N,P co-doped porous graphene with high electrochemical properties obtained via the laser induction of cellulose nanofibrils
Jie Wei,Weiwei Yang,Shuai Jia,Jie Wei,Ziqiang Shao
Beijing Engineering Research Center of Cellulose and Its Derivatives,School of Materials Science and Engineering,Beijing Institute of Technology,Beijing 100081,China
Keywords:Cellulose nanofibrils Laser induction Porous graphene Multiple lasing Supercapacitor
ABSTRACT Cellulose and its derivatives are natural materials with high carbon contents,but it is challenging to convert their carbon into high value-added carbonaceous materials (e.g.,graphene).Here,an approach to convert the carbon in cellulose into N,P co-doped porous graphene (LIG) materials via laser induction is proposed.Cellulose nanofibrils(CNFs),a cellulose derivative with high dispersion uniformity and abundant surface hydroxyl groups,were easily formed on a bulk substrate (thickness ≥5 mm) containing ammonium polyphosphate (APP).Then,a 10.6 μm CO2 laser was used to scribe for 1-5 passes on the CNFs/APP substrate under an ambient environment to produce N,P co-doped porous LIG.Upon increasing the number of laser scribing passes,the IG/ID of LIG first increased and then decreased,reaching a maximum of 1.68 at 4 passes.The good pore structure and low resistance also showed that 4 laser passes were ideal.Besides,the N,P co-doped LIG also showed excellent electrochemical performance,with a specific capacitance of 221.4 F·g-1 and capacitance retention of 89.9%.This method exploits the advantages of nanocellulose and overcomes the difficulties associated with directly compounding cellulosic materials,providing a method for the further development of biomass nanomaterials.
1.Introduction
The high-value-added and sustainable utilization of natural resources is a major concern due to increasing environmental pollution and environmental protection awareness [1].Hence,environmentally-friendly and cost-effective production routes based on‘green’materials and technologies have been established to synthesize biodegradable and biocompatible materials [2,3].Cellulose,one of the most sustainable,earth-abundant,and accessible green materials,can be converted to carbonaceous materials such as charcoal and activated carbon using hydrothermal carbonization processes [4-6].With continuous advancements in technology,the laser-induced production of porous graphene makes it possible to transform cellulose into two-dimensional graphene materials,thereby further enhancing the application value of biomass materials [7].
Laser-induced graphene(LIG)is a 3D network of carbon directly converted from carbon-containing materials by a laser-scribing process.It has been regarded as a highly efficient,patternable,convenient,and environmentally-friendly strategy for assembling graphene since it was developed in 2014 [8-10].Since then,LIG has been used in supercapacitors and has shown superior electrochemical performance with good specific capacitance and mechanical stability[11].Doping by different elements during preparation further improves the electrochemical performance of LIG [12,13].LIG has revealed potential environmental applications as an efficient adsorbent for the decontamination of pollutants from water[14].Materials used to produce LIG are usually commercial polymers like polyimide (PI),polyetherimide (PEI),sulfonated poly(ether ketone),polysulfone,and polyethersulfone [15-17].Creating graphene from biomaterials by laser induction would be highly desirable because they are environmentally-friendly alternatives to conventional plastics [18].
The formation of LIG on biomaterials such as cellulose is challenging because the five-or six-carbon sugar monomers in cellulose cannot be directly converted to LIG and begin to decompose above 330 °C [19].Tour’s group constructed LIG on wood using a 10.6 μm CO2pulsed laser in an atmospheric chamber without oxygen to avoid unexpected ablation and burning [20].Later,coated surfaces of heat-sensitive materials with high cellulose contents,such as paper or food with fire retardants,helped form LIG in ambient air by suppressing ablation [21].Zhanget al.[22] and Leiet al.[7] used polyvinyl alcohol (PVA) as an adhesive to form films of lignin with polymers (PC,PMMA,and PET) or urea (fire retardant)to ensure laser induction;therefore,simple and efficient methods for developing high-performance LIG from biomass resources is urgently needed.
Cellulose nanofibrils (CNFs) are derived from plants and have emerged as a promising precursor for carbonaceous materials[23-25].Herein,we report a versatile strategy to produce a LIG material doped with N and P elements prepared from a nanocellulose matrix in an ambient atmosphere by a 10.6 μm CO2pulsed laser.Considering their ability to form a stable suspension and engage in hydrogen bonding,cellulose nanofibrils were prepared[26] and then combined with ammonium polyphosphates (APP).APP acts as a source of N and P dopant atoms,while simultaneously protecting cellulose from ablation.Furthermore,its excellent electrochemical performance shows that doping improved the electrochemical properties of LIG.
2.Experimental
2.1.Materials
Wood pulp with 90% α-cellulose after removing non-cellulose components was provided by North Century Cellulose Technology Research &Development Co.,Ltd.Isopropyl alcohol (IPA),sodium hydroxide (NaOH),monochloroacetic acid (MCA),acetic acid(HAc),ethanol,polyvinylidene fluoride (PVDF),andN-methyl pyrrolidone (NMP) were purchased from National Pharmaceutical Group Chemical Reagent Co.,Ltd.(Beijing,China).Ammonium polyphosphate (APP:n>2000) was purchased from Qingyuan Pusaifurop Chemical Co.,LTD.All reagents are of analytical grade and used without further purification.
2.2.Methods
2.2.1.Preparation of CNFs/APP composites
Cellulose nanofibrils (CNFs) were prepared using a method reported in our previous study [26].As the raw material in this research,the low-cost and high-yield CNFs were the basis for the subsequent high value-added applications.
Wood pulp powder was first alkalized with 25% (mass) NaOH solution in a 1:1.2 ratio for 90 min.The ratio of wood pulp powder to the reaction solvent (isopropanol) was 1:17.Then,MCA (MCA/-wood pulp powder=0.3355) was added into the mixture and etherified at about 50°C for 80 min and 75°C for 30 min.After that,the pH of the mixture was adjusted to 7 by adding acetic acid and washing three times with 75% ethanol (v/v).The as-prepared product was then mechanically fibrillated using a microfluidizer(Nano DeBEE,BEE International,MA)at 206.85 MPa psi three times to produce CNFs in a 2.0%(w/w)water dispersion.Then,APP powder(40%(mass)of CNFs)was added into the CNFs suspension and mixed well.The mixtures were then placed in a drying oven at 60 °C to form CNFs/APP composites (thickness ≥5 mm).
2.2.2.Fabrication of LIG by multiple laser-scribing
LIG was formulated on CNFs/APP substrates by a 50 W 10.6 μm CO2laser in a Ketai 4060 laser cutter system (Shangdong Kaitai Laser Equipment Co.,Ltd.,Liaocheng,China).Composites were scribed under ambient conditions with the same power (5 W),image density(6,894,760 Pa),and scan rate (20 mm·s-1),but with different numbers of laser passes (1,2,3,4,and 5),corresponding to LIG-1×,LIG-2×,LIG-3×,LIG-4×,and LIG-5×,respectively.
2.2.3.Characterization of LIG
The morphology and microstructure of LIG samples were characterized by scanning electron microscopy(SEM;S-4800,Hitachi),and EDS spectra were acquired using the same equipment.A TEM system(JEM 1200EX,JEOL)was used to characterize the graphene.The surface elements and crystal structures were analyzed by Xray photoelectron spectroscopy (XPS;Thermo ESCALAB 250Xi)and X-ray diffraction (XRD;Bruker D8 ADVANCE,Karlsruhe,Germany),respectively.Raman spectra were recorded using a Raman spectrometer (Renishaw inVia).Based on the Raman spectra,La(the crystalline size along theaaxis of graphitic materials) of LIG was obtained using the following equation [10]:
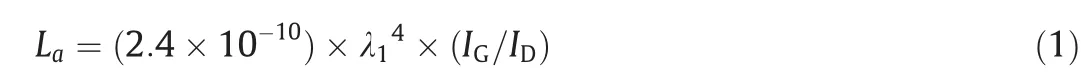
where λ1(λ1=532 nm)refers to the wavelength of the Raman laser.
2.2.4.Electrochemical measurements and calculations of LIG
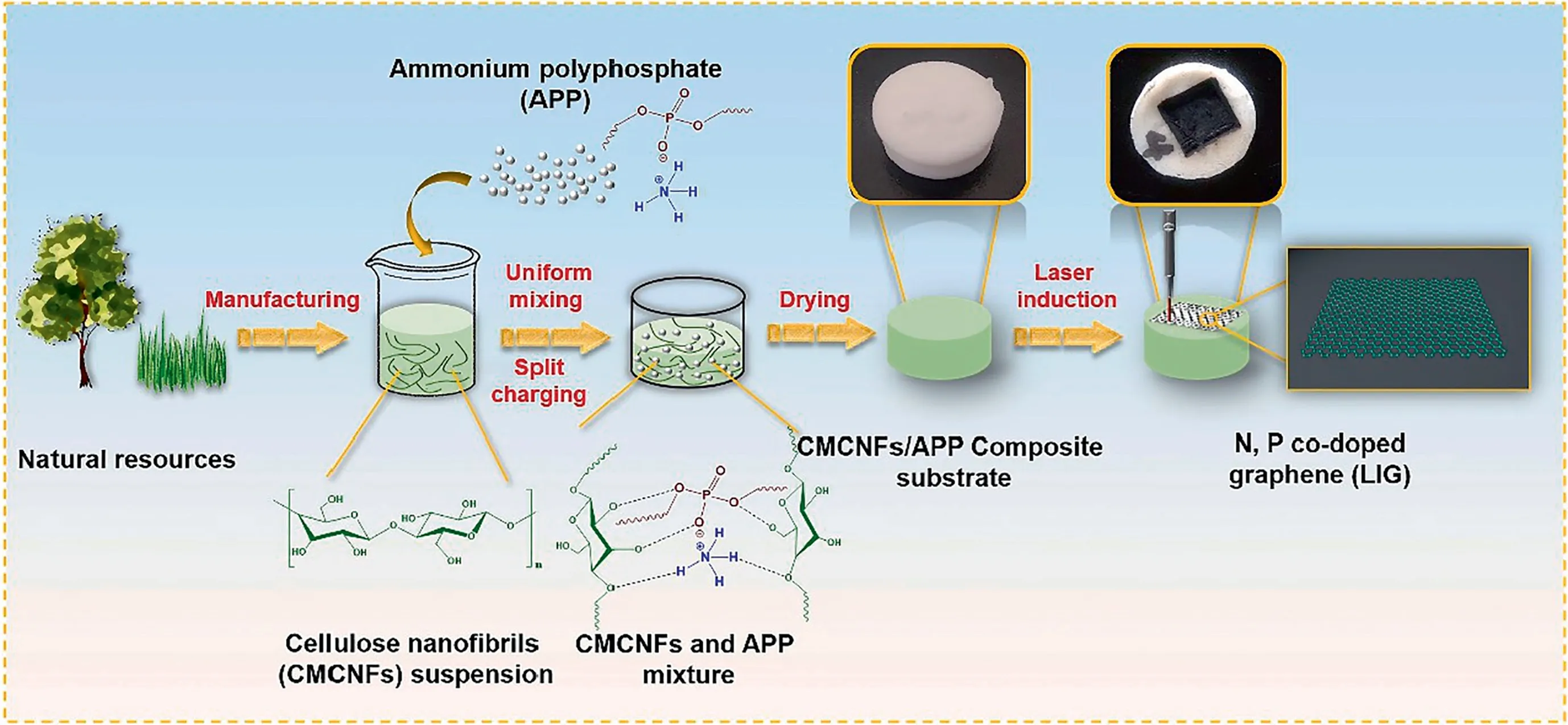
Fig.1.Schematic of the N,P co-doped LIG fabrication on CNFs and APP composite substrate.

Fig.2.SEM image of N,P co-doped LIG on the substrate (a).TEM image of few-layered N,P co-doped LIG (b and c) and local enlargement showing the ˜0.34 nm average lattice spacing (d).SEM image of N,P co-doped LIG and the corresponding EDS element maps of C,N,O,and P (e).
The electromechanical performance of LIG was evaluated by a typical three-electrode configuration on an electrochemical working station (CHI660E,Chenhua,Shanghai,China).The fabricated electrodes consisted of the LIG,conductive acetylene black,and PVDF with a mass ratio of 80:15:5.PVDF was first dissolved in NMP to form a 0.01 g·ml-1solution.The mixture was then magnetically stirred for 12 h to form a homogeneous slurry.After that,the electrodes were prepared by coating the resulting slurry onto titanium foil with a working area of about 1.0 cm× 1.0 cm,following by drying for 12 h at 80 °C [27].Cyclic voltammetry (CV) and galvanostatic charge-discharge(GCD)measurements were characterized in 1 mol·L-1H2SO4aqueous electrolyte,with a saturated calomel electrode(SCE)and a platinum sheet as the reference electrode and counter electrode,respectively.Electrochemical impedance spectroscopy (EIS) was performed in the frequency range of 0.01-100 kHz.The specific capacitances (Cs,F·g-1) were calculated from the GCD curves according to Eq.(2):

whereI(A)represents the discharge current,Δt(s)refers to the discharge time,ΔV(V) is the voltage window,andm(g) refers to the total mass of active materials used in the measurements.
3.Results and Discussion
3.1.Fabrication of LIG sheets
The fabrication of LIG co-doped with N,P elements on a CNFs substrate is schematically illustrated in Fig.1.After drying,the CNFs and water-soluble APP formed a uniform block due to strong hydrogen bonds and uniform dispersion.The addition of APP to the CNFs substrate promoted the efficient conversion of thermallyactivated biomaterials to N,P co-doped LIG without significant ablation or thermal damage,while simultaneously doping with N and P atoms.Finally,N,P co-doped porous LIG was fabricated on the CNFs/APP composite substrate.Furthermore,because the whole matrix is a uniform structure,after scraping it off the surface of graphene,the matrix can still be laser-treated to produce graphene.
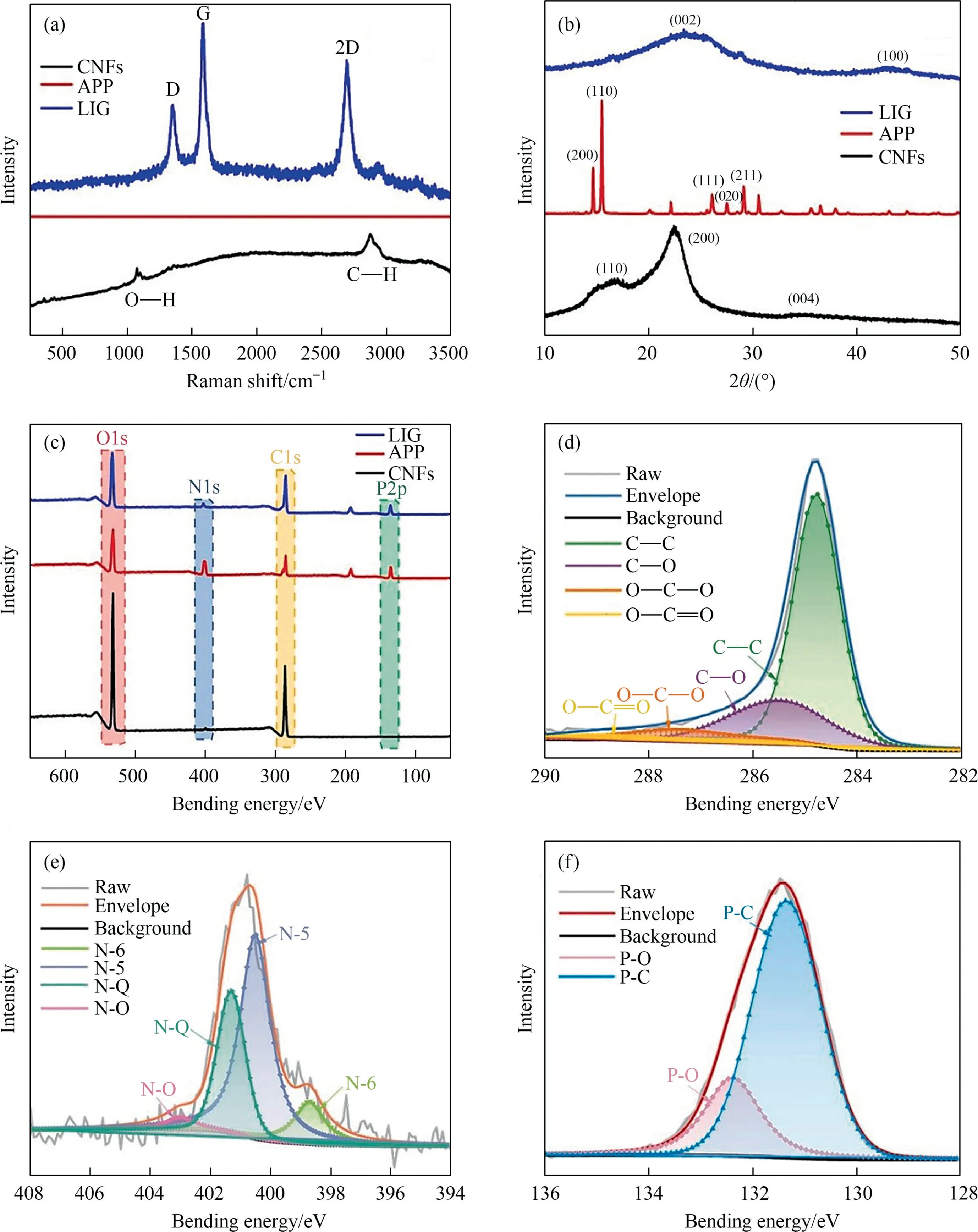
Fig.3.(a) Representative Raman spectrum,(b) XRD patterns,and (c) XPS survey spectra of substrate materials CNFs and APP,and N,P co-doped LIG scraped from the substrate.Typical high-resolution XPS C 1s,N 1s,and P 1s spectra of N,P co-doped LIG ((d),(e) and (f)).
According to the formation mechanism of LIG and the flame retardant mechanism of flame retardant APP,the laser-induced formation mechanism of LIG on CMCNFs/APP is as follows:CO2infrared laser can transform polymer into three-dimensional porous LIG in the air by photothermal effect.The photothermal effect introduced in the laser engraving process will lead to higher local temperature,combustion erosion and depolymerization of CMCNFs/APP composites.Due to the barrier effect of APP [28],APP degradation will produce phosphoric acid and metaphosphoric acid,which can catalyze CMCNFs to amorphous carbon.As the photothermal effect continues (the passes of laser-induced increases),most of the hydrogen,oxygen and a small amount of carbon elements in CMCNFs escape into gas products,and the remaining carbon elements are arranged into porous graphene in the form of SP2with porous produced by escaped gases [29].
3.2.Morphological characterization of LIG
The substrate irradiated by the laser was converted to N,P codoped LIG,while the rest was unchanged,as displayed in Fig.2(a).The morphology of the substrate and N,P co-doped LIG are quite different.Comparing the morphological changes of CNFs and N,P co-doped LIG (Fig.S1(a)),we can see that the tree-like CNFs matrix first formed a dense substrate with APP.After laser scribing,porous,fluffy,and flake-structured N,P co-doped LIG was transformed on the surface of the substrate.Further TEM observation (Fig.2(b) and (c)) showed that the flake surface of the N,P co-doped LIG sheet wall was severely wrinkled at the nanoscale,a phenomenon that has been reported in previous literature[30,31].The high-resolution TEM(HR-TEM)image in Fig.2(d)demonstrates that the average lattice spacing of N,P co-doped LIG is about 0.34 nm,which is expanded compared with conventional graphite.The expansion is due to the doping of nitrogen and phosphorus atoms[7].The energy-dispersive spectrometry(EDS)spectra display a uniform distribution of C,N,O,and P atoms embedded on the surface of the generated N,P co-doped LIG carbon framework with a 3D architecture (Fig.2(e)).
3.3.Structural characterization of the raw materials and LIG product
Fig.3 further confirms the successful fabrication of N,P codoped LIG on the composite substrate created from the CNFs and APP.As shown in Fig.3(a),the characteristic peak of CNFs at 2895 cm-1belongs to the -CH group [32].As for N,P co-doped LIG,the peaks around 1350,1580,and 2700 cm-1are attributed to the D,G,and 2D bands,respectively.Furthermore,the XRD spectra in Fig.3(b) show the crystal form changes between the substrate materials (CNFs and APP) and the N,P co-doped LIG.For CNFs,the XRD pattern shows a typical cellulose I structure,which has 2θ diffraction peaks at approximately 16°,22.5°,and 34.5°,respectively,corresponding to the(1 1 0),(2 0 0),and(0 0 4)reflection planes [26].The XRD pattern of APP exhibits four peaks at 2θ values of 14.2°,17.1°,18.6°,and 21.2°,which correspond to the(1 1 0),(0 4 0),(1 3 0),and(1 3 1)crystal planes,respectively[33].The XRD pattern of N,P co-doped LIG has a peak at 2θ=25.9°,giving N,P co-doped LIG an interlayer spacing(Ic)of about 0.34 nm between the(0 0 2)planes.This also indicates the high degree of graphitization.The peak at 2θ=42.9° is attributed to in-plane structure(1 0 0) reflections.
To further investigate the different elemental compositions,Xray photoelectron spectroscopy (XPS) measurements were carried out.The XPS survey spectrum of N,P co-doped LIG in Fig.3(c)shows four characteristic peaks atca.285,296,532,and 131 eV,corresponding to C 1s,N 1s,O 1s,and P 2p,respectively[34].Comparing the XPS spectra of CNFs and APP in Fig.3(c),it can be seen that carbon-forming matrix materials,CNFs,only contain C and O atoms,but not N and P.The presence of C,N,O,and P atoms in APP indicates that the N and P atoms in LIG came from APP.Further investigation showed that the C 1s (Fig.3(d)) spectrum of N,P codoped LIG contains four signals attributed to C-C,C-O,O-C-O,and O-C=O,respectively,at 284.7,285.6,287.5,and 289.1 eV[35].As for the N 1s spectrum (Fig.3(e)),the four peaks located at 398.7,402.0,401.4,and 403.7 eV were assigned to the N-6,N-5,N-Q,and N-O,respectively [13].The deconvoluted highresolution P 2p spectra show two peaks in Fig.3(f),which correspond to P-C (131.4 eV) and P-O (132.2 eV) bonds [34].These results strongly support that N and P atoms are successfully doped into graphene through laser induction.
3.4.Preparation process of LIG
The fabrication of LIG was affected by the laser power;thus,we further studied the properties of N,P co-doped LIG by changing the number of laser passes from 1 to 5 under 5 W at a 20 mm·s-1scan rate.Raman spectroscopy is often used to investigate and characterize the graphitization degree and defect formation of N,P codoped LIG [36].In Fig.4(a),three characteristic peaks (D-band,G-band,and 2D-band) of N,P co-doped LIG appeared around 1345,1586,and 2700 cm-1,respectively,which were associated with defects,heteroatom doping,and crystalline sp2carbon [37].As the number of laser-scribing passes increased from 1 to 4,the integrated intensities of G and D peaks (IG/ID) first increased from 0.90 to 1.68 (Fig.4(b));however,when more than 4 laserscribing passes were used,IG/IDbegan to decrease,and a scorched yellow ablated region emerged on the edge of the N,P co-doped LIG (red circle in Fig.4(b)),which may be due to oxidation in the air.TheLa(crystalline size along theaaxis) values calculated by Eq.(1) also show the same trend (Fig.S2),and theLaof LIG-4× is 32 nm.Moreover,the resistance (R) of N,P co-doped LIG (1.0 cm × 1.0 cm) decreased first and then increased slightly as the number of laser-scribing passes increased and reached a minimum of 31 Ω.The morphology of N,P co-doped LIG was affected by the number of laser passes because of the gaseous products generated(NH3etc.)during the pyrolysis of APP.Further SEM observations of LIG-1×,LIG-4×,and LIG-5×proved this(Fig.4(d)-(f)).The N,P codoped LIG layers were composed of many porous sheets.Neatlyarranged macropores replaced the chaotic micropore structure after increasing the number of laser-scribing passes,but an excessive number of laser-scribing passes reduced the number of pores;therefore,the suitable parameters to fabricate N,P co-doped LIG on the CNFs composite substrate were a laser power of 5 W with a 20 mm·s-1scan rate for 4 times.

Fig.4.Raman spectra(a),integrated intensities of G and D peaks (IG/ID) (b),resistance (1.0 cm× 1.0 cm),and SEM images(d-f) of N,P co-doped LIG samples produced by different numbers of laser passes.

Fig.5.Electrochemical performance of N,P co-doped LIG in a three-electrode system.(a)CV curves of N,P co-doped LIG at different scanning rates;(b)GCD curves of N,P codoped LIG at different current densities;Specific capacitance (c) of N,P co-doped LIG at different current densities;(d) Electrochemical impedance the electrochemical impedance spectroscopy(EIS)of LIG over a frequency range of 0.01-100 kHz;(e)cycling stability at a current density of 1 A·g-1;(f)specific capacitance of N,P co-doped LIG with different laser passes at 0.05 A·g-1 current density.
3.5.Electrochemical performance of LIG
The electrochemical properties of the N,P co-doped porous LIG prepared were tested using a three-electrode system.In Fig.5(a),the CV curves at different scanning rates show symmetric and quasi-rectangular shapes.When the scan rate increased from 800 mV·s-1to 8 V·s-1,the integral area of the CV curves increased,but their shape did not change significantly.This indicates that N,P co-doped LIG has good rate performance and ideal capacitance behavior[36],which may be attributed to its rich porous structure and doping by N and P atoms.
Fig.5(b) and Fig.S3 show the GCD curves of the N,P co-doped LIG electrode.It can be found that all GCD curves are not regular triangles,that is,the charge-discharge curves deviate from linearity,which is mainly due to the Faraday pseudocapacitance process on the electrode surface[38].Based on the GCD curves,the specific capacitances (Cs) of N,P co-doped LIG were calculated by Eq.(2)and are shown in Fig.5(c).From Fig.5(c),we can see that when the current density was 0.05 A·g-1,theCsof N,P co-doped LIG was 221.4 F·g-1.When the current density increased to 5 A·g-1,theCswas 190.4 F·g-1.The specific capacitance of our N,P codoped LIG is higher than undoped LIG and recently-reported GObased supercapacitors devices [39,40] and similar to that of a recently-reported doped LIG [12].The capacitance retention rate of N,P co-doped LIG is 89.9%,indicating its good rate performance.Moreover,the typical shape of GCD curves of N,P co-doped LIG electrodes exhibited small voltage drop (IR drop) of 0.05 V at 0.8 A·g-1.This indicates that the internal resistance of the N,P codoped LIG electrode is lower,which can also be demonstrated by EIS (Fig.5(d)).
The electrochemical impedance spectra (EIS) of the N,P codoped LIG electrode were measured to gain insight into the ion kinetics process.N,P co-doped LIG has a higher slope in the lowfrequency region,indicating better electrochemical performance[41].From the inset in Fig.5(d),the equivalent series resistance(ESR) of N,P co-doped LIG is 8.9 Ω,which is lower than the 26.1 Ω of a previously-reported pristine LIG [42].N,P co-doped LIG materials have good electromechanical properties,which are related to the specific surface area caused by a large number of wrinkles on the surface of the multilayered porous material,and also due to doping by N and P atoms.The cycling stability of N,P co-doped LIG was determined by performing a charge and discharge measurement at a current density of 1 A·g-1.As shown in Fig.5(e),although the specific capacitance of N,P co-doped LIG electrode decays after 10,000 times of charge and discharge,it can still maintain 88.4% of the initial specific capacitance,indicating its good electrochemical stability.The decrease of specific capacitance is related to the loss of oxygen and nitrogen functional groups on the surface of N,P co-doped LIG [41].Besides,we have carried out electromechanical performance of N,P co-doped LIG with different laser passes (1,2,3,4,and 5),and calculated the specific capacitance change trend chart when the current density is 0.05 A·g-1in Fig.5(f),finding that that the specific capacitance of supercapacitor is directly proportional to the graphitization degree (IG/ID) of N,P co-doped LIG.
4.Conclusions
Herein,we demonstrated a simple method to produce N,P codoped porous LIG on a CNFs/APP composite substratevialaser induction under ambient conditions.The results showed that 4 passes were optimal for producing N,P co-doped LIG.Using this number of passes,theIG/IDreached a maximum of 1.68 and the resistance reached a minimum of 31 Ω.The material showed a specific capacitance of 221.4 F·g-1and capacitance retention of 89.9% in a three-electrode supercapacitor,demonstrating its potential applications.This work provides a new opportunity for improving the utilization of carbonaceous biomass materials by using them to prepare high-performance carbon materials such as graphene.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The characterization results were supported by Beijing Zhongkebaice Technology Service Co.,Ltd.The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.07.025.
 Chinese Journal of Chemical Engineering2022年7期
Chinese Journal of Chemical Engineering2022年7期
- Chinese Journal of Chemical Engineering的其它文章
- Chemical reduction-induced fabrication of graphene hybrid fibers for energy-dense wire-shaped supercapacitors
- Preparation of aldoxime through direct ammoximation using titanium silicalite-1 catalyst
- Efficient synthesis of tyrosol from L-tyrosine via heterologous Ehrlich pathway in Escherichia coli
- Two-stage cyclic ammonium sulfate roasting and leaching of extracting vanadium and titanium from vanadium slag
- Conjugation of a zwitterionic polymer with dimethyl chains to lipase significantly increases the enzyme activity and stability
- Cycle temporal algorithm-based multivariate statistical methods for fault diagnosis in chemical processes
