A decellularized nerve matrix scaffold inhibits neuroma formation in the stumps of transected peripheral nerve after peripheral nerve injury
Shuai Qiu , Pei-Jun Deng , Fu-Lin He, Li-Wei Yan Zhe-Hui Tu, Xiao-Lin Liu , Da-Ping Quan , Ying Bai , , Can-Bin Zheng , , Qing-Tang Zhu ,
Abstract Traumatic painful neuroma is an intractable clinical disease characterized by improper extracellular matrix (ECM) deposition around the injury site. Studies have shown that the microstructure of natural nerves provides a suitable microenvironment for the nerve end to avoid abnormal hyperplasia and neuroma formation. In this study, we used a decellularized nerve matrix scaffold (DNM-S) to prevent against the formation of painful neuroma after sciatic nerve transection in rats. Our results showed that the DNM-S effectively reduced abnormal deposition of ECM, guided the regeneration and orderly arrangement of axon, and decreased the density of regenerated axons. The epineurium-perilemma barrier prevented the invasion of vascular muscular scar tissue, greatly reduced the invasion of α-smooth muscle actin-positive myofibroblasts into nerve stumps, effectively inhibited scar formation, which guided nerve stumps to gradually transform into a benign tissue and reduced pain and autotomy behaviors in animals. These findings suggest that DNM-S-optimized neuroma microenvironment by ECM remodeling may be a promising strategy to prevent painful traumatic neuromas.
Key Words: decellularized nerve matrix scaffold; extracellular matrix; fibrosis; functional recovery; microarchitecture; microenvironment; pain; peripheral nerve; tissue remodeling; traumatic neuroma 1Department of Microsurgery and Orthopedic Trauma, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong Province, China; 2Department of Orthopedic Surgery, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong Province, China; 3Department of Pediatric Orthopedics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, Guangdong Province, China; 4Guangdong Provincial Peripheral Nerve Tissue Engineering and Technology Research Center, Guangzhou, Guangdong Province, China; 5Guangdong Province Engineering Laboratory for Soft Tissue Biofabrication, Guangzhou, Guangdong Province, China; 6School of Chemistry, Sun Yat-sen University, Guangzhou, Guangdong Province, China; 7School of Material Science and Engineering, Sun Yat-sen University, Guangzhou, Guangdong Province, China
Introduction 664 Methods 665 Results 666 Discussion 668

Introduction
It is challenging to manage pathological pain following nerve injury in patients with orthopedic trauma. Painful traumatic neuromas occurs in 26% of amputees, causing intractability, increasing pathological pain symptoms that are often difficult to treat, and even requiring a second operation to remove the neuroma due to pain symptoms before fitting the prosthesis (Ives et al., 2018). Therefore, the issue of preventing nerve stump neuroma has attracted increasing attention in recent years (Poppler et al., 2018).
In contrast to other types of neuropathic pain, neuroma-related neuropathic pain originates from the anatomical destruction and malformation of peripheral nerves caused by traumatic lesions (Finnerup et al., 2021; Mao et al., 2021). Neuroma is a chronic reparative proliferative response of the nerve after neurotomy without anatomical reconstruction. The axonal transection of amputation triggers a sequence of events within the proximal nerve stumps and evokes massive axonal sprouting. Furthermore, a previous study found that pathologically disorganized extracellular matrix (ECM) architecture might be related to human neuroma formation (Mahan et al., 2019). Following a repairable nerve neurotomy, the portion of the axons distal to the injury site degenerates and then initiates nerve repair under a programmed process (Liu et al., 2019; Yuan et al., 2021). Nevertheless, in neuroma-related neuropathy, in the absence of distal nerve guidance, activated Schwann cells (SCs) and fibroblasts migrate diffusely and secrete a large amount of disorganized ECM to induce regenerative axons, SCs, non-neuronal cells and ECM fiber misfolding to form neuromas (Rotshenker, 2011; Neumeister and Winters, 2020). A traumatic microenvironment recruits inflammatory cells and reprograms SCs to an activated state and then drives regeneration (Rotshenker, 2011; Clements et al., 2017); however, pathological regeneration occurs in neuromas because the sprouting axons are always in the abnormal microenvironment, which is induced by mechanical stress and inflammatory pain factors (Finnerup et al., 2021). The ensuing vascular, scar and muscle fiber invasion leads to the development of intractable pathological pain (Oliveira et al., 2018; Neumeister and Winters, 2020).
Currently, a variety of grafts or caps in the clinic can be used to prevent the formation of traumatic neuromas by providing a physical barrier and restricting nerve regeneration. These methods can be divided into two categories. The first category is free autologous tissue capping, including autologous fat, muscle and blood vessels. These tissues create a protective envelope and a gliding layer for the nerve stump to avoid scar adherence and maintain the stability of local microenvironment and have achieved good clinical results (Galeano et al., 2009; Vaienti et al., 2012; Santosa et al., 2020). However, it may require extra and relatively complex surgical operations. The second category is the implantation of commercially available devices, such as Neuroflex Flexible Collagen Nerve Cuff (Collagen Matrix, Inc., Oakland, NJ, USA) and Axoguard Nerve Cap (Axogen, Inc., Alachua, FL, USA), providing a new permanent protective layer for the nerve end (Gould et al., 2013; Parker and Merced-O’Neil, 2016). These devices are safe and the operation is relatively simple in the management of painful neuromas. However, there is still no standard method for neuromas prevention in the clinic. Considering the effect of ECM on tissue repairing, a commercially available nerve graft that can intervene the pathophysiological ECM remodeling and correct the malformation of the nerve stump might be a promising technique for preventing traumatic neuroma formation.
Our previous study showed that gelatin blockers with the inhibitory ECM molecule chondroitin sulfate proteoglycans prevent traumatic neuroma formation by blocking irregular axon regeneration and disorderly collagenous fiber accumulation in the nerve end (He et al., 2020), which suggests that the proper ECM microenvironment plays an essential role in histological remodeling of the nerve end and pain-free recovery by inhibiting neuroma formation and discontinuing the traumatic microenvironment. However, the gelatin blocker cannot correct the irregular axon regeneration patterns effectively because it lacks the nerve-specific microarchitecture.
Therefore, we hypothesized that the bionic nerve scaffolds that contain ECM inhibitory components and microstructure of natural nerve would be able to provide the suitable microenvironment for the nerve end to avoid abnormal hyperplasia. Previously, we successfully developed a human decellularized nerve matrix scaffold (DNM-S) that maintains the natural three-dimensional neural macro/microstructure (He et al., 2015). In addition, we found that longitudinally oriented microchannels can guide neurite extension and SCs migration directionallyin vitro
andin vivo
(Lin et al., 2018). Knowing that the structural neurotomy reconstruction plays a key role in nerve injury and repair (Qiu et al., 2020; Rao et al., 2021), we attempted to induce the reconstruction of multiple anatomical microstructures for nerve stumps via ECM remodeling by the DNM-S, which might be promising for neuroma prevention.We hypothesized that ECM remodeling mediated by DNM-S may prevent neuroma formation on nerve stumps by switching the pathological evolution of the regenerative microenvironment and axonal regrowth patterns after neurotomy in a rat model of sciatic nerve injury. In the present study, the proteomics and microstructure of the DNM-S were analyzed, and the reconstruction effect on nerve stumps and neuroma prevention after transplantation was evaluated.
Methods
Preparation and evaluation of the DNM-S
This study was approved by the Animal Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University on October 29, 2019 (approval No. 2019[373]). All experiments were designed and reported according to the Animal Research: Reporting ofIn Vivo
Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020).Considering that mutual aggressive behavior in male rats may affect the accuracy of experimental results, we used female rats in this study. First, all specific pathogen-free female Sprague-Dawley rats weighing 180‒220 g (n
= 50, 6‒7 weeks old) were obtained from the Experimental Animal Center of the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China; license No. SYXK (Yue) 2020-0108). Animals were treated according to the National Research Council’s guidelines for the Care and Use of Laboratory Animals and had free access to rat chow and water (temperature 20‒26°C, relative humidity 40‒70%, alternating 12-hour light/dark cycle). The experimental procedure is shown inFigure 1
.
Figure 1 |Experimental flow diagram.DNM-S: Decellularized nerve matrix scaffold; SEM: scanning electron microscope; α-SMA: α-smooth muscle actin.
Briefly, 20 rats were anesthetized by intraperitoneal injection of 100 mg/kg sodium pentobarbital (Sigma-Aldrich, St. Louis., MO, USA) and then the bilateral sciatic nerve was exposed and cut off under aseptic conditions. Forty 20-mm sciatic nerves from the above 20 rats were subjected to decellularization and irradiation sterilization. The nerves were immersed in distilled water overnight, followed by two successive chemical extractions: agitated in 4% (w/v) Triton X-100 (Sigma-Aldrich) for 12 hours and then in 4% (w/v) sodium deoxycholate (Sigma-Aldrich) for 12 hours. After thorough washes in phosphate buffered saline, the DNM-Ss were sterilized byCo irradiation.
Forty DNM-Ss (~20 mm in length) were prepared. Two DNM-Ss were stained with Sirius red (Sigma-Aldrich) and ECM microstructure was observed with an Axio Scan Z1 slide scanner (Carl Zeiss, Goettingen, Germany). Three DNM-Ss were used for scanning electron microscope analysis, while 20 DNM-Ss were used to analyze the ECM components via protein identification and analysis by Matrisome DB (http://matrisomeproject.mit.edu/). The animal experiment was performed in the remaining 15 DNM-Ss.
Animal grouping and surgical procedures
Thirty female Sprague-Dawley rats were randomly divided into two groups: control (n
= 15) and DNM-S groups (n
= 15). All rats were anesthetized by intraperitoneal injection of 50 mg/kg sodium pentobarbital, after which the right sciatic nerve was exposed under aseptic conditions. The origin of the branch of the posterior gluteal nerve at the level of the sciatic notch was identified and labeled with a 10‒0 suture under a surgical microscope (Leica, Wetzlar, Germany) to determine location of the nerve end. Then, the sciatic nerve was sharply cut off and separated from the surrounding tissue. In all rats, a 1.0-cm length of the distal sciatic nerve stump was cut, leaving a defect after retraction of the nerve. After transection, distal nerve stumps were directly exposedin situ
, and the prepared DNM-Ss were sutured to the nerve stumps in the DNM-S group with 10‒0 monofilament nylon sutures. In the control group, the nerve stumps were left as they were and only marked with sutures only to make identification. In all groups, the muscles remained in place, and skin incisions were closed with 4‒0 sutures.Behavioral analysis
To assess pain symptom after sciatic nerve transection, rat autotomy behaviors were analyzed. At 8 weeks after surgery, all rats were anesthetized again. Before harvesting specimen, rat autotomy behaviors were evaluated in each group by two blinded observers. A modified Wall scale was adopted, with assigned points based on the severity of autotomy (He et al., 2020). Briefly, 1 point was assigned for two or more toenails (maximum, 1 point per limb), and 1 point was assigned for each half-digit (distal and proximal phalanges; maximum, 10 points per limb (n
= 10). The higher autotomy score indicated more severe autotomy behavior.Specimen preparation
At 8 weeks after surgery, all right sciatic nerve specimens and five normal left sciatic nerve specimens were harvested. Two-thirds of the specimens in each group were selected at random for histological and immunohistochemical analysis. Alpha-smooth muscle actin (α-SMA) expression was detected in neuromas and the proximal, middle, and distal parts of implanted DNM-Ss. All the specimens were preserved in 4% paraformaldehyde before being cut into longitudinal and cross-sectional sections. The remaining 1/3 of the specimens were used to perform toluidine blue staining and transmission electron microscopy (TEM) to observe the ultrastructure of regenerated axons and ECM fibers. All rats were euthanized via injection of an overdose of sodium pentobarbital.
Histological and immunohistochemical analyses
To assess the ECM microenvironment in sciatic nerve stumps, histological and immunohistochemical analyses were performed. After soaking in 4% paraformaldehyde for at least 24 hours, half of the experimental specimens and five normal left sciatic nerve specimens were paraffin-embedded and then cut into 4-µm thick longitudinal and cross-sectional sections. To standardize the site of staining or immunohistochemistry, the sections were selected from the proximal, middle and distal parts of the implanted DNM-S and the middle part of the neuroma. At least 30 sections were obtained from each sample after excluding small sections or poorly cut sections. Twenty sections were randomly selected, and five sections each were used for Masson’s trichrome staining, silver staining, and immunohistochemistry for α-SMA expression with imaging performed with an Axio Scan Z1 slide scanner (Carl Zeiss, Goettingen, Germany). For Masson’s trichrome staining, the sections were deparaffinated and stained with Masson’s trichrome (Servicebio, Wuhan, China) in accordance with the instruction of the kit. To show collagen formation and distribution by Sirius red staining, five sections were stained with Sirius red (Sigma-Aldrich) with imaging performed by polarized light microscopy (Nikon, Tokyo, Japan). To show the regenerated axons by silver staining, the sections were incubated in silver glycine at room temperature for 15‒30 minutes and then visualized by preheated reducing solution at 45°C. To evaluate pain-related marker α-SMA immunopositivity by immunohistochemistry, the sections were visualized using mouse anti-α-SMA antibody (1:1000, Servicebio, Cat# GB111364, RRID: AB_2910228) for 2 hours at 37°C after sealing in goat serum (Solarbio) for 10 minutes, washed with 0.01 M phosphate buffer saline (pH 7.4), and then incubated with biotinylated anti-mouse IgG secondary antibody (1:200, Servicebio, Cat# GB23301, RRID: AB_2904020) for 1 hour at 26°C. Immunoreactivity was determined by incubating sections with horseradish peroxidase conjugated streptavidin (1:200, Servicebio, Cat# G3431) and 3,3′-diaminobenzidine (Beyotime, Shanghai, China). Photographs of each nerve sample were quantitatively analyzed with ImageJ software version 1.50i (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012).
Toluidine blue staining and transmission electron microscopy
To assess the effect of DNM-S repairing on the axon extension and ECM pattern in sciatic nerve stumps, toluidine blue staining and transmission electron microscopy (TEM) were performed to observe the ultrastructure of regenerated axons and ECM fibers. The remaining specimens were immediately fixed in 2.5% glutaraldehyde for 3 hours after harvesting and post-fixed in 1% osmium tetroxide for 2 hours. Then, the samples were dehydrated in a graded ethanol series, embedded in epoxy resin, and cut into 1-µm semithin and 50-nm ultrathin sections. The semithin sections were stained with toluidine blue and the photographs were taken with an Axio Scan Z1 slide scanner (Carl Zeiss, Goettingen, Germany) and measured with Zen 2.3 blue edition software (Carl Zeiss Microscopy). The ultrathin sections were stained with 3% uranyl acetate-lead citrate and photographs were taken with a TEM (JEOL, Tokyo, Japan).
Statistical analysis
Sample size was designated based on previous studies (He et al., 2020; Pi et al., 2022). No animals were missing during the experiment. The raters were blinded to the assignments. Data are expressed as the mean ± standard error of mean (SEM), and were analyzed using SPSS version 20.0 (IBM, Armonk, NY, USA). Student’st
-test was performed to compare the differences between two different groups. Differences among three or more groups were evaluated by one-way analysis of variance, and the Bonferroni method was used to compare differences between two different groups.P
< 0.05 was considered statistically significant.Results
Engineering anatomy and proteomic analysis of DNM-S
After decellularization, the DNM-S retained intact nerve endoneurium and perineurium-epineurium components (Figure 2A
andB
). The Sirius red staining images showed complete nerve architecture without cellular constituents after decellularization (Figure 2C
). The perineurium-epineurium structure presented as a conduit that packaged the fasciculus (Figure 2D
andE
). The fasciculus consisted of a large number of longitudinal endoneurial tubes (Figure 2C
andF
), which can provide directed pathways to support axon and matrix cell directional extension. The identified proteins were annotated using Matrisome DB. There were approximately 60 types of ECM proteins present in DNM-S, including 13 types of collagens, 22 types of glycoproteins, 6 types of proteoglycans, 6 types of ECM-affiliated proteins, 12 types of ECM regulators, and 1 type of secreted factor (Figure 2G
andH
).
Figure 2 |Macrostructure and components of a DNM-S. (A, B) Appearance of an intact DNM-S. Black asterisk indicates the nerve endoneurium, and black arrow indicates the perineurium-epineurium. The nerve endoneurium area is semi-transparent and loose, while the perineurium-epineurium is white and relatively dense. (C) Sirius red image of cross-sectional a DNM-S. Black asterisk indicates the nerve endoneurium component, and black arrow indicates the perineuriumepineurium component. (D, E) The perineurium-epineurium contains a cylinderical sheath structure corresponding to the black arrows in (A) and (B). (F) The fasciculus (endoneurium) contains a longitudinal strands structure. Scale bars: 1 mm in A, B, D‒F. (G) Characterization of total number of proteins detected in DNMS tissue, categorized into core matrisome proteins. The listed proteins are the expressed proteins identified in DNM-S, grouped into clusters according to their function. (H) Histograms showing the number of proteins in different kinds of ECM. DNM-S: Decellularized nerve matrix scaffold; ECM: extracellular matrix.
Ultrastructure of different anatomical areas of DNM-S
The ultrastructure of DNM-S was observed and recorded by scanning electron microscope at high magnification (Figure 3A
). The basement membrane (BM) tubes (Figure 3B
; white star) were located in the inner layer of the endoneurial tubes (Figure 3C
; red arrow), whereas the endoneurial tubes consisted of assembled longitudinal nanofibers (Figure 3C
; green arrow). The perineurium was composed of dense longitudinal nanofibers (Figure 3D
; red star), while the epineurium was composed of a loosened fibrous structure (Figure 3D
). The medial surface of the perineurium was flat and smooth, while the lateral surface of the epineurium was rough and uneven (Figure 3E
andF
), indicating multiple distinctive hierarchies in the DNM-S.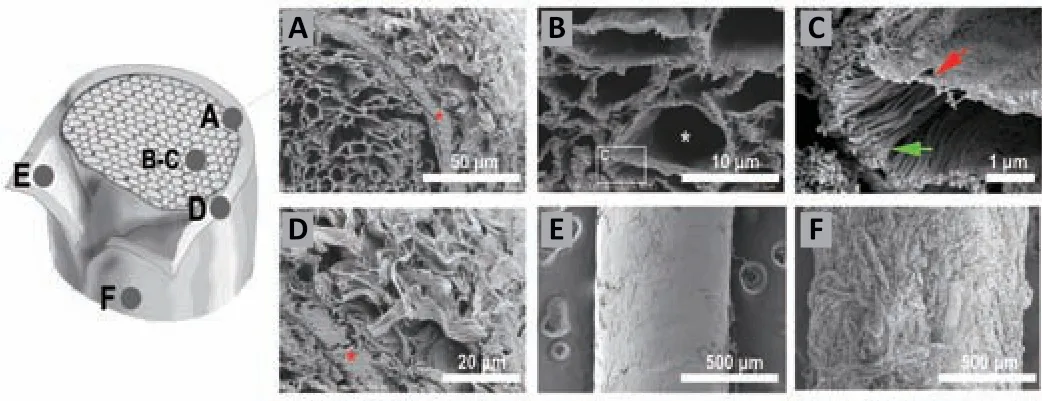
Figure 3 |SEM ultrastructure of DNM-S. (A‒D) Cross-sections of DNM-S (A) and its endoneurium area (B, C) and perineuriumepineurium area (D). The components of the myelin sheath and the axon were removed (white asterisk), whereas the endoneurium (green arrow), BM tube (red arrow) and perineurium (red asterisk) remained intact. (E) The medial surface of perineurium was flat and smooth. (F) The lateral surface of epineurium was rough and uneven. Scale bars: 50 µm in A, 10 µm in B, 1 µm in C, 20 µm in D, 500 µm in E and F. BM: Basement membrane; DNM-S: decellularized nerve matrix scaffold.
DNM-S mitigates the macropathology and autotomy in rats with traumatic nerve stump neuroma
After transection of the right sciatic nerve, the nerve stumps (indicated by the 8-0 suture line) were exposed to the nonneural connective tissue and hematoma in the control group (Figure 4A
). In the DNM-S group, the nerve stumps were sutured to a 2.0 cm DNM-S (Figure 4B
). At 8 weeks after surgery, typical bulbous neuromas could be found in the control group, and tissue scar adhesion was serious (Figure 4C
andD
; black arrowhead), while no typical neuromas were observed in the DNM-S group (Figure 4E
). Autotomy behavior was observed on the operative side (right foot) (Figure 4F
andG
). At 8 weeks after surgery, autotomy behavior score was lower in the DNM-S group than that in the control group (P
< 0.05;Figure 4H
andI
).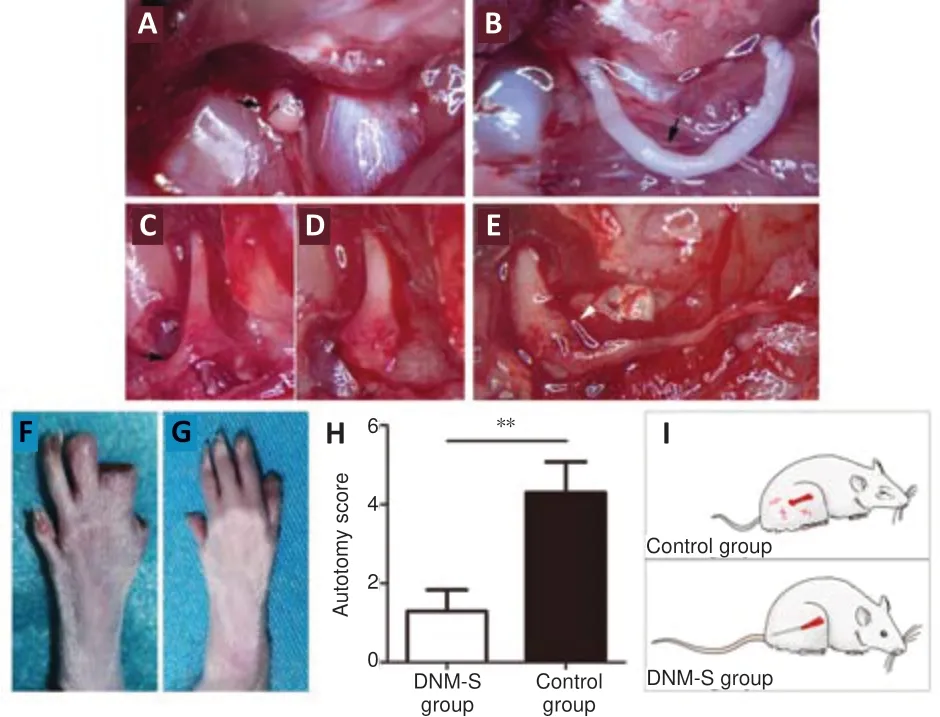
Figure 4 |Effect of DNM-S on neuroma formation and autotomy behavior in a rat model of sciatic nerve injury. (A) The nerve stump marked by suture. The black arrow indicates the suture. (B) The nerve stump repaired with a 2.0 cm DNM-S. The black arrow indicates the DNM-S. (C, D) Neuroma formation. Black arrow indicates the scar adhesion. (E) New neural tissue in the DNM-S group. White arrowhead indicates the anastomosis. White arrow indicates the implanted DNM-S. (F, G) Typical autotomy behavior of the control (F) and DNM-S (G) groups. (F) The distal digits on the 5th and 4th toes and the nails on the 3rd toe were chewed (5 points). (G) The nails on the 5th toe were chewed (1 point). (H) Assessment of the average autotomy score 8 weeks postoperatively. Data are expressed as the mean ± SEM (n = 10). **P < 0.01 (Student’s t-test). (I) Behavioral diagram of the two groups. Neuroma formation induces pain symptom in the control group, while DNM-S prevents neuroma formation in the DNM-S group. DNM-S: Decellularized nerve matrix scaffold.
DNM-S improves the transformation of the regenerative pattern of nerve fibers in rats with traumatic nerve stump neuroma
Silver and Masson’s trichrome staining showed that nerve fibers in the control group were disordered and arranged irregularly (Figure 5A
and
a1–3
), along with a haphazard expansion of collagen deposition (Figure 5A
anda4–6
) at 8 weeks. Immediately after neurotomy, the alignment of nerve fibers and collagen deposition were interrupted (Additional Figure 1A
). With muscle fiber invasion, the histopathological morphology of the nerve end transformed into traumatic neuroma (Additional Figure 1B
). DNM-S provided structural guidance and biological barriers, such as the endoneurium and perineurium, for regenerating neural tissue (Figure 5B
andb1–4
,Additional Figure 1C
andD
). The collagen deposition and regenerative nerve fibers were arranged in an orderly pattern in the DNM-S group, similar to the normal nerve (Figure 5C
andD
). It is worth noting that the perineurium/epineurium of the DNM-S gradually incorporates to create a physical barrier to the nerve end (Figure 5b3
), similar to the perineurium/epineurium of the normal nerve (Figure 5D
; black star). In the cross section, the spatial pattern of collagen deposition and regenerative nerve fibers was entangled and disorganized outgrowths in the control group (neuroma) compared with the DNM-S group and normal nerve tissue (Figure 5E–L
). In short, the ECM construction of nerve stumps was remodeled by the implanted DNM-S (Additional Figure 1E
andF
), which subsequently transformed the regenerative pattern of nerve fibers (Figure 5H
andL
).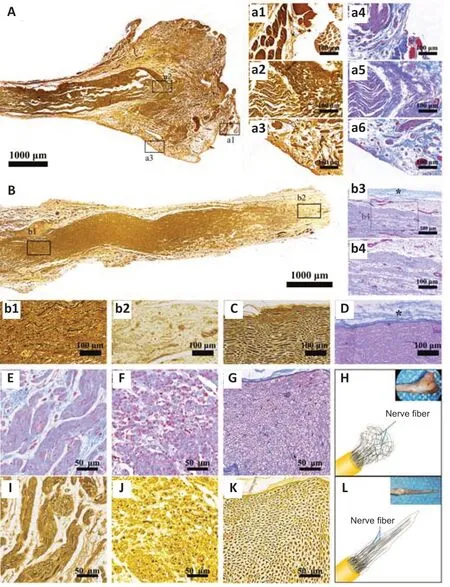
Figure 5 |Effect of DNM-S on histomorphology in a rat model of sciatic nerve injury. (A) Silver staining of the neuroma. (a1‒3) Partial enlarged view of A. The nerve fibers were disordered. (a4‒6) View of neuroma in Masson’s trichrome staining. The collagenous fibers were arranged irregularly with a haphazard expansion of deposition. (B) Silver staining of regenerated neural tissue induced by DNM-S. (b1, 2) Partial enlarged view of B. The nerve fibers were arranged irregularly. (b3, 4) View of regenerated neural tissue induced by DNM-S in Masson’s trichrome staining. The collagen deposition was arranged in an orderly pattern. The black star indicates the regenerated perineurium/epineurium. (C) Silver staining of the normal tissue in longitudinal section. (D) Masson’s trichrome staining of the normal tissue in longitudinal section. Black star indicates the normal perineurium/epineurium. (E) Masson’s trichrome staining of the neuroma in the cross-section. Collagenous fibers were entangled. (F) Masson’s trichrome staining of the DNM-S group tissue in the cross-section. Collagenous fibers were aligned and closer to normal morphology. (G) Masson’s trichrome staining of the normal tissue in the crosssection. (H) Disordered nerve fiber pattern of neuroma and its gross appearance. (I) Silver staining of the neuroma in the cross-section. Nerve fibers were disorganized outgrowths. (J) Silver staining of the DNM-S group tissue in the cross-section. The nerve fibers were orderly outgrowths. (K) Silver staining of the normal tissue in the cross-section. (L) The orderly nerve fiber pattern of the DNM-S group and its gross appearance. Scale bars: 1000 µm in A, B, 100 µm in a1‒6, b1, 2, 4, C, D, 200 µm in b3, 50 µm in E‒G, I‒K. DNM-S: Decellularized nerve matrix scaffold.
Sirius red staining was utilized to evaluate collagen formation and distribution by polarized light microscopy (Figure 6
). The results showed that two types of collagens coexisted in normal nerves (Figure 6A
anda1–a3
), regenerating neural tissue in DNM-S (Figure 6B
andb1
) and neuromas (Figure 6C
andc1–c4
). In the longitudinal section, the DNM-S group showed slight type dysplasia compared with the normal nerve group (Figure 5a1
andb1
). Similar to normal nerve (Figure 5A
anda2–a3
), type I collagen-positive area of perineurium/epineurium was greater than that of endoneurium area in the DNM-S group (Figure 6b2–b4
). Nevertheless, both type I and III collagen showed obvious hyperplasia in the basilar part of the neuroma (Figure 6c1
andc2
) and neuroma (Figure 6c3
andc4
). Type I collagen positive area in neuroma was significantly greater than that in the proximal, middle and distal parts of DNM-S (P
< 0.01;Figure 6D
). The mean area of type III collagen in the proximal, middle and distal parts of the DNM-S group were significantly less than that in the control group at 8 weeks after surgery (P
< 0.01;Figure 6E
).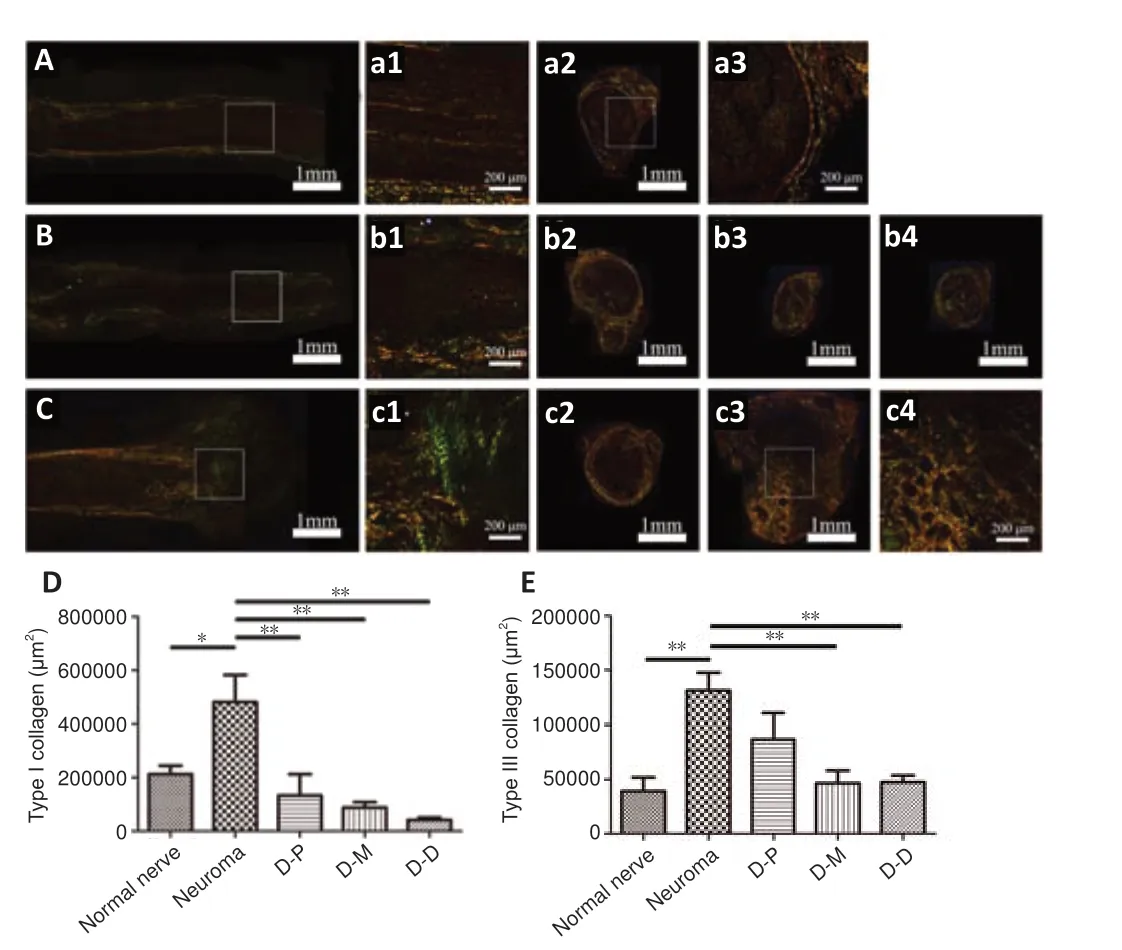
Figure 6 |Effect of DNM-S on collagen fiber in a rat model of sciatic nerve injury as shown by Sirius red staining. (A) Longitudinal section of a normal nerve. (a1) Partially enlarged view of A. (a2, a3) Cross-section of a normal nerve. The configuration of type I and III collagen corresponds to the nerve anatomical structure. (B) Longitudinal section of a regenerative nerve in the DNM-S group. (b1) Partially enlarged view of B. (b2‒4) Cross-section of the proximal, middle and distal parts of the regenerated in the DNM-S group. Both type I and III collagens showed slight hyperplasia. (C) Longitudinal section from the control group. (c1) Partial enlarged view of C. (c2) Cross-section of the basilar part of a neuroma. (c3) Cross-section of a neuroma. (c4) Partial enlarged view of c3. Both type I and III collagens showed severe hyperplasia. Scale bars: 1 mm in A, a2, B, b2-4, C, c2, c3, 200 µm in a1, a3, b1, c1, c4. (D, E) Mean area of type I and III collagen fibers. The proximal, middle, and distal parts of the regenerative nerve in the DNM-S group are shown in D-P, D-M and D-D, respectively. Data are expressed as the mean ± SEM (n = 5). *P < 0.05, **P < 0.01 (one-way analysis of variance followed by Bonferroni post hoc test). DNM-S: Decellularized nerve matrix scaffold.
DNM-S mitigates vascular and muscular invasion and pain-related marker expression in regenerative nerves in rats with traumatic nerve stump neuroma
Vascular and muscular invasion was assessed by Masson’s trichrome staining, muscle staining and immunohistochemistry for α-SMA (Figure 7
). There was no obvious vascular invasion in the basis part of neuroma in the control group (Figure 7A
anda1–3
), however, the large-sized, distorted, and proliferated blood vessels appeared in neuromas (Figure 7B
,b1–3
,C,
andc1–3
). Muscle staining demonstrated the presence of muscular invasion in the neuroma while it was not observed in the middle part of the implanted DNM-Ss (Figure
7B
andAdditional Figure 2
), suggesting that DNM-Ss possess a physical barrier structure to avoid the surrounding vascular and muscular invasion. α-SMA was expressed in myofibroblasts and blood vessels in the control group (Figure 7D
andd1–d3
), while it was mainly expressed in vascular wall in the DNM-S group (Figure 7E
ande1–e3
). In the DNM-S group, moderate vascularization resulted in vascular smooth muscle proliferation and α-SMA was mainly expressed in vascular wall (Figure 7F–H
, andf–h
). α-SMA-positive area in the proximal, middle and distal parts of the DNM-S was lower than that in the neuroma in the control group at 8 weeks after surgery (Figure 7I
).Ultrastructure of regenerated nerve tissues
At 8 weeks after surgery, the histomorphometry of axons and the area of axon regeneration at the different portions of the harvested tissue were quantified. Compared to the axons on the uninjured side (Figure 8A
), the perineurium structure and a small number of nerve bundles were regenerated in the implanted DNM-S (Figure 8B
). As the regeneration distance increased, fewer and fewer axons were observed in the implanted DNM-S, and no axons were observed in the distal region (Figure 8C
andD
). The outgrowth of regenerated axons reached outside of the perineurium in both the DNM-S and control groups (Figure 8C
andE
), suggesting that some axons would escape from the anastomosis. The outgrown axons in the neuroma were disordered and twisted (Figure 8F
,f1
, andf2
). The middle and distal parts of DNM-S contained significantly fewer axonal regions than the neuroma in the control group (P
< 0.05;Figure 8G
). The axes of the abnormal axons in the neuroma were longer than those of the proximal parts of the DNM-S (P
< 0.01;Figure 8H
). DNM-S seemed to have an obvious effect on the inhibition of axon outgrowth outside the perineurium, although the difference between the two groups was not statistically significant (Figure 8I
).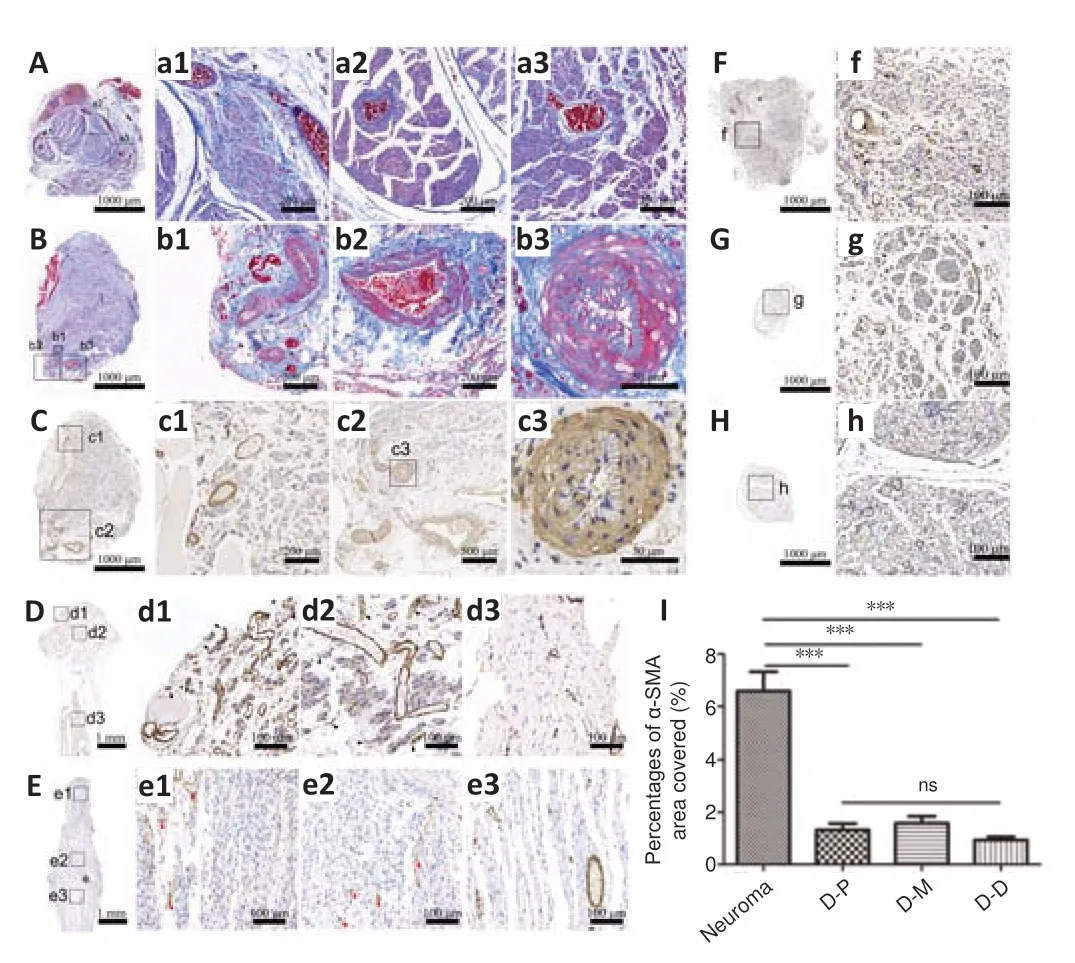
Figure 7 |Effect of DNM-S on the vascular malformation and α-SMA expression in a rat model of sciatic nerve injury. (A) Masson’s trichrome staining of the basis part of neuroma in the control group. (a1‒3) Partially enlarged view of A. There was no obvious vascular invasion. (B) Masson’s trichrome staining of the neuroma. (b1‒3) Partially enlarged view of B. The large-sized, distorted and proliferated vessels invade the neuromas. (C) α-SMA immunohistochemical staining of neuroma. (c1‒3) Partially enlarged view of C. (D) α-SMA immunohistochemical staining of longitudinal section of the control group. (d1-3) Partially enlarged view of (D). α-SMA was expressed in myofibroblasts and blood vessels (black arrow). (E) α-SMA immunohistochemical staining of longitudinal section of the proximal tissue in the DNMS group. (e1‒3) Partially enlarged view of E. α-SMA was mainly expressed in vascular wall (red arrow). (F‒H) α-SMA immunohistochemical staining of cross-section of the proximal, middle and distal nerve tissue in the DNMS group. (f‒h) Partially enlarged view of F‒H. α-SMA was mainly expressed in vascular wall. Scale bars: 1000 µm in A‒H, 200 µm in a1, a2, b1, b2, c1, 100 µm in a3, f, g, h, 50 µm in b3, c3, d1-d3, e1‒e3. (I) Statistical analysis of the percentages of areas of positivity for anti-α-SMA. The proximal, middle, and distal parts of the regenerative nerve in the DNM-S group, are represented as D‒P, D‒M and D‒D, respectively. Data are expressed as the mean ± SEM (n = 5). ***P < 0.001 (one-way analysis of variance followed by Bonferroni post hoc test). DNMS: Decellularized nerve matrix scaffold; ns: not significant; α-SMA: α-smooth muscle actin.
On TEM images, we found that in addition to the morphologically deformed myelinated fibers compared with the normal nerve (Figure 9A
anda
), there was also a mass of morphologically deformed unmyelinated fibers growing in the traumatic neuroma (Figure 9B
andb
). Consistent with the axon morphology, the ECM fiber configuration differed markedly between the DNM-S (Figure 9C
andc
) and the neuroma (Figure 9D
andd
). In the implanted DNM-S, the unmyelinated fibers were in the protective barrier of the perineurium (Figure 9E
ande
; blue arrows and green pseudocolor), which was absent in the neuroma. In the distal part of DNM-S, no myelinated or unmyelinated fibers were observed among the regenerated tissue (Figure 9F
andf)
.
Figure 8 |Effect of DNM-S on the myelinated axons in cross-sections in a rat sciatic nerve injury model at 8 weeks post-surgery under Toluidine blue staining. (A) Myelinated axons on the uninjured side. (a1, 2) Partially enlarged view of A. The axons are completely distributed in the endoneurial area. The black asterisk indicates the perineurium. (B) The regenerated tissue proximal to the implanted DNM-S. The perineurium structure is relatively intact. The regenerated axons are mainly distributed in the endoneurial area. The white arrow indicates the regenerated nerve bundle. (b1) Partial enlarged view of B. (C) The regenerated tissue of the basal part of the implanted DNM-S. (c1) Partial enlarged view of C. Some regenerated axons escaped from the endoneurium area. The white arrow indicates the regenerated axons outside of the perineurium (black asterisk). (D) Regenerated distal tissue of the implanted DNMS. (d1, d2) Partial enlarged view of D. The perineurium structure (black asterisk) is intact. Little regenerated axons grown into the distal part of DNM-S. (E) The basal part of the neuroma. (e1) Partial enlarged view of E. Some regenerated axons escaped from the endoneurial area. The white arrow indicates the regenerated axons outside of the perineurium (black asterisk). (F) Myelinated axons of nerve end in the control group. (f1, f2) Partial enlarged view of F. A large number of dysplasia axons were observed in neuroma. The black arrow indicates the dysplasia axons. Scale bars: 200 µm in A, D, F, 100 µm in B, C, E, 50 µm in a1, a2, b1, c1, d1, d2, e1, f1, f2. (G) Statistical analysis of axon distribution area. (H) Statistical analysis of the long axis of the axon from the proximal part of the implanted DNM-S in the DNM-S group and neuroma in the control group. (I) Statistical analysis of the axon area outside the bundle in the basis part of the implanted DNM-S in the DNM-S group and the basis part of neuroma in the control group. Data are expressed as the mean ± SEM (n = 5). *P < 0.05, ** P < 0.01 (Student’s t-test). DNM-S: Decellularized nerve matrix scaffold; ns: not significant.

Figure 9 |Effect of DNM-S on the regenerated axons in a rat model of sciatic nerve injury at 8 weeks after surgery as shown by TEM. (A) Myelinated axons on the uninjured side. (a) Partially enlarged view of A. (B) Typical view of a neuroma. (b) Pseudocolor image of a cross-section of the neuroma, and red pseudocolor image of disordered unmyelinated fibers. A mass of morphologically deformed myelinated and unmyelinated fibers grown in the traumatic neuroma. (C) Typical view of the regenerated tissue of the proximal DNM-S. (c) Partially enlarged view of (C). Blue pseudocolor image of orderly ECM fibers. (D) Typical view of neuroma. (d) Partially enlarged view of D. Blue pseudocolor image of disordered ECM fibers. A mass of deformed ECM fibers twisted in the traumatic neuroma. (E) Typical view of the regenerated tissue of the middle part of DNM-S. (e) Partially enlarged view of E. Pseudocolor image of the implanted DNM-S (red: muscle; green: perineurium; blue arrow: fasciculus). The blue arrowhead indicated the regenerated perineurium. (F) Typical view of the regenerated tissue of the distal DNM-S. (f) Partially enlarged view of F. No regenerated fiber was observed. Scale bars: 20 µm in A, B, C, D, E, 5 µm in a, b, c, d, e, 2 µm in f. DNM-S: Decellularized nerve matrix scaffold; ECM: extracellular matrix; TEM: transmission electron microscope.
Discussion
Peripheral nerves are composed of neural, vascular, and connective tissues. The axons that are grouped together into fascicles are surrounded by multiple hierarchical structures, such as the BM tube, endoneurium, perineurium and epineurium, which are peripheral nerve-specific connective tissues (Liu et al., 2018). Functionally, the endoneurium provides an endoneurial compartment for each axon independently, keeping them separate and providing structural support (Topp and Boyd, 2012). The perineurium-epineurium modulates external stretching forces and forms the blood-nerve barrier that can isolate external stimuli from the surrounding environment for the management of peripheral neuropathies, such as regional pain control (Hackel et al., 2012; Stubbs, 2020). Anatomical matching after reconstruction decreases the chances for inferior regeneration, fibrosis and neuromas (Roballo et al., 2022). In short, peripheral nerves require the integrity of anatomical structures to maintain physiological homeostasis and function correctly.
As a natural matrix network that plays a critical role in the dynamic homeostasis of nerve damage, the composition and structure of the ECM are associated with the recovery of structural and functional conditions following neurotmesis (Gao et al., 2013; de Luca et al., 2014). Neuroma formation begins with destruction of the anatomical microstructure, followed by improper intrinsic nerve repair (Galeano et al., 2009; Stokvis et al., 2010; He et al., 2020). Although the pathophysiological mechanism is still not clear, unorganized connective tissue ECM reconstruction and fascicular overgrowth are vital and conspicuous pathological responses of typical neurotraumatized neuropathy (Foltán et al., 2008; Mahan et al., 2019). The non-intervention of dysregulation of ECM modeling (i.e., scarring) in the regenerative microenvironment might markedly alter the regenerative potential toward structural pathological changes resulting in dysfunction, such as pain symptom (Yi et al., 2018).
Repairing the nerve stump is necessary to protect the sprouting axons from scar invasion and prevent the formation of traumatic neuroma. Autologous fat, muscle or vessel capping would be technically demanding, which may require extra preparation time and relatively complex surgical operations. The bioabsorbable nerve conduit might not correct the irregular extension and reduce pathologic interaction of axons effectively because it lacks the multiple longitudinally aligned channels which can guide axon regeneration. DNM-S preserves the engineering characteristics and ECM network of natural nerves. Implantation of a DNM-S could provide an off-the-shelf endoneurium and perineurium-epineurium scaffold for the sprouting axons in the nerve end (Qiu et al., 2020). The proteomics data demonstrated that 60 species of ECM protein were present in the DNM-S, indicating that the DNM-S could construct a complex regulatory network for tissue repair. Compared with single-component scaffolds, DNM-S has the advantage of ECM diversity that is close to that of natural nerves. In addition to substances that promote nerve regeneration, such as collagen, laminin and fibronectin, DNM-S also contains inhibitory factors such as versican. Versican is the core protein of chondroitin sulfate proteoglycans, which can block irregular axon regeneration in neuromas proactively and effectively, based on our previous study (He et al., 2020).
Hong et al. (2021) found that a DNM-S of a certain length attached to the proximal end of an injured nerve can control neuroma formation via axon growth limitation and that gene expression associated with regeneration and pain sensitization was decreased in the dorsal root ganglia (Pan et al., 2021). In our study, we found that another important role of the DNM-S in controlling neuroma formation was remodeling of the ECM environment. Clinical studies have revealed that pathologically disorganized BM tubes and endoneurium are vital pathophysiological processes of neuromas (Karsy et al., 2018; Mahan et al., 2019). From inside to outside, the BM tube, endoneurium, perineurium and epineurium constituted the peripheral nerve-specific ECM architecture of the DNM-S. Under the guidance and induction of these architectures, the uncontrollable traumatic condition of the nerve stump gradually transformed into a benign tissue repair process without scar deposition and axon tangles. Correction of the alignment of endoneurium axons and ECM fibers together with the exhaustion of axonal regenerative capacity effectively eliminates the pathologic niche of neuroma.
It is worth noting that some scholars have asked the following question: is it possible to reduce neuropathic pain without reducing peripheral nerve regeneration (Xie et al., 2017). From the point of view of nerve regeneration, DNM-S reduces neuropathic pain by improving the reconstruction process instead of reducing or restraining regeneration. Our study provides two points of evidence: first, the sprouted axon extended approximately a 10 mm long distance in implanted DNM-S at 8 weeks after surgery; second, the recellularized endoneurium reduced the pathologic interaction of axons and continuously guided these sprouted axons until they lost endogenous regenerative power for extension. Axonal growth arrest is the result of SC senescence and neurotrophic factor deficiency in the long DNM-S environment (Poppler et al., 2016) instead of the reduction of nerve regeneration directly. At this time, the regenerated perineurium-epineurium layers that serve as protective barriers for nerve stumps would minimize the risk of pathologic irritation and pain onset. In this respect, it is similar to the entubulation of terminal nerve stumps within artificial caps that reduces the risk of pain attack by modeling the soft tissue layer for the nerve end (Galeano et al., 2009; He et al., 2020). Therefore, our study confirms that DNM-Ss, as nervous ECM scaffolds, could reverse tissue pathophysiologic reconstruction and suppress neuroma formation at the nerve end via ECM remodeling to recover the hierarchical structure.
Uncontrolled fibrosis is a result of excessive deposition of fibrous ECM components during the neuroma formation process (Yi et al., 2018). Histological staining revealed that the levels of types I and III collagen major constituents of fibrous connective tissue were markedly reduced at 8 weeks. It is worth noting that the end of the implanted DNM-S expressed the lowest levels of types I and III collagen, indicating that fibrosis was effectively eliminated. A lower degree of fibrosis suggests that fewer anagenetic axons would be exposed to the chronic mechanical stimulation caused by myofibroblasts (Foltán et al., 2008). Then, histomorphology analysis was carried out to assess vascular malformation and α-SMA expression. α-SMA was expressed in myofibroblasts and vessels in the control group, while it was mainly expressed in vascular wall in the group. α-SMA expression was shown to be an indirect biomarker of autotomy behavior and neuroma-associated pain (Yan et al., 2012). In our experiments, higher α-SMA expression was measured in the neuroma of the control group.
Vascular and muscular malformations were not observed in the basal parts of the neuroma, indicating that these vascular and muscular components come from the surrounding scar, muscle and blood vessels. Therefore, in general, the protective barrier of the perineurium-epineurium in the prevents vascular invasion, and acellular limits vascular extension. This suggests that the mild microenvironment constructed by has optimized anatomical physiological remodeling and pain-free recovery, which discontinued the traumatic microenvironment and suppressed neuroma formation. Therefore, we supposed that in traumatic amputee cases in the clinic, can be implanted to the nerve stumps by epineurial microsurgical suture to prevent neuroma formation and might avoid second operation to remove the neuroma before fitting the prosthesis.
The limitations of our study should be noted. First, the ECM composition and microstructure of the neuroma were not evaluated in this study. The pathological ECM proteomics and ultrastructure of painful neuroma should be characterized in further studies to reveal the pathogenesis of neuroma formation at the ECM molecular level. Second, a single neuropathic pain animal model was used in the short-term follow-up period in our study. Several models show advantages over the others in terms of specific aspects of neuroma physiopathology, prevention or treatment; thus, a single model may be not enough to provide a comprehensive reference. Peripheral nerve injury and regeneration are complex pathological processes. Therefore, to further confirm the validity of the in preventing neuroma formation, more animal models should be tested for long-term therapeutic effectiveness.
Our study demonstrates that peripheral nerve-specific microarchitecture of can optimize the ECM microenvironment in nerve stumps to inhibit traumatic neuroma formation and relieve pathological pain by preventing the invasion of vascular and muscular scar tissue and correcting irregular ECM deposition and axon regeneration patterns. This finding provides valuable evidence that acellular bionic nerve grafts can protect nerve stumps. Overall, optimizing the ECM remodeling process with this may be a promising strategy to prevent traumatic painful neuroma formation in the clinic.
Author contributions:
Study conception and design: QTZ, CBZ; experiment implementation: SQ, PJD, FLH; data analysis: SQ; preparation and nerve morphological detection: LWY, ZHT; manuscript draft and revision: SQ, PJD. All authors discussed the conceptual and practical implications of the methods, and approved the final version of the manuscript.
Conflicts of interest:
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to
influence the work reported in this paper.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:
Francesco De Francesco, Azienda Ospedaliero Universitaria Ospedali Riuniti di Ancona, Italy; Maria Easler, University of Padova, Italy.
Additional files:
Open peer review reports 1 and 2.
Masson’s trichrome staining of nerve tissues.
Muscle staining of the middle part of the implanted DNM-S (A) and neuroma (B).
- 中国神经再生研究(英文版)的其它文章
- Inflammation and retinal degenerative diseases
- Synaptic alterations as a common phase in neurological and neurodevelopmental diseases: JNK is a key mediator in synaptic changes
- Brain-derived neurotrophic factor in main neurodegenerative diseases
- The best of both worlds: mastering nerve regeneration combining biological and nanotechnological tools
- Exosomal miR-23b from bone marrow mesenchymal stem cells alleviates oxidative stress and pyroptosis after intracerebral hemorrhage
- Chlorogenic acid alleviates hypoxic-ischemic brain injury in neonatal mice

