Thermochemical decomposition of phosphogypsum with Fe-P slag via a solid-state reaction
Lei Sun,Zhongjun Zhao,Xiushan Yang,Yan Sun,Quande Li,Chunhui Luo,Qiang Zhao,
1 School of Chemical Engineering,Sichuan University,Chengdu 610065,China
2 School of Mechanical Engineering,Chengdu University,Chengdu 610106,China
3 State Key Laboratory of Long-Life High Temperature Materials,Deyang 618000,China
4 Dongfang Electric Corporation Dongfang Turbine Co.,Ltd,Deyang 618000,China
Keywords:Phosphogypsum Decomposition Mechanism Thermodynamic Roasting conditions
ABSTRACT Phosphogypsum is a solid waste sourced from the wet-process phosphoric acid industry,which causes severe environmental damages.Its utilization was limited by its high decomposition temperature and high energy consumption.Herein,an Fe-P slag,which is a solid waste that mainly comprises iron phosphide (FeP) and diiron phosphide (Fe2P),can dramatically decrease the decomposition temperature of phosphogypsum.It was found that the Fe-P slag and CaSO4 can react as shown in the following reaction equation:2Fe1.5P+3CaSO4+6CO2 →Ca3(PO4)2+Fe3O4+3SO2+6CO.Its reaction mechanism was further determined using the thermodynamic method.It was found that CaS was the key intermediate for this reaction.The CaSO4 conversion for this method can reach approximately 97%under the optimized roasting conditions:the molar ratio between Fe1.5P and CaSO4 of 2:3,roasting temperature of 900°C,a roasting time of 8 h.
1.Introduction
Phosphogypsum is a solid waste of the wet production process of phosphoric acid.It is primarily composed of CaSO4and a certain number of impurities,such as phosphates,fluorides,silica,heavy metals ions,and organic substances.More than 250 million tonnes of phosphogypsum are estimated to be produced worldwide annually.However,less than 15%of this amount has been utilized thus far[1-3].Phosphogypsum is usually dumped or stored in the open air without any treatment as solid waste.Thus,it often causes severe environmental pollution [4,5].Traditionally,phosphogypsum has been utilized as an additive in building materials[6,7],cement retarders [8-10],and soil fertilizers [11].Moreover,phosphogypsum can be effectively reused by transferring the sulfur element in phosphogypsum to SO2that can be used for producing sulfuric acid [1,12,13].Also,calcium obtained from phosphogypsum can be used to produce additives for cement [1,14].Efforts have been increasingly made to develop a reliable decomposition method for phosphogypsum and understanding its reaction mechanism.Some chemicals such as coal [15],CO [16,17],H2[18] and sulfur[1]were reported as reducing agent for CaSO4conversion.It is generally accepted that CaSO4can be reduced to CaS in the presence of reducing agents below 1000 °C and further converted to SO2by CaSO4or oxidative gas above 1000 °C [19,20].The decomposition of CaSO4was found to be an extremely complex chemical process.More specifically,it may include up to 20 simultaneous chemical reactions between CaSO4and coal.These reactions can reduce efficiency and make their control very difficult.What’s more,such a high reaction temperature and low-value CaSO4decomposition products inhibit its successful industrial applications.
The Fe-P slag used herein is the by-product of the commercial yellow phosphorus industry having a low cost (no more than 150 USD·t-1)and massive production(more than 140 thousand tonnes per year in western China)[21,22].Fe-P slag primarily comprises of equal amounts of iron phosphide (FeP) and diiron phosphide(Fe2P).In our previous work,it was referred to as Fe1.5P [23].Herein,Fe-P slag was introduced as a novel reductive agent to decompose phosphogypsum.The thermochemical decomposition test of the mixture of phosphogypsum and Fe-P slag was performed in the CO2atmosphere.The solid roasting products were investigated by X-ray diffraction analysis (XRD),whereas the gaseous products were determined using an online sensor and chemical titration,respectively.Furthermore,the possible chemical reactions that occurred in this chemical system were proposed and studied using the thermodynamic method.Finally,the effects of the molar ratio of raw materials,roasting temperature,and roasting time on the roasting process were investigated to optimize the roasting conditions for this method.
2.Experimental
2.1.Material
The phosphogypsum sample used throughout this work was provided by Sichuan Lomon Titanium Industry Co.,Ltd.(Deyang,China) and it was dried overnight in a vacuum oven under 110°C before use.XRF and XRD were served as chemical composition analysis of the as-obtained phosphogypsum (Table 1 and Fig.S1).It shows that the major component of the as-obtained phosphogypsum sample is CaSO4·2H2O.Fe-P slag,used as a reductive agent herein,is the molten industrial solid waste discharged from the phosphorus thermal process.The gas flow was crushed to obtain Fe-P slag powder with a particle size below 8.0 μm(2000 mesh).All the other chemical reagents used in this work were of analytical grade without the need for further purification before use (Kelong chemical group,China).
2.2.Roasting experiments
Fe-P slag and phosphogypsum were mixed thoroughly by ballmilling in different molar ratios.This molar ratio was calculated based on the actual CaSO4content in phosphogypsum and Fe1.5P in Fe-P slag.Then the mixture was roasted in an electric tube furnace in a CO2atmosphere.In a typical roasting process,Fe-P slag and phosphogypsum were mixed with as a molar ratio of 2:3 (between CaSO4and Fe1.5P) and roasted at 800 °C for 10 h in a CO2atmosphere.Moreover,the solid and gaseous products were collected for further analysis.
2.3.Analytical method
The chemical composition analysis of the as-obtained solid roasting products was carried out using X-ray diffraction analysis(XRD,Philips X Pert Pro,Holland).The XRD analysis was made with Cu Karadiation(40 kV,30 mA)and Ni filter,with increments of 0.1 degrees and a counting time of 1 s per step.A home-made online CO analysis system was used to determine the CO content in the gaseous product,as detailed in our previous papers [21].The gaseous products were determined using vacuum ultraviolet ionization time-of-flight mass spectrometry (VUV-TOFMS).In this test,the VUV ion source was operated in electron ionization mode with a 10.6 eV Kr lamp and produced about 20 eV electron the photoelectric effect.
The sulfur content in the mixture before and after the roasting test was determined by an Eschka method [24].Eschka agent was used to ensuring that all the sulfur was transferred to sulfate,and gravimetric analysis was used to evaluate the total sulfur content.Then,the CaSO4conversion (the molar ratio between the SO2in gaseous product and CaSO4in the added initially for the roasting test) for the roasting test can be calculated as follows [24]:

Here,γ is CaSO4conversion in %;M1is the mass of the mixture after the roasting process,in g;St1is the corresponding sulfur content in the mixture in %;M2is the mass of the mixture before the roasting process in g andSt2is the corresponding S content in the mixture in %.
2.4.Thermodynamic analysis
The stoichiometric reaction for the decomposition of phosphogypsum by Fe-P slag was also studied using the thermodynamic simulation analysis method.Some thermodynamic parameters for possible reactions in this system were calculated using HSC chemistry 6.0 software.The criterion employed for the simulation was the minimization of Gibbs free energy according to two hypotheses.The first hypothesis is that all input substances are considered as ideal,and two or multiple substances are assumed to be completely mixed when they can be defined as the same phase.The second hypothesis is that the initial system should finally reach equilibrium[25].The thermodynamic data used to calculate the enthalpy change in the reactions are shown in Table 2.

Table 1 Chemical composition of the phosphogypsum

Table 2 Thermodynamic properties of the pure phases used in the simulation
3.Results and Discussion
3.1.Chemistry in phosphogypsum decomposition
First of all,the thermogravimetric analysis (TGA) was performed to investigate the reaction between Fe-P slag and phosphogypsum.The TGA curve for phosphogypsum and Fe-P slag mixture in a CO2atmosphere was shown in Fig.1(a).In particular,the mass of the mixture has not changed until the temperature reached about 600 °C (Fig.1(a)).The mass of the mixture showed an increase of 12% at approximately 606 °C.Considering this,the as-solid chemical system used in this study,CO2sourced from the gas,can be captured during the reaction.As shown in Fig.1(a),the mass of the solid materials decreased to about 94% of the initial mass when the temperature exceeded 80 is supposed to be a gas emission process with a temperature of about 800°C.Also,the mass loss was slightly higher than that in the CO2capture process at about 606 °C.Also,TGA of the mixture of phosphogypsum and Fe-P slag was performed in a nitrogen atmosphere(Fig.S2).The TGA curve in the nitrogen atmosphere was different from that in the CO2atmosphere.As shown in Fig.S2,there was no mass increase below 600 °C.The latter indicated that CO2participated in the chemical reaction during roasting.Furthermore,roasting tests were carried out by calcining the mixture of phosphogypsum and Fe-P slag in the CO2atmosphere.After cooling to room temperature,the roasting products were collected.The XRD pattern of the obtained solid roasting product is shown in Fig.1(b).It demonstrates that the main components of the roasting products are Fe3O4and Ca3(PO4)2.Thus,phosphogypsum could react with Fe-P slag in the in a CO2atmosphere.Furthermore,the roasting tests were conducted by calcining the mixture of phosphogypsum and Fe-Pa slag in a nitrogen atmosphere.The XRD pattern of the asobtained solid roasting product is shown in Fig.S3.As can be seen,the main components of the roasting products were Ca10(PO4)6S,FeS,and unreacted Fe2P.While Ca10(PO4)6S can be treated as a complex of Ca3(PO4)2and CaS.It indicated that FeS and CaS can be possible intermediate species in the decomposition of CaSO4.
Next,the gaseous products collected when the mixture roasted under 900 °C.VUV-TOFMS was employed to analyze the as-obtained gaseous products.The as-obtained mass spectra are shown in Fig.2(a).It demonstrated that the gaseous product was dominated by CO2,CO,and SO2,where the contents of CO and SO2were comparable.The content of CO2was higher than that of CO and SO2,as CO2was the atmosphere for roasting and carrier gas for the sample collecting.And the concentration of CO is comparable to that of SO2.At the same time,the absorbing solution of the gaseous products in 5 mol·L-1NaOH solution was determined by ion chromatography.The corresponding chromatograms were shown in Fig.2(b).

Fig.1.TG analysis of Fe-P slag and phosphogypsum mixture under a CO2 atmosphere,(b) XRD pattern of the roasted product of Fe-P slag and phosphogypsum under a CO2 atmosphere.
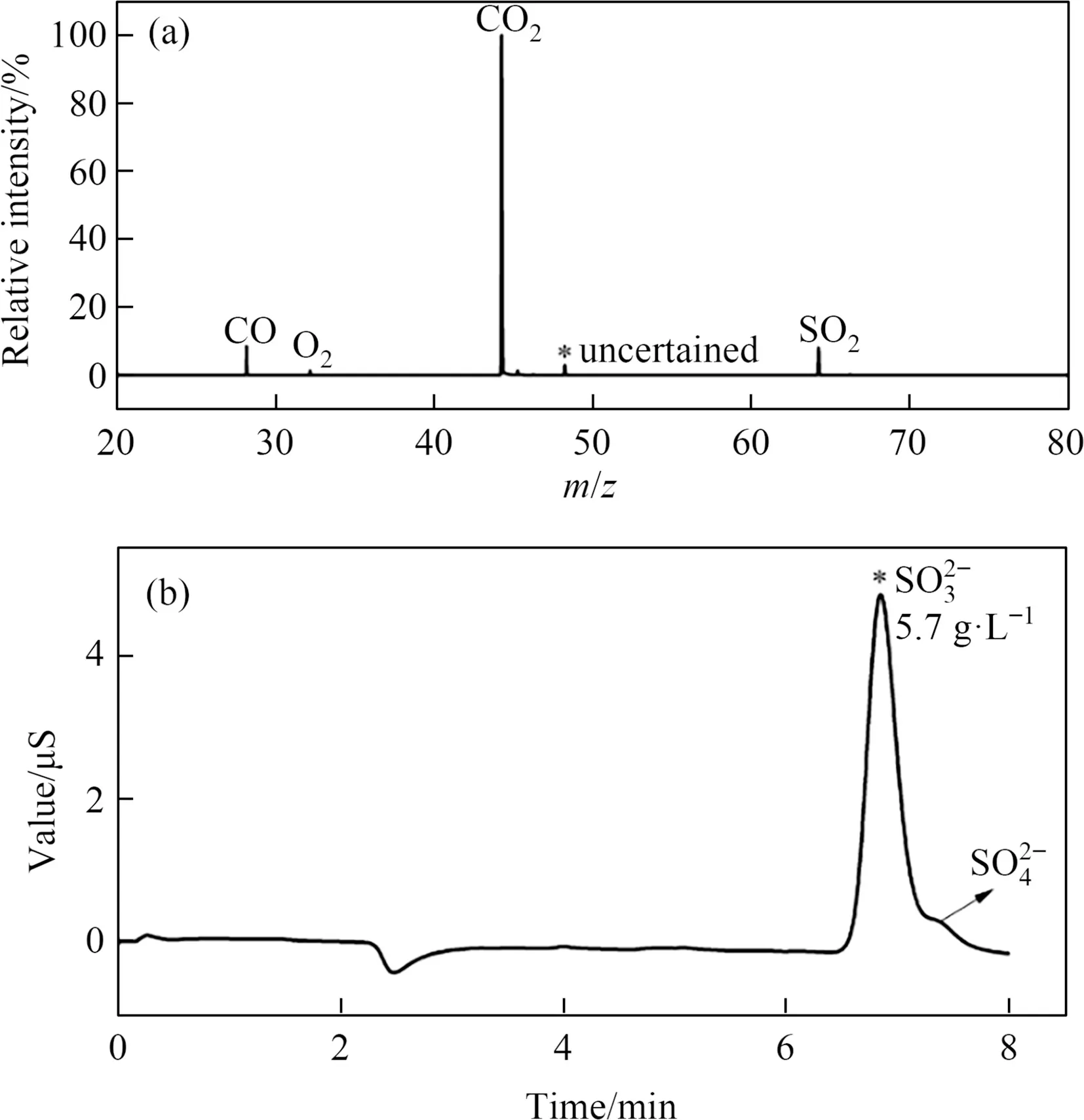
Fig.2.(a) Mass spectra of the gaseous products in the roasting test.(b) The chromatograms of SO32- in as-obtained absorbing solution.
A home-made online CO test system was used to analyze the gaseous products.The schematic diagram of this system was illustrated in Fig.3(a).
In this system,a coil heat exchanger was employed to cool down the gaseous products,followed by its pumping into a highprecision CO sensor (Membrapor,Switzerland).The concentration of CO in the gaseous products during the roasting test is shown in Fig.3(b).In this test,the temperature drift of the sensor was tested.In a typical experiment,a 4-g mixture of Fe-P slag and phosphogypsum was roasted in the tube furnace.As shown in Fig.3(b),there was an obvious CO emission process when the roasting temperature raised to 600°C,indicating that CO2was reduced to CO by the mixture of Fe-P slag and phosphogypsum during roasting.
3.2.The reaction mechanism of phosphogypsum decomposition
According to the analysis of the roasting products,possible solid products were Fe3O4and Ca3(PO4)2,whereas the possible gaseous ones included CO and SO2.Moreover,the Fe-P slag used herein was comprised FeP and Fe2P.Therefore,the possible chemical reaction that occurred during roasting can be ascribed as follow:


Fig.3.(a) Schematic diagram of the in-situ CO testing system.1—tube furnace;2—thermocouple;3—intelligent temperature controller;4—sample crucible;5—SiC heating component;6—quartz tube;7—coil heat exchanger;8—CO sensor.(b) CO generated while roasting Fe-P slag in CO2.
Therefore,the overall chemical reaction can be ascribed as blew.

Furthermore,the HSC chemistry software was employed for the thermodynamic analysis for the enthalpy change (ΔH) and the Gibbs free energy change (ΔG) of these reactions.The ΔGand ΔHfor reactions (1) and (2) were calculated,and the results were shown in Fig.4(a).In particular,as can be seen in Fig.4(a),both chemical reactions have negative Gibbs free energy change.Thus,both reactions can occur spontaneously for the entire temperature range considered herein.Moreover,the enthalpy changes for reaction (1) and reaction (2) amounted to approximately 30 kJ·mol-1and 80 kJ·mol-1,respectively.Consequently,both reactions were endothermic.It is also worth noting that reaction (1) has both lower ΔHand ΔGcompared to reaction (2),which suggests that it’s more favorable.
The chemical reactions that occur in the decomposition of CaSO4are rather complicated [20,29].The Gibbs free energy change (ΔG) of the possible reactions in the chemical system of Fe-P slag,CaSO4and CO2are calculated to speculate the reaction pathway for the decomposition of CaSO4.First of all,some possible intermediates are taken into consideration to speculate possible reactions.It was previously reported that CaS and CaO were typical intermediate species for the decomposition of CaSO4.Fe-slay served as a reducing agent in this process and was probably oxidized to iron oxides and phosphate.Thus,FeO,Fe2,Fe3O4and FePO4were considered as possible oxidation products.Fe-P slag was proved to be a mixture of FeP and Fe2P in our previous study.Therefore,FeP and Fe2P are discussed separately in the analysis of the reaction mechanism between Fe-P slag and CaSO4.Herein,the possible reactions between FeP and CaSO4are listed below.The Gibbs free energy change(ΔG)of the reactions(4)-(11)can be calculated using the reaction equations module of HSC chemistry.The effects of temperature on the ΔGof the reactions (4)-(11) are shown in Fig.4(b).

As shown in Fig.4(b),reactions (4)-(7) take place with a temperature below 1000 °C.However,reactions (8)-(11) do not seem to take place for the temperature below 1000 °C.It indicates that the intermediate component of CaSO4decomposition in this method is CaS rather than CaO.Also,reaction(4)seems to be more favourable than other reactions under the temperature below about 900°C.It indicates that FeP is likely to be oxidized to FePO4.Moreover,FePO4seems to be decomposable beyond this temperature and generates P2O5and iron oxides.Also,the possible reactions between Fe2P and CaSO4are listed below.CaS,CaO,FePO4and iron oxides are taken into consideration as possible products.The ΔGof the reactions(12)-(17)can be calculated using the reaction equations module of HSC chemistry.The effects of temperature on the ΔGof the reactions (12)-(17) are shown in Fig.4(c).

Fig.4.(a) Variation of ΔH and ΔG with temperature for the reactions (1) and (2);(b) Variation in ΔG with temperature for the reactions (4)-(11):(c) Variation of ΔG with temperature for the reactions (12)-(17) and (d) Variation of ΔG with temperature for the reactions (18)-(22).

As can be seen from it,reactions(12)-(14)occurs at the temperature below 1000 °C.It indicates that CaS seems to be the decomposition product of CaSO4in its reaction with Fe2P,while Fe2P can be oxidized to FePO4and iron oxides.According to the roasting products analysis,the final products are Ca3(PO4)2,Fe3O4and CO.Therefore understanding the conversion of the intermediate product CaS in the subsequent reactions is vital to building a complete reaction pathway.Herein,CaSO4and CO2are considered as possible oxidants in this chemical system.The possible reactions are listed below.


The ΔGof the reactions (18)-(22) can be calculated using the reaction equations module of HSC chemistry.The effects of temperature on the ΔGin reactions (18)-(22) are shown in Fig.4(d).It demonstrates that reactions(18)and(19)have positive ΔGwith a temperature below 1000 °C,that indicates that CaS may not be oxidized by CaSO4and CO2.However,when introducing FePO4as a reactant in reaction (20),it seems to be favorable to be driven it to the right beyond a temperature of about 700°C.Also,reaction(20)can be considered as an overall reaction of(18),(21)and(22).It seems that the strong interaction between the calcium ion and phosphate radical helps the oxidization of CaS in a CO2atmosphere,that can be crucial in decreasing the decomposition temperature of CaSO4.
3.3.Effect of roasting process on CaSO4 decomposition
In this work,the CaSO4conversion was considered as a critical parameter to evaluate the decomposition ratio of phosphogypsum.The CaSO4conversion was calculated by the decrease rate of the total S in the mixture during roasting.As for the decomposition process,the roasting condition has a significant influence on CaSO4conversion.Therefore,the effects of the molar ratio of raw materials,roasting temperature and roasting time on the CaSO4conversion were considered in the following study.

Fig.5.Effect of molar ratio between Fe1.5P and CaSO4 from phosphogypsum on the CaSO4 conversion.
The mixture of Fe-P slag and phosphogypsum was calcined in the different molar ratios by fixing the roasting temperature of 900 °C and the roasting time of 10 h.Herein,the molar ratio was defined as the molar ratio of Fe1.5P from Fe-P slag and the CaSO4from phosphogypsum.The CaSO4conversion with different raw materials added is shown in Fig.5.In general,the CaSO4conversion increases with the increase of Fe-P slag addition when the molar ratio is less than 2:3,which is the molar ratio from its equation(reaction (3)).When the molar ratio is 2:3,the CaSO4conversion can reach 97.14%.After that,the CaSO4conversion shows a slight downwards trend when more Fe-P slag was added.Also,we performed the roasting test using the mixture of analytical reagent CaSO4and Fe1.5P(Fig.S5).The CaSO4conversion is almost the same when the molar ratio between Fe1.5P slag and CaSO4is lower than 2:3.Also,the CaSO4conversion is constant when excess Fe1.5P added.Comparing these results,it can concluded that the impurity substance in phosphogypsum can suppress the release of SO2.It was noted that the solid roasting products have a color change when varying the initial addition of Fe1.5P.The digital images of the roasting products with different Fe1.5P addition are shown in Fig.S6.As can be seen from it,when the molar ratio between Fe1.5P slag and CaSO4from phosphogypsum is higher than 2:3,the roasting products appear to be of a black color,while it turns to a brown color when the molar ratio is lower than 1:3.XRD analysis was employed to study the chemical composition of these roasting products,and the results were shown in Fig.S7.As shown in Fig.S7,the solid roasting products when inadequate Fe1.5P added (molar ratio of 0.5:3) contain a considerable amount of Fe2O3beyond Fe3O4,which is probably to be an important iron compound in the roasting products when insufficient Fe1.5P added in this test.The generation of Fe2O3makes the corresponding solid roasting product appears a brown color as shown in Fig.S6.
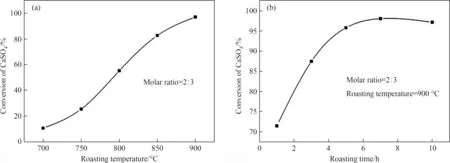
Fig.6.Effect of (a) roasting temperature and (b) roasting time on the CaSO4 conversion.

Fig.7.Schematic diagram of the roasting process.
The effect of roasting temperature on the CaSO4conversion was investigated,and the result was shown in Fig.6(a).The molar ratio between Fe1.5P slag and CaSO4from phosphogypsum was fixed at 2:3,and the roasting time was fixed at 10 h.The effect of roasting time on the CaSO4conversion was shown in Fig.6(b).The molar ratio between Fe1.5P and CaSO4from phosphogypsum was fixed at 2:3,and the roasting temperature was fixed at 900°C.It is obvious that the extent of reaction has a positive relationship with the roasting temperature and time.Also,the corresponding test using analytical reagent CaSO4was also conducted,and the result was shown in Fig.S8 and Fig.S9.As can be seen from these figures,the roasting temperature and roasting time showed a similar influence on the CaSO4conversion with that of phosphogypsum.Considering the actual reaction requirement,we supposed the molar ratio between Fe1.5P slag and CaSO4from phosphogypsum is 2:3,roasting temperature is 900 °C,roasting time of 8 hours was the optimum roasting condition,while the corresponding CaSO4conversion was 97.14%.
3.4.The process flow of decomposition phosphogypsum by Fe-P slag
Based on this,we proposed a process flow for decomposition phosphogypsum using Fe-P slag,that can eliminate phosphogypsum pollution and generate some useful products such as sulphuric acid,Fe3O4,and CaSO4.Also,the CO produced in this process can be utilized as fuel to preheat the raw materials to reduce its energy consumption.The flow diagram for this method was shown in Fig.7.There are four processes for this method,including raw materials mixture,roasting in a CO2atmosphere,gaseous product separation,and solid product separation.The primary reaction conditions for the roasting process were investigated in this work.We suggest using a molar ratio between Fe1.5P slag and CaSO4from phosphogypsum of 2:3 to promote the liberation of SO2and making Fe-P converted to the high-value Fe3O4.
As for the separation of Fe3O4and Ca3(PO4)2,the strong ferrimagnetism of Fe3O4allows the separation from its mixture with Ca3(PO4)2by a magnetic separation method.As for the gaseous separation,SO2is not difficult to separate from its mixture with CO owing to their remarkable difference in physical and chemical properties.Considering the primary application of SO2as producing sulfuric acid,the mixture can be catalytically oxidized to SO3before absorbed by dilute sulfuric acid.In this process,some commercial catalysts,such as V2O5[30],may be applied to oxidize SO2to SO3while it has not any catalytic activity toward the oxidation of CO.After that,the mixture gas of CO and CO2can be used as fuel and reactant gas for the decomposition of phosphogypsum.
4.Conclusions
Herein,we have demonstrated that Fe-P slag can decrease the decomposition temperature of phosphogypsum in a CO2atmosphere to 800°C.The solid and gaseous products of this decomposition process were confirmed and it was found that the reaction between phosphogypsum and Fe-P slag can be described as 2Fe1.5-P+3CaSO4+6CO2(g)→Ca3(PO4)2+Fe3O4+3SO2(g)+6CO(g).The reaction mechanism was investigated using the thermodynamic method.CaS was suggested as the key intermediate product of CaSO4decomposition.It was shown to be further oxidized by CO2in the presence of FePO4.The effect of the roasting condition on the CaSO4conversion was investigated.It was found that with a molar ratio between Fe1.5P slag and CaSO4from phosphogypsum of 2:3,the roasting temperature of 900 °C,the roasting time of 10 h,and the CaSO4conversion could reach approximately 97%.This novel method is a promising one to protect the environment by transforming two low-value by-products into some high-value products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (22108185,51702027),the Fundamental Research Funds for the Central University in China(20826041B4126) and project of Sichuan Chengdu Science and Technology Bureau (2019-YF05-02170-SN).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.06.025.
 Chinese Journal of Chemical Engineering2022年7期
Chinese Journal of Chemical Engineering2022年7期
- Chinese Journal of Chemical Engineering的其它文章
- Chemical reduction-induced fabrication of graphene hybrid fibers for energy-dense wire-shaped supercapacitors
- Preparation of aldoxime through direct ammoximation using titanium silicalite-1 catalyst
- Efficient synthesis of tyrosol from L-tyrosine via heterologous Ehrlich pathway in Escherichia coli
- N,P co-doped porous graphene with high electrochemical properties obtained via the laser induction of cellulose nanofibrils
- Two-stage cyclic ammonium sulfate roasting and leaching of extracting vanadium and titanium from vanadium slag
- Conjugation of a zwitterionic polymer with dimethyl chains to lipase significantly increases the enzyme activity and stability
