Enhanced endoscopic ultrasound imaging for pancreatic lesions: The road to artificial intelligence
Marco Spadaccini, Glenn Koleth,James Emmanuel, Kareem Khalaf, Antonio Facciorusso, Fabio Grizi, CesareHassan, Matteo Colombo, Benedetto Mangiavillano, Alessandro Fugazza,Andrea Anderloni, Silvia Carrara,Alessandro Repici
Abstract Early detection of pancreatic cancer has long eluded clinicians because of its insidious nature and onset. Often metastatic or locally invasive when symptomatic, most patients are deemed inoperable. In those who are symptomatic,multi-modal imaging modalities evaluate and confirm pancreatic ductal adenocarcinoma. In asymptomatic patients, detected pancreatic lesions can be either solid or cystic. The clinical implications of identifying small asymptomatic solid pancreatic lesions (SPLs) of < 2 cm are tantamount to a better outcome. The accurate detection of SPLs undoubtedly promotes higher life expectancy when resected early, driving the development of existing imaging tools while promoting more comprehensive screening programs. An imaging tool that has matured in its reiterations and received many image-enhancing adjuncts is endoscopic ultrasound (EUS). It carries significant importance when risk stratifying cystic lesions and has substantial diagnostic value when combined with fine needle aspiration/biopsy (FNA/FNB). Adjuncts to EUS imaging include contrast-enhanced harmonic EUS and EUS-elastography, both having improved the specificity of FNA and FNB. This review intends to compile all existing enhancement modalities and explore ongoing research around the most promising of all adjuncts in the field of EUS imaging, artificial intelligence.
Key Words: Pancreatic ductal adenocarcinoma; Pancreatic cancer; Endoscopic ultrasound; Contrast-enhanced endoscopic ultrasound; Endoscopic ultrasound contrast agents; Endoscopic ultrasound elastography;Artificial intelligence; Fractal analysis; Endoscopy; Imaging
lNTRODUCTlON
Pancreatic ductal adenocarcinoma (PDAC) is the 5thmost fatal cancer globally, and those receiving a diagnosis have only a 6% 5-year survival rate[1]. Fortunately, early detection of a PDAC, especially when smaller than 1 cm, along with timely resection can aggrandize 5-year survival rates by up to 80.4%[2]. This disease's increasing incidence and mortality trend have brought different specialists (i.e.radiologists and gastroenterologists) together to create a framework to enhance early detection.Computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS)have all emerged as essentials in the multidisciplinary diagnostic approach of PDAC. EUS images of the pancreas are distinct from all modalities above because of their increased spatial resolution, making it 94% sensitive for PDAC detection[3]. Successive generations of EUS imaging transducers and postprocessing software have markedly improved the spatial resolution of conventional B-mode EUS images. When used alone, it boasts a superior diagnostic accuracy over multidetector CT and MRI,singularly for solid pancreatic lesions (SPLs), but a meager specificity when differentiating pancreatic cancer from other pathologies[3,4]. However, with the advent of image-enhancing technologies such as contrast-enhanced EUS (CE-EUS) and EUS-elastography (EUS-E), there is evidence to suggest that even smaller SPLs are now detectable with good sensitivity and specificity[5-7]. Although promising, these advances' overarching impact in the face of early pancreatic cancer detection and diagnosing benign pancreatic diseases is still largely underwhelming. Pancreatic cancer remains a leading cause of death in industrialized populations, and the new case burden is estimated to grow steadily for the next 20 years[8]. Thus far, we know that the diagnostic accuracy of EUS, when coupled with EUS-guided tissue sampling, holds good outcomes, but reproducing its performance with ease remains a global challenge.The learning curve to master and competently perform an EUS examination is steep[9,10]. In this context, the introduction of artificial intelligence (AI), alone or in combination with existing enhanced EUS imaging technologies, may contribute to filling the gaps in accurately and consistently diagnosing pancreatic diseases[11]. This commentary highlights existing enhanced EUS imaging technologies while paving the way for AI, postulating its possible role in this arena.
EUS-E
Elastography is an ultrasonographic tool to measure the stiffness of the desired study area. The desired study area for a suspicious pancreatic lesion is also known as a region of interest (ROI). Akin to the clinical aspect of palpating a mass by hand and describing its characteristics, elastography does this similarly, but instead for deeper structures[12]. The two types of elastography include strain elastography (SE) and sheer wave elastography (SWE). However, SWE is still unstable in its use to measure the elasticity of SPLs and will not be discussed further in this article[13].
The type of elastography used in EUS is quasi-static or SE. It measures tissue displacement from a compressive force generated internally by the patient’s physiology like breathing and vascular pulsations or externally by the tip of the echoendoscope pressing against the GI wall[14]. The compressive force deforms the tissue momentarily. The amount the tissue deforms or the degree to which it gets displaced is known as strain. Softer or more elastic tissue,i.e.more benign, will have more displacement while stiffer, inelastic, and generally more malignant tissue, will have less strain[15]. The images can be seen side by side on the EUS display, with the EUS-E overlayed on the B mode image.
We present examples for PDAC (Figure 1A and B) and for a metastatic lymph node (Figure 1C). The color spectrum represents a qualitative measurement, blue being the hardest and red being the softest.Quantitative measurements are seen on the strain histogram or valued as the strain ratio (SR; the strain of the larger round-shaped ROI area A divided by the strain of smaller round-shaped region of surrounding normal tissue area B). Suggested surrounding normal homogenous tissue to be used as reference include either healthy surrounding pancreatic parenchyma (pSR) or healthy GI tract wall(wSR). Suggested elastography SR values are automatically computed and estimated using the built-in software found in the Olympus EU-ME2 processor and the Hitachi United States machines. However,the reference areas have yet to be standardized.
Outcomes
One of the earliest prospective multicenter studies by Giovannini[16] evaluated the elastography of pancreatic masses from 121 patients on initial EUS referenced against final histological diagnoses.Elastography successfully demonstrated “intense blue” on qualitative measurement for all PDAC,endocrine tumors, pancreatic metastasis, and pancreatic sarcomas. According to his scoring system of 1 through 5 (5 being the stiffest and most intense blue), scores 1 and 2 had a negative predictive value(NPV) of 77.4%, whilst scores 3 and 4 had a positive predictive value (PPV) for malignancy of 92.8%[16].
In the interest of detecting smaller ductal adenocarcinomas (< 15 mm), which if resected earlier, have shown a 5-year survival mark in PDAC survivors to exceed 30%-60%, Igneeet al[7] evaluated SRs for SPLs of < 15 mm from 218 patients across 13 international centers. They found that EUS-E patterns of a small lesion (average size 11 ± 3 mm), if found to be soft, could confidently rule out malignancy (NPV 98%). Although PDAC was diagnosed in these small lesions with a sensitivity of 96%, elevated stiffness in a detected lesion had a specificity of only 67% with a PPV of 56% when diagnosing malignancy[7].These small lesions’ final etiological percentage breakdown was 66% benign, while 52% were neuroendocrine tumors (NETs). Notably, 36% of the NETs were stiff lesions, while 64% were soft compared to surrounding tissue. The remaining 23% were PDAC, 17% other entities, and 8% metastasis (which showed stiffness only 59% of the time)[7].
In 2012, Peiet al[17] and in 2013, Yinget al[18], the Asian groups conducted separate metanalyses, but with additions to pooled data on the subject matter of EUS-E’s diagnostic accuracy, a more recent metaanalysis of 17 studies analyzing 1544 lesions from 1537 patients was conducted in 2017. From their pooled results, EUS-E reported an accumulated sensitivity of 97% (95%CI: 95%-99%) and a sensitivity of 67% (95%CI: 59%-74%) for qualitative methods; 97% sensitivity (95%CI: 95%-98%) and 67% specificity(95%CI: 61%-73%) for Strain Histograms, and lastly, 98% specificity (95%CI: 96%-99%) and 62%sensitivity (95%CI: 56%-68%) for strain ratios, suggesting its value as a complement to EUS-guided tissue sampling,i.e.EUS-FNA[19].
Similarly, guidelines proposed by the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) advocate using elastography as a complementary tool for focal pancreatic lesion characterization[20]. When a EUS-FNA sample is negative, but the index of suspicion for malignancy remains, the EFSUMB recommends using elastography and CE-EUS for repeat tissue sampling or referring the patient directly to the surgeons for operative intervention without further delay[20]. We demonstrate the clinical application of EUS-E (with CE-EUS) to target FNB sampling in a patient with a degenerated intraductal papillary mucinous neoplasm (IPMN) under our surveillance (Figure 2). This patient had positive cytology, warranting early surgery. Although documented to be helpful in the diagnosis of early autoimmune pancreatitis due to an idiosyncratic distribution of tissue stiffness, the guidelines cannot recommend the use of elastography in differentiating advanced chronic pancreatitis from pancreatic cancer[20].
One of EUS-E’s many inherent limitations is evident here: Its low specificity and inability to consistently distinguish between pancreatic diseases (inflammatoryvsneoplastic) or even types of pancreatic malignancies.
Inbuilt elastography software reduces intra-observer variability by overcoming the selection bias of images by providing the operator with a real-time movie of all recorded frames. However, there remains significant inter-observer variability when differentiating NETs from normal pancreatic parenchyma, as both may appear greeni.e.soft.
Here are a few other scenarios where misdiagnoses occur: (1) If the endoscopist places too much compressive pressure between the probe and the examined tissue; and (2) False image reconstruction due to the heterogeneity of the examined tissuei.e.in the case of pancreatic cancer where some areas within the lesion are either necrotic, fibrous from desmoplasia, or have become inhomogeneous from vessel infiltration.

Figure 1 Endoscopic ultrasound-elastography. A and B: Endoscopic ultrasound-elastography (EUS-E) use in demonstrating stiffness of pancreatic ductal adenocarcinoma tissue (encircled A) against normal pancreatic parenchyma (encircled B); C: EUS-E use in demonstrating stiffness of metastatic lymph nodes in patient from B. The nodes in B-mode when seen with elastography are blue, indicating that it’s hard and potentially malignant.

Figure 2 The clinical application of endoscopic ultrasound-elastography (with contrast-enhanced endoscopic ultrasound) to target fine needle biopsy sampling in a patient with a degenerated intraductal papillary mucinous neoplasm under our surveillance. A: Case demonstration of a degenerated intraductal papillary mucinous neoplasm and combination use of endoscopic ultrasound-elastography (EUS-E) to direct fine needle biopsy (FNB) sampling. Pre-EUS-E, the area of concern was iso-echoic, resembling normal parenchyma; however, under EUS-E, the area is stiff (blue); B: Contrast use post-FNB showing hypo-enhancement of the area of concern, confirming the area previously highlighted by EUS-E; C: Post-EUS-E and contrast-enhanced EUS guided FNB sampling in the same patient, allowing targeted sampling of the hard area.
Other pitfalls include: (1) A lack of standardization for cut-off values for strain ratios, although some experts propose using parenchyma-to-lesion SR of > 9.10 (PPV 89.7%; NPV 76.9%) or the gastric wall-tolesion SR of > 16.2 (PPV 86.5%; NPV 80%)[21]; (2) Border delineation once the lesion becomes larger[22]; (3) Inability to measure strain if the lesion is too far away from the probe, and lastly; and (4) Image acquisition when fluid is present,i.e.with cysts[22].
Carraraet al[21] demonstrated the use of fractal geometry analysis (Figure 3) to quantify the surface roughness of 2 tissues that share the same mechanical properties on elastography, and in doing so,overcome a significant limitation of EUS-E, but further trials are required[21]. Studies are also underway to evaluate the use of EUS-E in differentiating actual vessel wall infiltration by tumor tissue from that caused by an inflammatory reaction. The European Elastography Group, amongst others, is also designing and training deep learning platforms for future use in reducing inter-operator variability.
CE-EUS lMAGlNG
CE-EUS imaging is a non-invasive technique that utilizes a contrast agent during a EUS examination to improve diagnostic imaging. Contrast use in ultrasound imaging was first pioneered in 1986 by Matsuda and Yabuuchi with the infusion of CO2as a medium. Kato heralded its subsequent application into EUS in 1991, confined only to angiographic examinations[23]. In the mid-1990s, the use of Doppler function and sonicated serum albumin expanded the application of CE-EUS beyond the premise of angiographic studies[24].
In CE-EUS, the mechanical index (MI) alludes to the reaction of microbubbles in response to a stimulus in the form of an acoustic wave[25]. From this response, CE-EUS is typed into two: (1)Contrast-enhanced high-MI EUS (CEHMI-EUS), not requiring specific interpretive software; and (2)Contrast-enhanced low-MI EUS (CELMI-EUS), requiring a contrast-specific software mode.
A significant advantage of CELMI-EUS over CEHMI-EUS is its higher resolution and visibility of the contrast enhancer bubbles. In contrast, CEHMI-EUS's ability to display pancreatic macro-vessels (vessels with a diameter of approximately 0.2 mm and higher) comes at the expense of microbubble destruction and, as a result, CEHMI-EUS's use for Doppler enhancement is preferred[26].
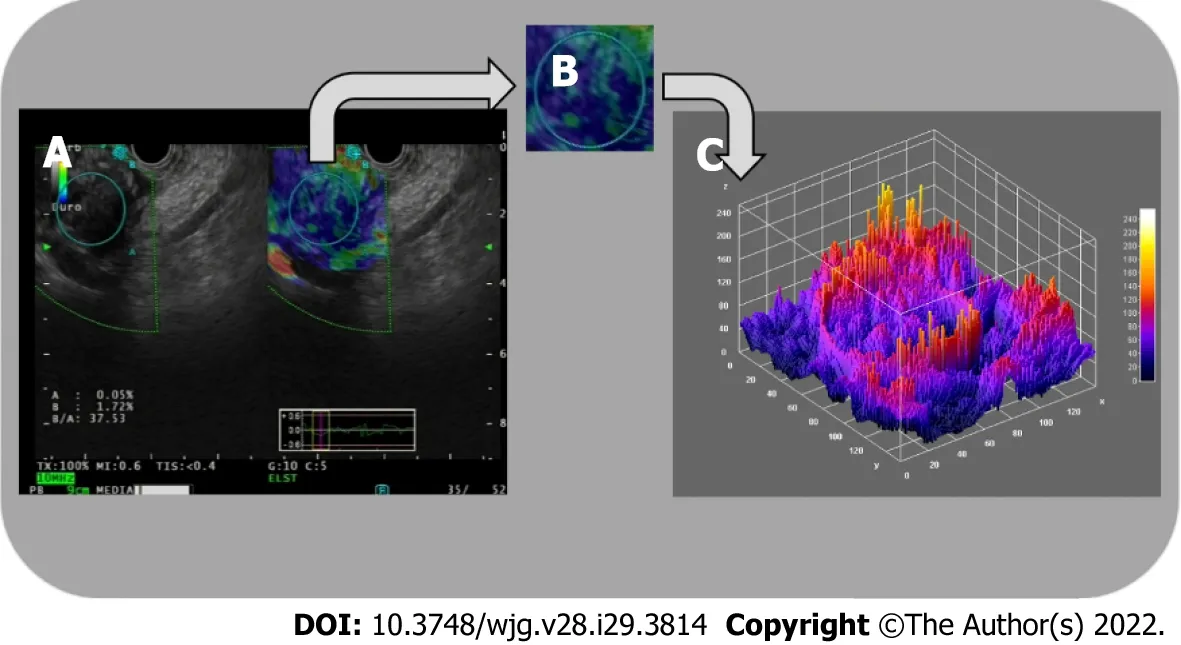
Figure 3 Three-dimensional surface fractal dimension estimate. A and B: Endoscopic ultrasound-elastography images (A) are used to highlight representative parenchymal regions (B) of solid pancreatic lesions; C: A computer-aided image analysis system generates an irregularly-shaped three-dimensional surface as a “shape matrix” of points with the column and row numbers proportional to the x and y coordinates and with the depth information z (x, y) stored as a matrix element. The three-dimensional fractal dimension, which is an index of the “surface roughness”, is automatically determined using the box-counting algorithm.The fractal dimension of a surface is expressed by a real number greater than 2 (the Euclidean dimension of a two-dimensional surface) and less than 3 (the Euclidean dimension of a solid). A surface with a higher surface fractal dimension is wrinkled than one with a lower dimension.
In collaboration with European experts in a recently issued guideline, the Asian Federation of Societies for Ultrasound in Medicine and Biology (AFSUMB) concluded that the MI should be between 0.2 and 0.4. They added that the 0.1 to 0.4 range used in published reports was influenced by several factors, including EUS probe type (radial or linear), image processing hardware, and software, phaseinversion harmonics, amplitude modulations, and focal points[27].
Contrast-enhanced harmonic EUS imaging
Tissue harmonic imaging (THI) is an ultrasonographic technique, first introduced in 1997, that utilizes nonlinear propagation of ultrasound (US) waves to generate images that are superior in quality to the fundamental B-mode imaging mode[28]. This tool was incorporated into the EUS imaging arsenal approximately a decade later[29].
The two image processing techniques developed to generate harmonic images were the band filtering method and phase inversion method. The ability of harmonic imaging to improve signal-to-noise ratio and reduce artifacts produced by side lobes, grating lobes, and reverberation allowed it to outperform earlier imaging applications, such as Color Doppler and power Doppler, which were susceptible to motion artifacts[30]. Furthermore, this modality allowed for visualization of parenchymal perfusion and microvasculature of the pancreas[31].
AFSUMB, in their summary recommendations, alluded to the benefit of contrast-enhanced harmonic EUS (CH-EUS) for characterization of solid pancreatic masses, pancreatic cancer staging in patients with suspected major vessel involvement, identification of mural nodules in cystic pancreatic lesions, and detection of subtle pancreatic lesions[27].
Nevertheless, THI is saddled with certain intrinsic technical limitations. The narrow bandwidth decreases axial resolution when applying the band filtering method. This limitation gave rise to the conception of the phase inversion method. However, the latter method necessitates the transmission of two US pulses, thus decreasing the frame rate and raising the possibility of motion artifacts[30].
Contrast agents
The first reported use of intravenous ultrasound contrast agents (UCAs) in ultrasonography was for image enhancement during echocardiography. Its adoption followed this in transabdominal ultrasonography before it was eventually introduced in EUS[32]. EFSUMB’s recommendation on contrast for CEEUS in non-liver applications highlighted its similarity to its use in transabdominal CEUS[33]. Worth noting is that the modalities, as mentioned earlier, represent the only imaging techniques that allow a dynamic observation of the CE phases[34]. UCAs are used to augment their ability in lesion characterization and intervention guidance.
During the evolutionary phase of CE-EUS, various agents were explored to advance the diagnostic performance of this modality. To render stability and durability, all commercially available agents are composed of gas-filled microbubbles encapsulated by a phospholipid or albumin shell. Each agent is aptly categorized based on its half-life and ability for transpulmonary passage[32].
The AFSUMB working group strongly recommends the following for use as UCAs: SonoVue(Lumason, sulfur hexafluoride micro-bubbles; Bracco, Milan, Italy; available in Europe, China, India,Korea, Hong Kong, New Zealand, Singapore, and Brazil); Definity (octafluoropropane microbubbles;Bristol- Myers Squibb Medical Imaging, New York, NY, United States; available in the United States,Canada, and Australia), and Sonazoid (perfluorobutane microbubbles; GE Healthcare, Chicago, IL,United States; Daiichi Sankyo, Tokyo, Japan; available in Japan, South Korea, Taiwan and Norway).They further stated that neither of the agents had a superior diagnostic ability over the other[27].
Outcomes
A significant emphasis has been placed on researching the adjunctive role of CE-EUS in evaluating solid pancreatic masses. The result is a wealth of data that supports its use in assessing the pancreas and related pathologies[33]. Meiet al[35] affirmed this in a recent meta-analysis (n= 1497) to evaluate the accuracy of CE-EUS in discriminating between benign and malignant pancreatic masses with a reported pooled sensitivity, specificity, and diagnostic odds ratio of 0.91, 0.86, and 69.50, respectively[35].
Another meta-analysis that included 1139 patients across 12 studies highlighted that both high and low MI techniques had a 94% sensitivity and 89% specificity in differentiating pancreatic adenocarcinoma from other pathologies, with an area under the receiver operating characteristic curve of 0.9732.Subgroup analysis by excluding the outliers provided a sensitivity and specificity of 93%[36].
Săftoiuet al[37] analyzed the diagnostic accuracy of combining CE-EUS and elastography to differentiate focal pancreatic masses and highlighted its influence in decision making when confronted with a negative EUS-FNB result with a strong clinical suspicion of malignancy. A similar study by the same authors assessed the "synergistic" performance of CE-EUS with EUS-EG in 50 patients with a negative FNA of pancreatic masses. The results determined 19 to be pancreatic adenocarcinoma and 31 to be pseudo-tumoral chronic pancreatitis. CE-EUS reported a specificity of 100% and an accuracy of 93% in the cohort of 25 patients where EUS-EG demonstrated high lesion stiffness, diagnosing malignancy[38].
Buxbaumet al[39], in a prospective tandem-controlled trial involving 101 cases of focal pancreatic lesions, concluded that CE-EUS increased the diagnostic yield compared to conventional B-mode EUS with an odds ratio of 7.8 (95%CI: 2.7-30.2). Ninety-one percent of lesions were correctly characterized in the validation cohort, with an improved yield compared to unenhanced EUS[39]. In a real-life clinical setting, we demonstrate its use in a case of a degenerated IPMN showing how its use allows differentiation of tumor vegetation from mucus within a cyst (Figure 4).
Al
AI can level the playing field for endoscopists globally by making this highly sensitive and specific, but technically challenging, diagnostic tool we know as EUS more user-friendly and quicker to master.
The existing image enhancing techniques, CH-EUS and EUS-E, are available to the endoscopist currently as stand-alone software plug-ins. They require prior training before use with a low to moderate learning curve to master given that the user is already proficient with an echoendoscope.
The potential of AI is seen here in its ability to harness the diagnostic power of EUS while unifying all existing and future image-enhancing techniques “under one roof.” This unifying software can aid the endoscopist by highlighting areas of concern while prompting suitable image-enhancing methods.
This added edge of diagnostic confidence can help overcome lesion ambiguity and improve lesion specificity, regardless of the years of prior endosonographic training.
Principle of AI
AI is a divaricate under the broader umbrella of computing methodology and information sciences. It utilizes autonomous properties from rapidly advancing technological developments in deep learning as a segue to mimic human behavior ultimately. Over the last decade, AI applications have quickly matured. Their iterations in the daily practice of various fields within medicine are expanding with success.
The development and training of computer-aided diagnosis (CAD) systems revolve around the aim of autonomously distinguishing features that the human eye and mind may unintentionally miss.Identifying and interpreting changes of varying subtleties, particularly in medical images, has been the high point of AI. The objectivity of a trained CAD system could potentially seek to overcome one of the most significant limitations of any physician or endoscopist: The lack of an intra- and inter-observer agreement. Suppose an AI system can distinguish between neoplastic and non-neoplastic lesions with near perfection after training, it would seem reasonable to imagine that the burden from pathological examinations may be considerably relieved very soon. Thus far, colonic adenoma detection and differentiation have gained tremendous attention from several CAD systems utilizing deep learning and Artificial Neural networks. Due to their promising results, these systems have gained popularity in dayto-day endoscopy usage[40-43].
AI and EUS
The most common application of AI in EUS is creating machine learning architectures that extract EUS images and analyze them for their textures (boasting accuracy of up to 93%-94%) to create an artificial neural network that can ultimately serve as a clinical decision support tool. It can do one of 2 things: (1)Using the station approach to familiarize the endoscopist with normal anatomy, as demonstrated by Kannoet al[44]; and (2) Non-invasively differentiating pancreatic parenchymal pathologies when EUSFNB may not be clinically feasible or have a poor diagnostic yield in the case of cysts or AIP[40].
Figure 4 Use of contrast-enhanced endoscopic ultrasound to study the evolution of a degenerated intraductal papillary mucinous neoplasm and the vegetations within, allowing differentiate between mucus and vegetations as only tumor vegetations will show inhomogeneous enhancement.
In 2008, two studies emerged providing the diagnostic ability of AI integrated EUS in reporting pancreatic diseases. Săftoiuet al[45] reported 68 cases in a prospective study using a neural network EUS to compare PDAC and chronic pancreatitis. The authors reported 95% accuracy in the system’s diagnostic ability. In the second study, Daset al[46] reviewed 319 images with a neural network system and performed a principal component analysis to compare acute and chronic pancreatitis, from pancreatic carcinoma. The study reported the area under the curve indicating the systems’ diagnostic ability to be 93%. In 2013, Zhuet al[47] studied 288 cases retrospectively with a support vector machine and reached an accuracy of 94%.
Additionally, Săftoiuet al[48], in a prospective multicenter study, reported 167 cases using a neural network system, reaching a diagnostic accuracy of 94%. Ozkanet al[49] reported an accuracy of 93% in reviewing 332 images in retrospect using a neural network. A deep learning architecture specific to EUS noteworthy of mentioning is one used to differentiate high-grade dysplasiavsmalignancy for IPMN with an accuracy of 94%[42]. In a single-center retrospective study that trained a deep learning algorithm using 3970 still images, deep learning was superior to human ability in its diagnostic accuracy(95%vs56%), proving an objective method such as is this is more accurate in diagnosing malignancies of IPMN origin in comparison to conventional EUS features[42].
AI demonstrates its usefulness again in increasing diagnostic specificity using occlusion heatmap analysis to differentiate AIP from PDAC. The result is overcoming a delay in diagnosis, initiating immunosuppressive or chemoradiotherapy early, and preventing unwarranted resections (90%sensitive, 85% specific)[50]. It is worth mentioning that these studies use a cross-validation method with an internal control check, thus inflating the actuality of the diagnostic accuracy. In its applications to actual EUS fine-needle biopsy histopathological samples, AI was able to bring diagnostic clarity to difficult-to-analyze samples with an accuracy of 94.1% despite cellular paucity and contamination.
In centers with a lack of dedicated GI pathologists, AI software can quickly close this gap of diagnostic delay and eliminate repeat sampling at second or third centers[11]. One setback of AI is the black-box phenomenon, which can cause judgment errors without an explanatory basis. That said, a trained convolutional neural network model with extracted visual features from various pancreatic diseases, namely chronic pseudo-tumoral pancreatitis, NET, and PDAC, can accurately produce a realtime diagnosis[51].
SUMMARY RECOMMENDATlONS
For a suspected pancreatic mass, we propose utilizing both EUS-E and CE-EUS to augment conventional B-mode imaging (Figure 5).
Their advantages in defining nodules and ambiguous ROIs allow FNA/FNB sampling precision,making them crucial adjuncts in day-to-day practice, whether for first encounters or follow-up visits.
Validation studies are still underway to evaluate promising non-contrast-based high-definition imaging modalities. The modalities are in-built into the echo processor for ease of microvasculature study. They carry great potential in supplementing and possibly surrogating UCAs in conditions, such as pregnancy or compromised cardiopulmonary status[52]. However, until then, EUS-E and CE-EUS

Figure 5 Graphical representation of existing diagnostic applications to endoscopic ultrasound imaging in the form of a fractal tree,fractal analysis with artificial intelligence being the possible future of enhanced endoscopic ultrasound imaging. AI: Artificial intelligence; CHEUS: Contrast-enhanced harmonic endoscopic ultrasound; FNB: Fine needle biopsy.
coupled with B-mode imaging will remain the choice stratagem in improving the early detection of pancreatic cancer.
CONCLUSlON
In the EUS world of greys, colors emerged from elastography and patterns from contrast use, improving the overall accuracy of pancreatic mass differentiation. AI could be the rainbow that bridges us to a more precise diagnosis.
FOOTNOTES
Author contributions:Spadaccini M provided the outline; Koleth G with Spadaccini M performed most of the writing and equally contributed to this paper; Emmanuel J wrote on contrast-enhanced endoscopic ultrasound; Carrara S provided valuable oversight, all EUS pictures, and concluded the manuscript; Grizzi F provided the picture for fractal analysis; Khalaf K, Colombo M, Mangiavillano B, Fugazza A, Anderloni A, Facciorusso A, and Repici A provided input in writing the paper.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORClD number:Marco Spadaccini 0000-0003-3909-9012; Glenn Koleth 0000-0002-2939-6876; James Emmanuel 0000-0001-7154-461X; Kareem Khalaf 0000-0002-5534-7533; Antonio Facciorusso 0000-0002-2107-2156; Fabio Grizzi 0000-0003-0925-742X; Cesare Hassan 0000-0001-7167-1459; Matteo Colombo 0000-0003-0715-8233; Benedetto Mangiavillano 0000-0003-0611-7448; Alessandro Fugazza 0000-0003-0485-4903; Andrea Anderloni 0000-0002-1021-0031; Silvia Carrara 0000-0003-4206-9463; Alessandro Repici 0000-0002-1621-6450.
S-Editor:Fan JR
L-Editor:Filipodia
P-Editor:Fan JR
 World Journal of Gastroenterology2022年29期
World Journal of Gastroenterology2022年29期
- World Journal of Gastroenterology的其它文章
- Mechanistic and functional extrapolation of SET and MYND domain-containing protein 2 to pancreatic cancer
- Clinical challenge for gastroenterologists-Gastrointestinal manifestations of systemic mastocytosis: A comprehensive review
- Structural changes of proteins in liver cirrhosis and consequential changes in their function
- Epidemiologic and socioeconomic factors impacting hepatitis B virus and related hepatocellular carcinoma
- Qingyi decoction attenuates intestinal epithelial cell injury via the calcineurin/nuclear factor of activated T-cells pathway
- High-fat diet aggravates colitis via mesenteric adipose tissue derived exosome metastasis-associated lung adenocarcinoma transcript 1
