiority for selective adsorption of toluene compared to benzene under humid condition.This work provides certain insight into the development of high-performance activated carbon with uncompromising adsorption performance and thermal stability through rationally controlling the synthesis process.
2.Experimental
2.1.Chemicals and materials
The carbon adsorbent precursors were purchased from a local supermarket.Potassium hydroxide was obtained from China Sun Specialty Products Co.Ltd.Concentrated hydrochloric acid 37%(mass),benzene,and toluene (>99.0%) were purchased from Yangzhou Shanghai Chemical Reagent Co.Ltd.All involved chemicals were used without further purification.
2.2.Synthesis of carbonaceous adsorbents
Firstly,carbon adsorbent precursor was pre-carbonized for generating more void space to accommodate the activated agent in the subsequent activator-implant process.The detailed procedure was depicted as follows:shaddock peel was washed,dried for 24 h,then put into a tube furnace for calcination in a nitrogen condition at 450°C with the raised temperature speed of 5°C·min-1,and kept for 2 hours.After cooling to room temperature,the collected sample was ground and then thermal treated at 150°C for 12 h.The pre-carbonized powder derived from shaddock peel was completely mixed with KOH(controlled mass ratio of 1:1,1:2,1:3,and 1:4) and additional proper amount of deionized water,respectively.Ensuingly,the resultant mixture was dried in the oven at 110°C for 24 hours.Afterward,the collected mixtures were further calcined in N2atmosphere with the controlled procedure.The furnace was first raised from room temperature to 450°C with a heating rate of 5°C·min-1,kept for 90 min,then increased to 600°C,700°C,800°C,and 900°C at a rate of 5°C·min-1,respectively,and kept for 2 hours.After cooling,it is washed to neutral with HCl solution of 1 mol·L-1,then washed with deionized water,and finally dried in the oven at 120°C.The final sample was uniformly marked asCx-T,wherexrepresents the KOH/carbon ration,andTrepresents the activation temperature.
2.3.Characterizations
The micro-morphology of the synthesized samples was observed by field emission scanning electron microscope (FESEM,German Zeiss Merlin).Crystalline states of samples were ascertained by X-ray diffraction (Shimadzu XRD-6000 diffractometer with CuKα radiation source (=0.154178 nm).The test speed is 3(°)·min-1,ranging from 5° to 20°.The infrared spectrum was characterized by Fourier transform infrared spectrometer(Agilent).The sample was prepared by potassium bromide pressing method,and the wavenumber 400-4000 cm-1was recorded.Thermogravimetric analysis of samples was performed under nitrogen atmosphere (Pyris Diamond TG-DTA analyzer,America PerkinElmer)with a heating rate of 10°C·min-1.The operation temperature ranges from 25°C to 800°C.The specific surface area was tested by Belsorp Vapor Pretreatment instrument(MicrotracBEL Corporation,Japan).Before the test,the sample was activated in vacuum at 180°C for 12 h,the test range was 0.1 MPa,the test temperature was -196°C,and the purity of nitrogen was 99.99%.The pore structure and specific surface area were analyzed according to the nitrogen adsorption curve:the specific surface area of the sample was calculated by BET method at the adsorption data of relative pressure 0.05 to 0.35.The pore volume was calculated by the adsorption data at the relative pressure of 0.99.Nonlocal density functional theory(NLDFT)Pore size distribution analysis was used to analyze the overall pore size distribution and the micropore specific surface area and micropore volume were calculated by the t-curve formula.
2.4.Benzene and toluene adsorption test
According to the standard static capacity method,the adsorption test was carried out by using the Belsorp Vapor Pretreatment instrument (MicrotracBEL Corporation,Japan).The benzene(toluene)solution of 20 ml was put into the stainless steel constant temperature volatilization chamber,and the benzene (toluene)solution was treated with liquid nitrogen and boiled water for more than three times to remove the excess gas in the volatilization chamber.The 30 mg-50 mg of adsorption materials were precisely weighed and treated with vacuum degassing at 180°C for 12 h,and the quality of the pretreated materials was recorded.The sample tube containing the adsorbent is placed in the adsorption test position,and the constant temperature water bath position is adjusted to immerse the sample tube.The vapor pressureP0(in mm Hg column) of volatile organic compounds at test temperature is calculated by using Antoine equation:lgP0=A-B/(T+C),whereA,B,Cis a constant,and the corresponding constants of different substances can be obtained in the website.WhenP0is converted into kPa international units,P0value is the saturated vapor pressure of VOCs gas at ambient temperature.0.1P0,0.2P0,0.3P0.0.9P0andP0are set as the air pressure valuePto be measured.Finally,the corresponding standard gas volumeVa(unit:cm3·g-1) under different pressurePis obtained,and the corresponding gas adsorption capacity is obtained by using formula 1 [39].

Fig.1.Schematic illustration for fabricating carbonaceous adsorbent from waste shaddock peels.

Fig.2.Representative SEM images of dried shaddock peel (A,a),pre-carbonized shaddock peel (B,b),and activated shaddock peel-derived activated carbon (C,c).

ma:The adsorption capacity of the gas,unit:mg·g-1;
Mg:The relative molecular mass of the adsorbed gas.
TakingP/P0as Abscissa and ma as Abscissa,the corresponding isothermal adsorption curve was made.For the isothermal adsorption test of benzene and toluene at different temperatures,it is only necessary to set the saturated steam pressure at the corresponding temperature as the final test pressure and adjust the constant temperature water bath to the corresponding test temperature,which is generally 15-45°C.
2.5.Isothermal adsorption test of water vapor
The steam adsorption test is carried out by using the modified steam adsorption instrument.Firstly,the pipeline is purged at 50°C with helium for 30 min,then 20 ml of deionized water is extracted by freezing heating and pumping cycle,and at the same time,the constant temperature heating sleeve is connected and put into the volatilization chamber.The 10 mg of sample is weighed by microbalance for vacuum degassing treatment at 180°C for 12 h.The comparison table of water-saturated vapor pressure and the temperature is found,and the saturated vapor pressure of the corresponding temperature is input in the instrument.Because of the poor volatility of water,the temperature range of isothermal adsorption test is 25-45°C.The corresponding operation method refers to the isothermal adsorption test of VOCs gas.
3.Results and Discussion
3.1.Morphology and structural characteristics
Fig.1 shows the synthetic illustration for fabricating carbonaceous adsorbent from waste shaddock peels,further validating the detailed synthetic procedure.Ensuingly,Fig.2(A,a)shows field emission scanning electron microscope images of dried shaddock peel.The plenty of holes with near 10-100 μm size can be observed through the whole material,which accounts for the porosity of the shaddock peel.The original loose structure of shaddock peel is conducive to the implant of KOH into the internal structure of precursor and promotes their contact and activation reaction at high temperature,and triggers rich micropores to enhance the adsorption properties.Fig.2(B,b) gives the micromorphology of biochar after pre-carbonization.Note that the original macrostructure of the shaddock peel is destroyed,and most of the pore structure disappears and finally forms a smooth flake structure.Fig.2(C,c) shows the morphology of final activated carbon after activation.We observed the microstructure of activated carbon was further destroyed after activation process,and the previous biochar precursor became further fractured owing to the drastic reaction between KOH and biochar.The above morphology variation revealed the forming process of activated carbon from biomass.

Fig.3.XRD pattern (a) and (b) FTIR spectrum of activated carbon C4-800.
Fig.3(a) depicted the wide-angle XRD pattern of activated carbon of C4-800.Two diffraction peaks at about 26° (002) and 44°(100) were observed and indicated the amorphous state of activated carbon,whose weak crystal plane diffraction intensity reflects graphite structure degree [39].Fig.3(b) shows the representative infrared spectrum of activated carbon C4-800 obtained by Fourier transform infrared spectrometer.The absorption band at 3419 cm-1represents the surface-OH group on the activated carbon,ascribed to the physicochemical adsorbed moieties.The appearance of 1633 cm-1and 1129 cm-1absorption peak is caused by the bending vibration peak of —NH.The presence of a peak of 1392 cm-1is derived from the out-of-plane bending absorption peak of hydroxyl compounds.The benzene monocycle of C4-800 showed a characteristic peak at ca.618 cm-1[40].The above observation indicates the specific featured structure of the resultant activated carbon.
3.2.Thermal stability and structural properties
Generally,the application field and scope of activated carbon adsorbent tend to be limited by their applicable temperature,therefore,the thermal stability of activated carbon was regarded as the important consideration factor of adsorption.Here,Fig.4(a) collects and shows TG thermal decomposition curves of the resultant activated carbon prepared at different temperatures in the air environment.Note that the higher preparation temperature of activated carbon contributed to the tardive thermal decomposition temperature of the resultant activated carbon.Specifically,the samples derived from 600°C begin to decompose after 300°C,while the samples prepared at 900°C begin to decompose after 500°C in air atmosphere,indicating that higher activation temperature induces the higher aromatization degree and better stability of activated carbon.Because the higher thermal treatment temperature makes the carbon precursor better transfer into the state affording lower surface energy after removing more surface polar moiety,thereby exhibiting better thermal stability.Fig.4(b)shows the statistical results on the thermal stability of various reported biomass activated carbon at their respective obtained surface area,where B4-800 and B4-900 afford the most desirable character including the highest specific surface area and best thermal stability(603°C)compared with other biomass activated carbon[41,42].This phenomenon suggests the prepared activated carbon affords large potential in the practical engineering adsorption case.Herein,we further suggest the activated mechanism of KOH assisted gasification process,and the activated reactions are depicted as follows(Eqs.(2)-(7)).KOH would react with the surface carbon in the carbon precursor toetch away some surface organic moieties,and pores appeared in theetched position.Note that the introduced KOH could suppress the formation of tar and improves the yield of activated carbon adsorbent.Meanwhile,KOH makes the actual temperature of the activation reaction decrease by about 100°C,and also accelerates the removal of non-carbon atoms including N,H,andetc.
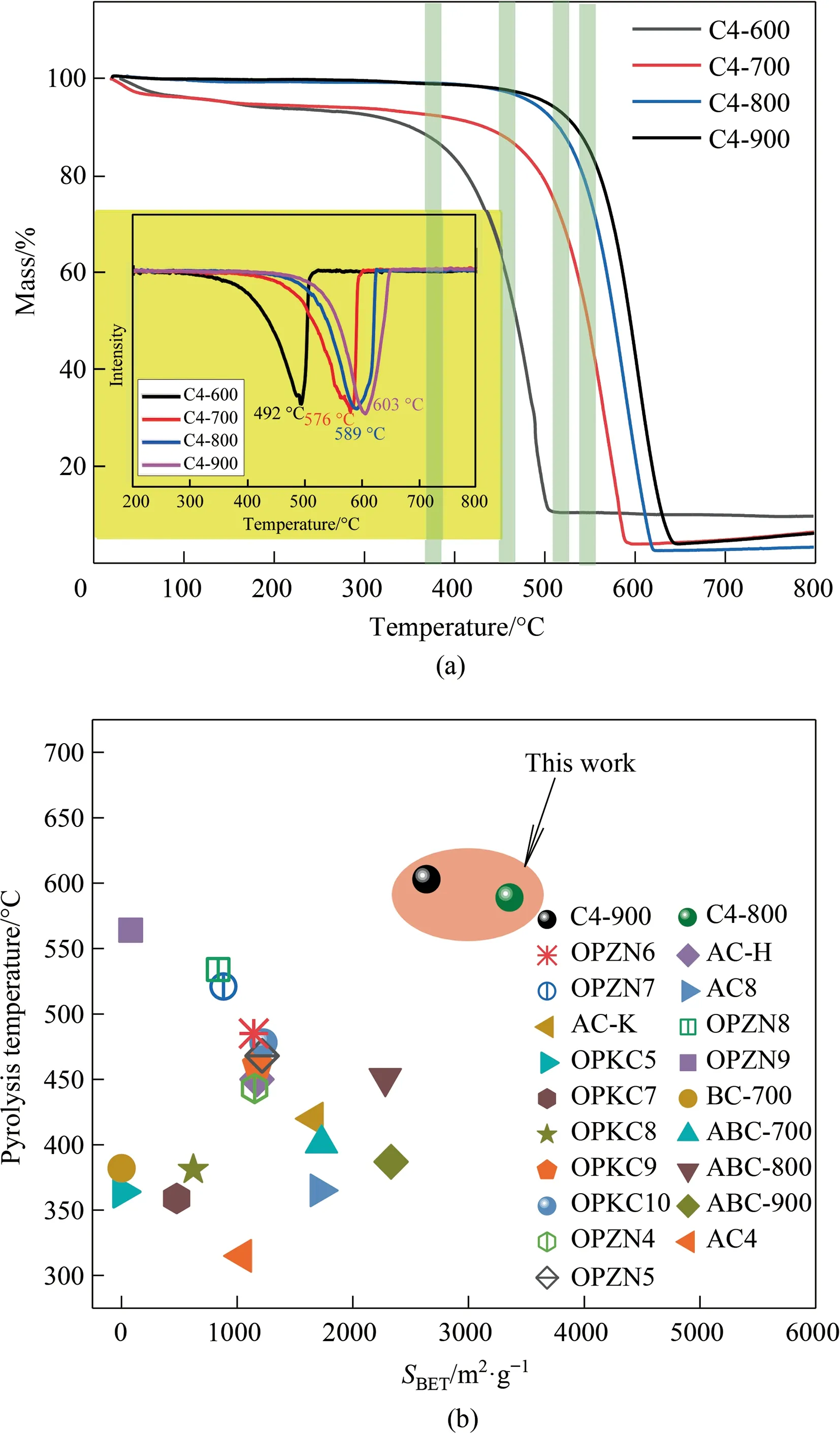
Fig.4.(a) TG profiles and DTA curves (insert) of activated carbon in C4 group,and(b) related comparison of thermal stability and specific surface area of various biomass activated carbons.

Apart from the thermal stability,the adsorbent with excellent structural properties is expected to afford better adsorption performance.Especially,the proper adsorption properties usually have a high specific surface area and pore structure suitable for the diameter of adsorbed gas molecules.Fig.5 shows the nitrogen adsorption and desorption curves of activated carbon prepared at different KOH ratios and activation temperatures.As shown,the nitrogen adsorption capacity of activated carbon materials exhibits the rapid adsorption jump at the low-pressure region with type I character,indicating the presenting microporous structure formation.Regarding the medium pressure and high-pressure region,the enhancement of adsorption capacity of all the materials excluding C3-900 and C4-900 is unremarkable,indicating that these activated carbon adsorbents are dominated by micropores.As a contrast,the adsorption capacity of C3-900 and C4-900 activated carbon increased in varying degrees at the relative pressure of 0.02-0.99,revealing that these two materials have a mesoporous and mesoporous hierarchical structure.Based on above observation,we suggest,at the same KOH/C ratio,the higher the activation temperature contributed to a higher adsorption capacity of adsorbent.As such,in the same activation temperature range,the higher KOH/C ratio also triggers a higher nitrogen adsorption capacity,indicating that high temperature and higher activator ratio are beneficial to the formation of activated carbon pores.In the light of C3 and C4 group activated carbon,we noticed that the nitrogen adsorption capacity of C4-900 in the low-pressure region is less than that of C4-800,indicating the C4-800 affords the better lowpressure adsorption performance,while the later obvious increment in the medium and high-pressure region contributes to the last superiority of C4-900 than C4-800.Such the phenomenon suggests the well-defined hierarchical structure bearing the micropores and mesopores in the resulted activated carbon.In contrast with C4-900,the lower KOH/C ratio derived C3-900 also shows certain hysteresis loop phenomenon at medium pressure region,but obviously weaker than that of C4-900,hinting the only formation of a small number of mesopore even treated by identical high temperature.The above results demonstrated together the synergetic effect of activated temperature and activated agent on the structure optimization in the proper match state.
To better identify the structural properties of the obtained absorbents,Table 1 lists the relevant textural parameters of specific surface area,total pore volumes,and micropore volumes calculated by BET formula,Langmuir formula,andTformula respectively according to the nitrogen adsorption and desorption data of activated carbon.By comparing the specific surface area of the adsorbent derived from the specific KOH/C mass ratio,we found that the specific surface area of C1 group activated carbon increases with the raised activated temperature,indicating that the activation temperature is favorable for KOH to react with surface carbon and corrode the material to form the pore under the condition of a lower amount of activator.For C2,C3 and C4 activated carbon,the specific surface area increases at first and then decreases with raised activated temperature,in which the specific surface area of adsorbent prepared at 800°C is the highest,which is coincided with nitrogen adsorption result,indicating that,under the condition of a higher amount of activator,only appropriate activation temperature is beneficial to the formation of micropores,and too high temperature makes micropores disappear to form mesoporous or macropore.From the pore volume results,except for C3 group activated carbon,the total pore volume and micropore volume of adsorbent increase with the increased activated temperature.The rich micropore structure is conducive to the rapid adsorption of VOCs gas at low pressure in the environment,while mesoporous adsorption can have multi-layer adsorption and condensation of gas at high pressure.On the whole,the above results demonstrated that all the activated carbon prepared are dominated by micropores,which is beneficial to the adsorption of VOCs in low-pressure conditions.While the C4-900 bearing the micropore and mesopore affords the integrated adsorption ability for VOCs resources in the whole pressure range.

Table 1 Textural properties of activated carbon adsorbents at different activation temperatures.
Fig.6 shows the main aperture distribution calculated by the NLDET method.These observed pores are mainly distributed within 5 nm.Through the lateral comparison of activator ratio,with the increasing proportion of activator,the pore diameter became bigger and accompanied with the wider distribution range of pore diameter,because the activator filled in the pore diameter further corrodes the pore surface of the material rendering the widening effect.By comparing different activation temperatures at the same proportion of longitudinal activators,we found the higher activation temperature causes the wider pore size distribution and the partial increase in pore volume,indicating the synergetic effect of activated temperature and activator dosage for optimizing the structure properties.Finally,through NLDFT pore distribution analysis,it can be concluded that the chemical activation method is conducive to the efficient increment of pore volume and pore size to accommodate the adsorbed molecules.

Fig.7.3D colormap surface diagram of KOH/C mass ratio and activated temperature co-dependent adsorbed capacity of benzene (a) and toluene (b) over various resulted adsorbents at a relative pressure of 0.6.
3.3.Adsorption test of benzene and toluene
Fig.7 depicts a 3D colormap surface diagram of KOH/C mass ratio and activated temperature co-dependent saturated adsorbed capacity of benzene and toluene over various resulted adsorbents.As observed,these two 3D surface diagrams give a great description of how the KOH/C mass ratio and activated temperature synergetically affect the adsorbed properties of the resultant activated carbon adsorbents.In general,the higher activated temperature and KOH/C ratio together benefit to contribute to excellent VOCs adsorption performance,due to the great structural properties and characters,which is coincided with the above N2adsorption/desorption results.On the other hand,Fig.8 and Fig.9 display the detailed adsorption isotherms of benzene and toluene on activated carbon series material at 25°C,to identify the relative pressure-dependent adsorption behavior of benzene and toluene.Note that the adsorption of benzene and toluene at low-pressure region below 0.1 ofP/P0is obvious in the case of all the samples,which ascribed to the dominant microporous adsorption ability for VOCs molecules.Besides,the detailed evolutive variation trend for benzene and toluene adsorption was identified for the adsorbent prepared at specific KOH/C ratio with different activated temperature,it could be found that the adsorption capacity of adsorbents in the low-pressure region was sharply enhanced as the increased activated temperature.This suggests more micropores were created under high-temperature driven activated process because the higher temperature providing enough energy for triggering the more robust reaction between KOH and surface carbon species of biochar to form micropores.However,we also noticed that the over-high activated temperaturee.g.900°C causes the downshift of benzene adsorption capacity at low-pressure range and the newly-emerged further medium pressure adsorption behavior.This should ascribe to the mesopores in the adsorbent because the high-temperature driven thermal-reaction progress was too violent and induced the more serious surface etching of activated carbon and made the formed micropore crosslink and widen to mesopore size.Ulteriorly,we also suggest the adsorption capacity of adsorbent was remarkably enhanced with the gradually-increased activator proportion at the specific activated temperature.
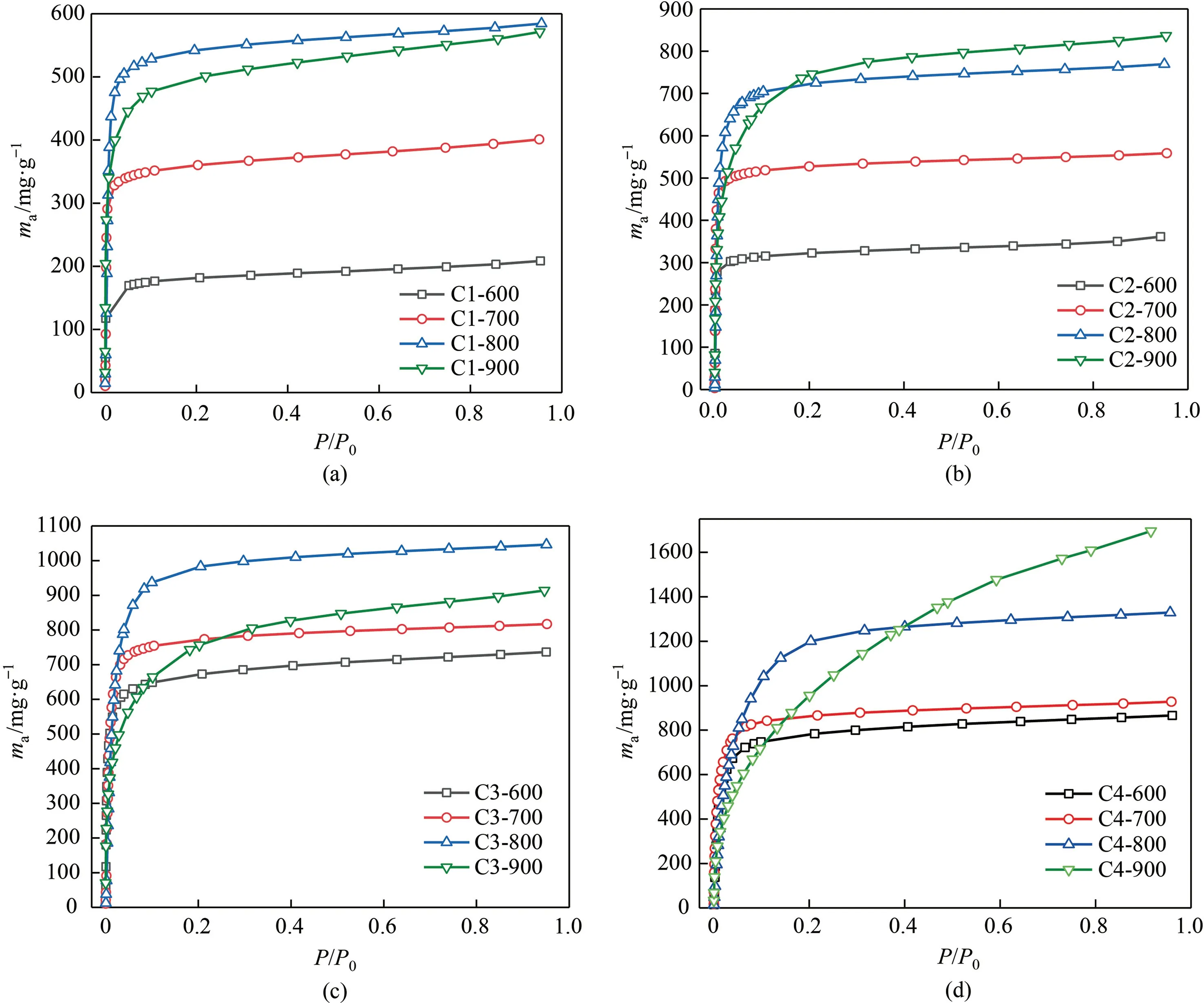
Fig.8.Benzene adsorption isotherms of activated carbon in groups (a) C1;(b),C2;(c) C3;and (d) C4,at 25°C.
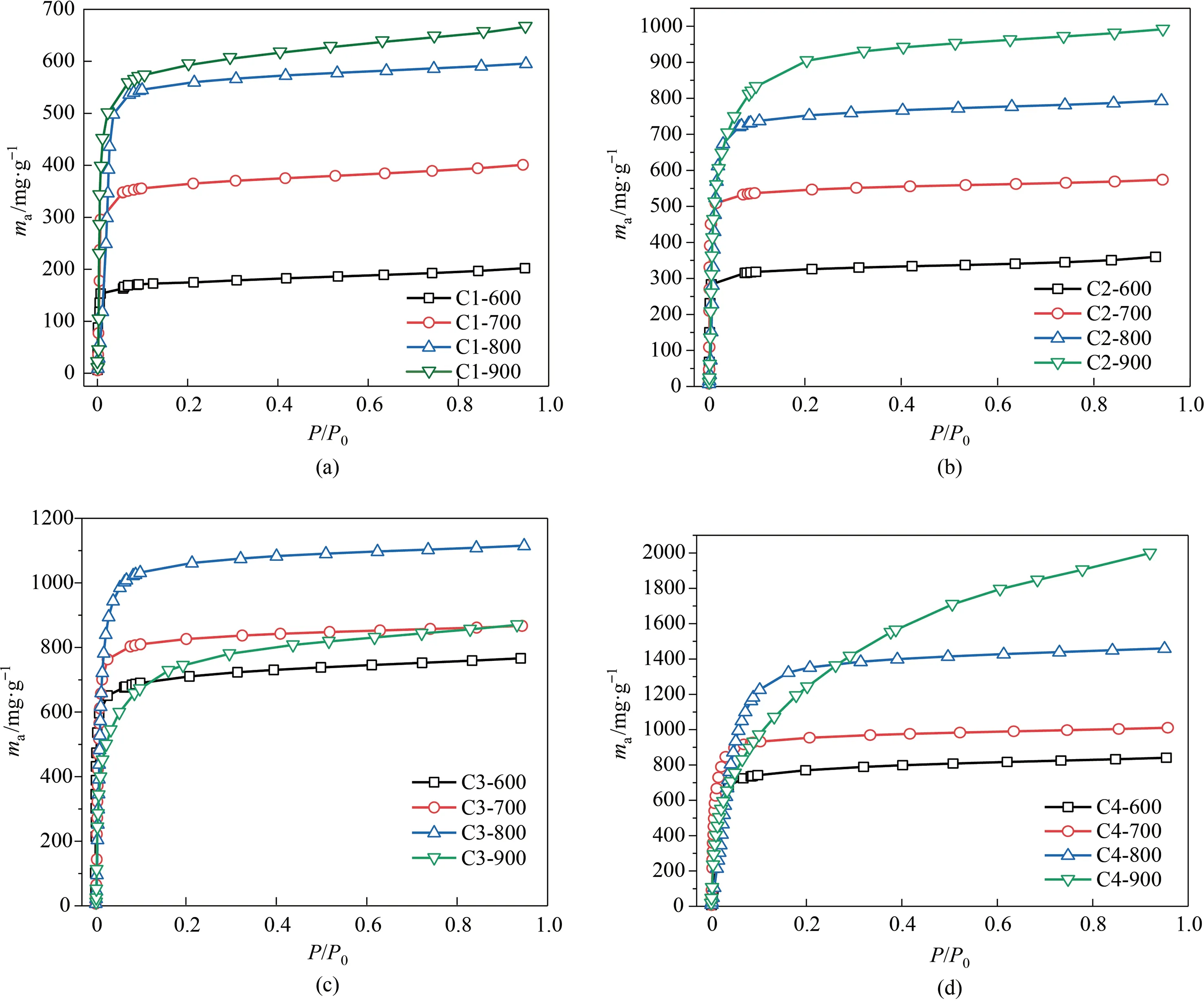
Fig.9.Toluene adsorption isotherms of activated carbon in groups (a) C1,(b),C2,(c) C3,and (d) C4,at 25°C.
To further determine the relationship between adsorption performance and structural properties of the resultant adsorbents,we listed the detailed adsorption capacity of various adsorbents(Table 2),and conducted the tentative fitting results of textural properties of adsorbents and adsorption capacities of benzene and toluene such asSBETsurface areavs.adsorption capacities,Smicrosurface areavs.adsorption capacities,Vmicropore volumevs.adsorption capacities,andVtotalpore volumevs.adsorption capacities.Fig.10(a),(b) reveal that the adsorption capacity of benzene and toluene over the resulted adsorbents is not highly linearly correlated with BET specific surface area and Langmuir specific surface area as a whole.The fitting degree is only ca.0.7 in the light of adsorption capacity and surface area.This indicates the VOCs adsorption is still affected by other factors.By observing Fig.10(c),(d),it can be found that the adsorption capacity of benzene and toluene is significantly influenced by the microporous pore volume and the total pore volume,and the linear correlation is relatively high.The adsorption capacity of the adsorbent to benzene and toluene gradually increased with the increase of micropore volume and total pore volume.The above fitting results further demonstrated the excellent VOCs adsorption properties should mainly derive from the present rich microporous pore volume under the efficient activated process using the synergetic function of rational activated temperature and KOH/C ratio.

Table 3 The comparison in adsorption capacities of benzene and toluene over C4-800 and other some known materials
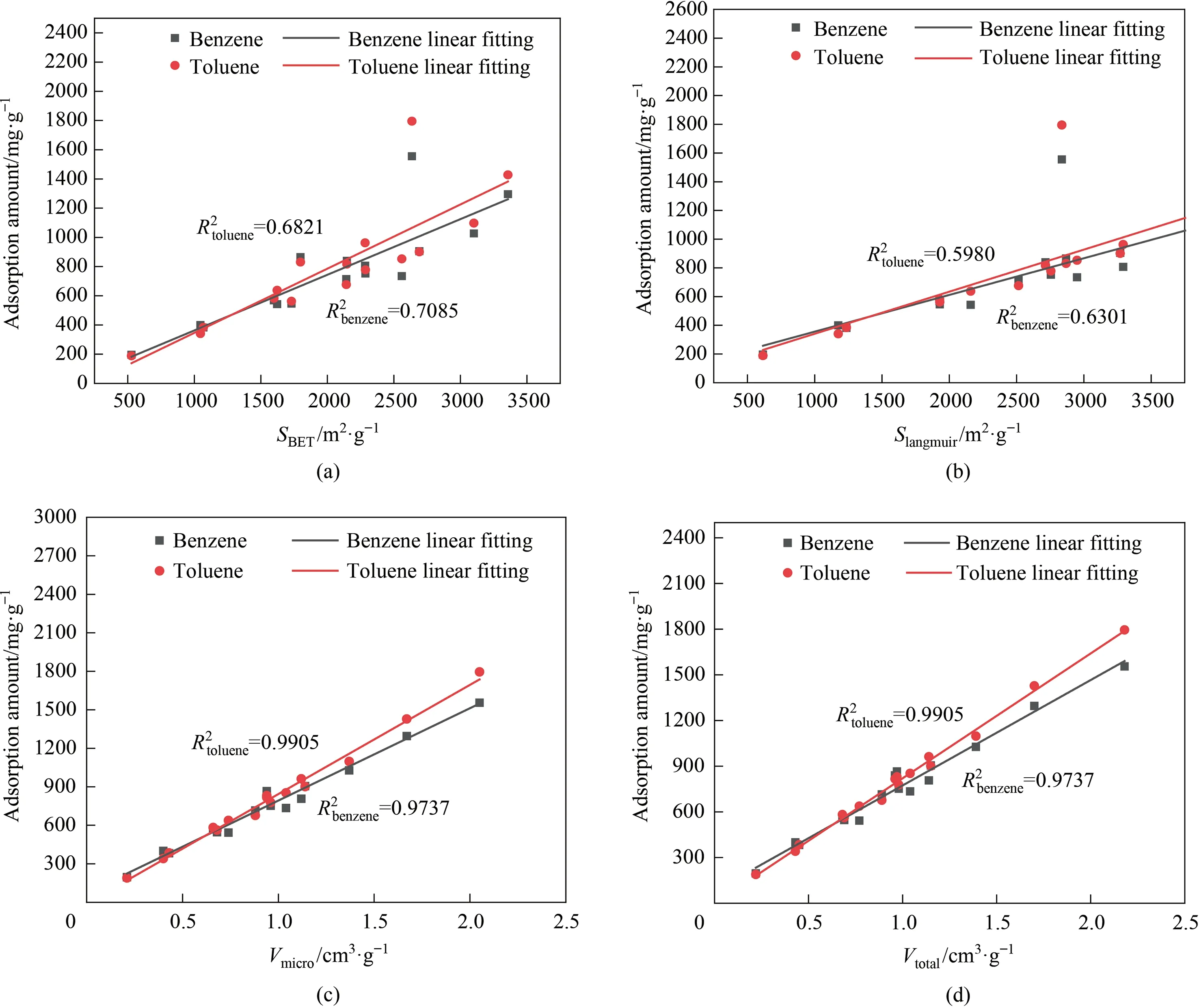
Fig.10.The tentative fitting results of textural properties of adsorbents and adsorption capacities of benzene and toluene (a) SBET surface area,(b) SLangmuir surface area,(c)Vmicro pore volume,and (d) Vtotal pore volume.
To further known the adsorption performance level of the developed activated carbon adsorbent derived from shaddock peel,Table 3 shows the comparison of the adsorption capacity of C4-800,C4-900,and some other reported materials for benzene and toluene.It can be seen from the table that C4-800 and C4-900 have best adsorption properties compared with other counterparts and traditional activated carbon materials.Fig.S1 (Supplementary Material) displays that the toluene adsorption cycle test of C4-800 samples showed no change in adsorption capacity and high reusability.This suggests our developed activated carbon has a good prospect in the application of VOCs adsorption in the future.
3.4.Comparison of adsorption behavior of benzene and toluene
To further determine the adsorption difference of benzene and toluene on the activated carbon especially at the low-pressure region,Fig.S1(a) shows the adsorption and desorption curve of benzene and toluene obtained by steam pressure control of C4-800.It should be noted that the prepared activated carbon material has no obvious desorption lag,indicating that the pore type of activated carbon is open pore,which can be recycled in industrial application.Fig.S1(b) shows an enlarged diagram of isothermal adsorption curves of benzene and toluene at low pressure.It is found that the adsorption amount of benzene is higher than that of toluene when the relative pressure is less than 0.04.This is because the molecular diameter of benzene is relatively smaller and easier to enter the pore of the adsorbent.Upon the relative pressure is greater than 0.04,the filling effect decreases gradually,while the combination of VOCs gas and activated carbon surface plays a major role,and the surface binding degree of toluene and activated carbon is better than benzene.Therefore,the adsorption capacity of toluene is higher than that of benzene in the later adsorbed stage.Combined with the previous results,we found that the adsorption between adsorbents and adsorbates is produced by a variety of interactions,not only the simple physical bond but also the role of the chemical bond.Besides,we further fitted the adsorption isotherms using Langmuir model (Fig.S2,Table S2),which exhibits high correlation between real adsorption curves and fitted curves and thereby confirms the monolayer adsorption behavior of VOCs molecules over the developed activated adsorbent.
3.5.Isothermal adsorption test of water
In the real adsorption process of VOCs,water vapor in the air will produce adsorption competition and interfere.In order to explore the adsorption capacity of activated carbon to water,the water vapor adsorption test was carried out at 25°C.The adsorption isotherm of water vapor is shown in Fig.S2 based on the used C4-800 activated carbon material.As shown,the material is very hydrophobic,and the C4-800 hardly adsorbs water vapor when the relative pressure is below 0.6.With the gradual increase of humidity,the adsorption capacity increases sharply,which is that water vapor begins to conduct multi-layer adsorption and condensation in the pore under a high humidity environment.When the relative pressure is close to 0.9,the adsorption capacity is saturated,and the adsorption capacity is 1500 mg·g-1.The adsorption capacity is larger than that of benzene and toluene because the water vapor molecule is smaller than the VOCs molecule and easier enters into some partial ultramicropore.Also,the present results indicate the possible selective adsorption of real VOCs under certain relative humid atmospheres because of the hydrophobicity of surface.
3.6.Calculation of adsorption heat of benzene,toluene,and water
To explore the adsorption heat of various adsorbates on the developed activated carbon,the adsorption isotherms of C4-800 for benzene,toluene,and water at different temperatures are collected and shown in Fig.11.Note that,in the low-pressure region,the adsorption amount of benzene and toluene increases sharply with the increased pressure,indicating that the activated carbon material has a strong adsorption force for benzene and toluene,while water only affords very low adsorption capacity in the low-pressure area,which shows that the adsorption force of the material to water is weak.When increasing adsorption temperature,the adsorption capacity of benzene and toluene gradually decreased and the low-pressure rapid adsorption area became wider,which is a typical characteristic of physical adsorption,indicating that the adsorption of VOCs over activated carbon adsorbents are dominated by physical adsorption.

Fig.11.Adsorption isotherms of different adsorbates over C4-800 adsorbent at different temperatures (a) benzene,(b) toluene,(c) water;(d) Adsorption isotherms for benzene,toluene,and water over C4-800 at 25°C.
According to the above results,the adsorption heat correlation calculation is carried out by using the fitting formula,in which the adsorption isotherms of benzene and toluene at different temperatures were fitted by Langmuir formula 8.The specific fitting diagram is shown in Fig.S4.The calculation of the adsorption heat of water is more complex,therefore,the adsorption heat of related water is directly obtained by the test software,and the relevant fitting parameters of benzene and toluene are shown in Table S2.

whereqa(mmol·g-1)is the equilibrium adsorption capacity,pis the equilibrium relative pressure of toluene and benzene,qm(mmol·g-1) is the theoretical maximum adsorption capacity andBis Langmuir constant which is related to the adsorption rate [39].
According to the above fitting curves,the adsorption equilibrium pressure at different temperatures with the same adsorption amount is calculated,and the linear relationship between lnp andT-1is obtained by using Eq.(9).The related results are shown in Fig.S4.

whereQst(kJ·mol-1)is the isosteric heat,R(kJ·mol-1·K-1)is the universal gas constant,T(K)is the temperature,p(kPa)is the pressure,andCis an integral constant [39].
Finally,the adsorption heat is calculated according to the linear slope,and the relationship between the adsorption heat and the adsorption capacity is shown in Fig.12(a),and the related adsorption heat of water derived by the software is also shown in the diagram.We noticed that the adsorption heat of toluene and benzene is always greater than that of water,which shows that the interaction effect of toluene and benzene over activated carbon is greater than that of water in the adsorption process.This could ascribe to the surface hydrophobicity of activated carbon.Even with little adsorption capacity,the adsorption heat of toluene and benzene on the activated carbon is very high.This can be explained that,at the beginning of adsorption,the gas coverage degree on the surface of activated carbon is low,the adsorbate molecule directly interacts with bare adsorption sites.When the adsorption sites are gradually occupied,the weakening adsorption force contributes to the decreased adsorption heat.Besides,we noticed that the weakening degree of adsorption heat of benzene is higher than that of toluene,indicating that the binding force of toluene and activated carbon is stronger.In the later adsorption selectivity calculation,the adsorption heat called is the obtained average value of the adsorption process,and the average adsorption heat of benzene,toluene and water is calculated as 83.00 kJ·mol-1,95.56 kJ·mol-1and 43.318 kJ·mol-1.
3.7.Calculation of competitive adsorption of toluene and benzene to water
To be closer to the actual application of the adsorbent,the adsorption separation efficiency of the adsorbent was evaluated by calculating the adsorption selectivity.But even today,the direct measurement of adsorption equilibrium and selectivity of mixtures remains the most difficult experimental technique.Therefore,it is still necessary to make predictions with the help of theoretical models or semi-empirical models.In this paper,the DIH (equipotential heat difference) equation was selected to predict the selectivity of C6H6/H2O and C7H8/H2O on C4-800.The main equation is as follows formula 10 [49]:

whereqi(p)andqj(p)are static adsorption amounts of pure componentiorjatp.is equal to the difference between isosteric heats of componentiandj.R(=8.314×10-3kJ·mol-1·K-1)is the molar gas constant andT(K) presents temperature.In this work,we tried to use the DIH equation to estimate the adsorption selectivity of C6H6/ H2O and C7H8/H2O on the developed engineering activated carbon.

Fig.12.The calculated adsorption heat curves with increased adsorption amount (a) and DIH equation prediction on the selectivity of toluene and benzene to water on C4-800 (25°C).
Fig.12(b) shows the adsorption selectivity of toluene and benzene compared to water at 25°C based on the DIH calculation results.The result indicates that the adsorption selectivity of toluene is much better than that of benzene in the environment of water vapor.From the formula,the range of adsorption selectivity of toluene to water is 1833-12400,and that of benzene is 273-1671.The adsorbed selectivity of toluene to water is near threefold that of benzene to water.This would be very significant for the practical adsorption separation in the mixed VOCs sources.We suggest the generated appreciated adsorbed selectivity on the VOCs/water should benefit from the high temperature induced great surface hydrophobicity under the KOH assisted activated process.Especially,the surface polarity of activated carbon was further weakened at 800°C and make the surface of activated carbon more matched with the VOCs molecules compared to polar water.Besides,toluene molecules affords the certain polarity compared to benzene,which seems more suitable with the surface of activated carbon with certain surface oxygen moieties,thereby exhibit higher adsorption selectivity compared to benzene molecule.
The service life of the adsorbent is one important evaluation index of applied functional materials [50-53] was further ascertained to identify the reversible adsorption process.Therefore,the adsorption and desorption cycles of toluene were tested 5 times (Desorption method:the sample after adsorption was pretreated at 150°C in a vacuum,and the processing time was 2 h.).Fig.S5 suggests the C4-800 works very stably and the adsorption performance was well reserved during several adsorption-desorption cycles,which indicates its potential practical industrial value.
4.Conclusions
In summary,the optimized synthesis process for the activated carbon was adopted for reaching one outstanding engineering carbon adsorbent endowed with ultrahigh structural properties(SLangmuir=4962.6 m2·g-1,Vmicro=1.67 cm3·g-1) and uncompromising thermal stability (603°C).The comprehensive results suggest the micropore volume linearly correlated with the VOCs capture value,which claims the dominant effect for the VOCs adsorption.Besides,detailed results indicate 800°C derived activated carbon afford the dominant ultralow pressure adsorption effect which is promising for practical adsorption application,while the higher activated temperature triggered the emerged mesopore and caused more medium pressure adsorption.Using the DIH equation to predict adsorption selectivity of benzene and toluene in the mixed atmosphere bearing water vapor,the highest adsorption selectivity of activated carbon C4-800 for benzene and toluene is 1671 and 12,400 respectively,which exhibits the huge potential for practical selective adsorption separation of VOCs.From the point of view of the utilization of carbon source,the difficulty of preparation process,the VOCs adsorption performance,and the adsorption selectivity of mixed gas,we developed engineering carbon adsorbent deliver a good industrial application prospect.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21908085),Natural Science Foundation of Jiangsu Province,China (BK20190961),Postdoctoral Research Foundation of Jiangsu Province (2020Z291),Foundation from Marine Equipment and Technology Institute for Jiangsu University of Science and Technology,China (HZ20190004),and High-tech Ship Research Project of the Ministry of Industry and Information Technology,China (No.[2017] 614).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.02.013.
 Chinese Journal of Chemical Engineering2022年7期
Chinese Journal of Chemical Engineering2022年7期
- Chinese Journal of Chemical Engineering的其它文章
- Chemical reduction-induced fabrication of graphene hybrid fibers for energy-dense wire-shaped supercapacitors
- Preparation of aldoxime through direct ammoximation using titanium silicalite-1 catalyst
- Efficient synthesis of tyrosol from L-tyrosine via heterologous Ehrlich pathway in Escherichia coli
- N,P co-doped porous graphene with high electrochemical properties obtained via the laser induction of cellulose nanofibrils
- Two-stage cyclic ammonium sulfate roasting and leaching of extracting vanadium and titanium from vanadium slag
- Conjugation of a zwitterionic polymer with dimethyl chains to lipase significantly increases the enzyme activity and stability
