Exosomal miR-23b from bone marrow mesenchymal stem cells alleviates oxidative stress and pyroptosis after intracerebral hemorrhage
Liu-Ting Hu , Bing-Yang Wang, Yu-Hua Fan , Zhi-Yi He, , Wen-Xu Zheng
Abstract Our previous studies showed that miR-23b was downregulated in patients with intracerebral hemorrhage (ICH). This indicates that miR-23b may be closely related to the patho-physiological mechanism of ICH, but this hypothesis lacks direct evidence. In this study, we established rat models of ICH by injecting collagenase VII into the right basal ganglia and treating them with an injection of bone marrow mesenchymal stem cell (BMSC)-derived exosomal miR-23b via the tail vein. We found that edema in the rat brain was markedly reduced and rat behaviors were improved after BMSC exosomal miR-23b injection compared with those in the ICH groups. Additionally, exosomal miR-23b was transported to the microglia/macrophages, thereby reducing oxidative stress and pyroptosis after ICH. We also used hemin to mimic ICH conditions in vitro. We found that phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was the downstream target gene of miR-23b, and exosomal miR-23b exhibited antioxidant effects by regulating the PTEN/Nrf2 pathway. Moreover, miR-23b reduced PTEN binding to NOD-like receptor family pyrin domain containing 3 (NLRP3) and NLRP3 inflammasome activation, thereby decreasing the NLRP3-dependent pyroptosis level. These findings suggest that BMSC-derived exosomal miR-23b exhibits antioxidant effects through inhibiting PTEN and alleviating NLRP3 inflammasome-mediated pyroptosis, thereby promoting neurologic function recovery in rats with ICH.
Key Words: bone marrow mesenchymal stem cells; exosomal miRNAs; intracerebral hemorrhage; miR-23b; neuroinflammation; NLRP3 inflammasome; Nrf2; oxidative stress; PTEN; pyroptosis 1Department of Neurology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong Province, China; 2Department of Neurology, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning Province, China; 3Geriatric Department of Dalian Friendship Hospital, Dalian, Liaoning Province, China
Introduction 560 Methods 561 Results 562 Discussion 566
Introduction
Intracerebral hemorrhage (ICH) is a cerebrovascular disease that presents with high morbidity and mortality, and it accounts for 15% of strokes globally (Xi et al., 2006; Deng et al., 2021; Zhu et al., 2022). Brain injury caused by the hematoma mass mechanical effects, which can cause neurologic injury, and ICH secondary brain injury can cause further serious and persistent neurological disorders. The mechanisms of ICH secondary brain injury are complicated and include oxidative stress, neuroinflammation, excitotoxicity, and cytotoxicity. These mechanisms are connected to each other and collectively lead to brain edema and brain injury (Zhu et al., 2019). Pyroptosis, a process of programmed inflammatory death, has crucial effects in ICHinduced neuroinflammation through activating the nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3)-caspase-1 inflammasomes or other inflammasomes (Fang et al., 2020). Studies demonstrated that ICH-induced NLRP3 inflammasomes could be activated by robust reactive oxygen species (ROS) overproduction, and the ROS scavenger alleviated the NLRP3 inflammatory response through reducing oxidative stress, which indirectly protected neuronal cell from death and further reduced ICH injury (Tang et al., 2020; Chen et al., 2022). Thus, effective therapeutic strategies to alleviate pyroptosis and oxidative damage, therefore to protect neuronal cells from death are urgently needed.
Exosomes are important in bone marrow mesenchymal stem cell (BMSC) communication, and they exhibit protective roles in various diseases including ICH, and the researchers showed that exosomes effectively promoted neurological recovery after ICH by alleviating neuroinflammation and inhibiting neuronal apoptosis (Duan et al., 2020; Shi et al., 2021). Exosomes can to convey information by transferring microRNAs (miRNAs), long noncoding RNAs, and proteins, and thus, attention should be paid to miRNA effects and function, long non-coding RNAs, and proteins in exosomes (Zhang et al., 2015). miRNAs are small non-coding RNA molecules that exert their biological function in various diseases by reducing target gene expression through translational inhibition or mRNA destabilization (Bushati and Cohen, 2007). We showed down-regulated miR-23b expression in patients with ICH in a previous study, which suggested the possible biological functions of miR-23b in ICH (Zhu et al., 2015; Wang et al., 2016). Besides their involvement in different cancers (Lei et al., 2021; Yang et al., 2021), miR-23b was recently found to play various anti-inflammatory roles in multiple neurological diseases (Zhang et al., 2018; Hu et al., 2019). Moreover, miR-23b in exosomes were shown to be derived from BMSCs, which can prevent intracranial aneurysm formation by maintaining the Th17/Treg immune balance. However, evidence regarding the implication of BMSC-exosomal miR-23b in ICH is lacking. Therefore, this study aimed to explore the functions of BMSC-exosomal miR-23b in ICH-induced oxidative stress and pyroptosis and to investigate the molecular mechanisms using animal and cell models.
Methods
Animals
The animal experiments were approved by the Animals Ethics Committee at China Medical University (No. 2017008) on March 8, 2017. All experiments were designed and reported in accordance with the Animal Research: Reporting ofIn Vivo
Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020). A previous epidemiology study (Roquer et al., 2016) showed that more men than women had ICH (52.4%vs
. 47.6%). Because of the higher incidence of ICH in men compared with that in women, we chose male rats for our study. One hundred and forty-four male Wistar rats (SPF level, 250‒280 g, 8‒12 weeks old) were obtained from Chang Sheng Biotechnology Co. Ltd. (Benxi, Liaoning, China; license No. SCXK (Liao) 2019-0001). All rats were housed under standard conditions (temperature 25 ± 1°C; humidity, 50 ± 5%), a regular 12-hour light/dark cycle was used, and they had free access to food and water. There were three rats in each cage. The study procedure is shown inFigure 1
.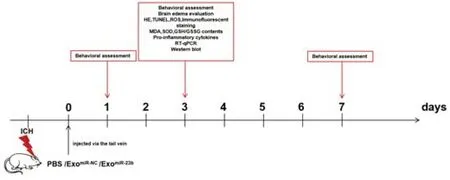
Figure 1 |Animal experimental flow chart.ExomiR-23b: exosomes derived from BMSCs transfected with miR-23b; ExomiR-NC: exosomes derived from BMSCs transfected with miR-NC; GSH: reduced glutathione; GSSG: oxidized glutathione disulfide; HE: hematoxylin-eosin; ICH: intracerebral hemorrhage; MDA: malondialdehyde; PBS: phosphate-buffered saline; ROS: reactive oxygen species; RTqPCR: reverse transcription quantitative polymerase chain reaction; SOD: superoxide dismutase; TUNEL: terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling.
Cell preparation and culture
Wistar rat BMSCs (Cat# 170908I32) that had been isolated and cultured were obtained from Cyagen Biosciences Inc. (Santa Clara, CA, USA), and microglia BV2 cells (Cat# 1101MOU-PUMC000063) and hippocampal neuronal HT22 (Cat# BNCC338235) were obtained from Cell Resource Center of the Peking Union Medical College and the Bena Culture Collection (Xinyang, China). BMSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% exosome-free fetal bovine serum (Gibco, Sidney, Australia). BV2 cells were cultured in DMEM containing 10% heat-inactivated fetal bovine serum (Gibco). HT22 cells were cultured in DMEM/F-12 containing 10% fetal bovine serum. Hemin (MedChemExpress, Shanghai, China) stimulation (60 µM for 24 hours) was applied to mimic ICH conditionsin vitro
(Hu et al., 2020). N-acetylcysteine (NAC), a thiol-containing redox modulatory compound, is a known antioxidant. As previously described (Karuppagounder et al., 2018), 1 mM NAC was delivered to cells 2 hours after hemin stimulation to observe the change of intracellular ROS levelsin vitro
.Cell transfection
Lentivirus carrying miR-23b or the negative control (miR-NC) was obtained from GeneChem Company (Shanghai, China). We separately transfected BMSCs (1 × 10) with individual lentivirus with a multiplicity of infection of 20. MiR-23b mimics and inhibitors and pcDNA3.1-phosphatase and tensin homolog deleted on chromosome 10 (PTEN) plasmids were obtained from Genepharma (Suzhou, Jiangsu, China).
Exosomes isolation, characterization and labeling
ExoQucik-TC isolation kit (System Bioscience, Palo Alto, CA, USA) was used to isolate the exosomes (McNicholas and Michael, 2017). After isolation, exosomes from BMSCs were resuspended in phosphate-buffered saline (PBS). Exosomes were visualized under a transmission electron microscope (JEOL, Tokyo, Japan). The expression levels of exosome-specific biomarkers (CD63, CD81 and TSG101) were evaluated using Western blot. Kits were obtained from the following manufacturers: mouse anti-CD63 (Thermo Fisher Scientific, Waltham, MA, USA, Cat# 10628D, RRID: AB_2532983), mouse anti-CD81 (Thermo Fisher Scientific, Cat# MA5-13548, RRID: AB_10987151), and mouse anti-TSG101 (Thermo Fisher Scientific, Cat# MA1-23296, RRID: AB_2208088). Exosomes were labeled using the PKH67 green fluorescent linker Mini Kit (Sigma, St. Louis, MO, USA).
ICH models and exosomes administration
Rats were randomly divided into the following four groups: (1) sham group, in which a micro-syringe was inserted without an injection; (2) ICH model group, in which ICH induction was performed, and rats were injected with an equivalent amount (1 mL) of PBS; (3) exo miR-NC group, in which ICH induction was performed, and rats were injected with exosomes that were derived from BMSCs after transfection with lentivirus carrying miR-NC; and (4) exo miR-23b group, in which ICH induction was performed, and rats were injected with exosomes that were derived from BMSCs after transfection with lentivirus carrying miR-23b.
The rats underwent ICH surgical procedures after being induced by collagenase VII (Sigma), as described previously (Hu et al., 2020). Two microliters of collagenase VII (0.25 U/µL) was slowly injected into the right basal ganglia (stereotaxic coordinates were 1.0 mm posterior, 3.0 mm lateral, and 5.8 mm below the horizontal plane of the bregma) (Hu et al., 2020). Twenty-four hours after ICH, BMSC-miR-NC-exosomes (100 µg/mL, 100 µg), BMSC-miR-23b-exosomes (100 µg/mL, 100 µg), or 1 mL PBS was injected via the tail vein.
Behavioral assessment and brain edema evaluation
To evaluate neurological behavioral function in rats including the sensory and motor function, corner tests and forelimb placement tests were performed on days 1, 3, and 7 after the exosomes were administered. Briefly, for the corner test, rats were approached in a 30 ° corner and had to turn to the left or right to exit the corner. We recorded the choice of turning side for ten trials per rat. Forelimb placement tests were performed as described in the previous study (Xi et al., 2018). Each rat was tested for ten trials. The percentage of trials in which the rat placed the appropriate forelimb on the edge of the countertop in response to vibrissae stimulation was calculated. Rats were anesthetized using 50 mg/kg 2% sodium pentobarbital (Cat# P3761, Sigma) via intraperitoneal injection, and they were then sacrificed by decapitation. Ipsilateral hemisphere tissue was dissected on day 3 and weighed immediately on an electronic balance (Sartorius, Gottingen, Niedersachsen, Germany) to determine the brain wet weight. The tissue was then heated at 100°C for 1 day to determine the dry weight. The water content (%) was obtained as (wet weight ‒ dry weight) / wet weight × 100.
Hematoxylin-eosin staining
Rats were sacrificed at day 3 for the histology study and perfused with normal saline and 4% paraformaldehyde. Brain samples were dissected, soaked with graded sucrose, and frozen sequentially by a slicer. A hematoxylin-eosin (H&E) staining kit (G1120-3, Solarbio, Beijing, China) was used to stain brain coronal slides (8 µm). Briefly, the slides were stained with hematoxylin for 4 minutes and washed with distilled water. Next, the slides were incubated with the differentiation fluid for 20 seconds and washed with water for 30 minutes. Finally, the slides were stained with eosin for 30 seconds and sealed with neutral balsam.
Terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling and immunofluorescence staining
The terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling (TUNEL) assay was used to identify apoptotic cells from perihematomal brain region using in situ death detection kits to detect TUNEL-positive cells, as previously described (Hu et al., 2019) (No. 11684795910, Roche, Grenzach Wyhlen, Germany). Briefly, after fixation, the slides were permeabilized for 5 minutes and washed with PBS three times. The slides were then incubated in 50 µL of TUNEL reaction solution for 1 hour in a dark chamber. Next, slides were washed with PBS and counterstained with 4′,6-diamidino-2′-phenylindole (DAPI). For immunofluorescence staining, the frozen brain slides were rewarmed at 37°C and then incubated with 0.3% Triton X-100 and blocked. Rabbit anti-ionized calcium binding adapter molecule 1 (Iba1; 1:200, Abcam, Waltham, MA, USA, Cat# ab178846, RRID: AB_2636859) was applied as the primary antibody and incubated with the sections at 4°C overnight. Goat anti-rabbit IgG(H+L)-Alexa Fluor594 (1:500, Thermo Fisher Scientific, Cat# A-11012, RRID: AB_141359) were incubated with the brain slides for 1 hour at 37°C. We selected three slides per rat and counted the numbers of positive cells in five random fields per slice under the fluorescence microscope (Olympus, Tokyo, Japan).
Reactive oxygen species staining
Frozen slides were stained using the GENMED kit to evaluate the ROS in the perihematomal brain region (GMS10016.1, GENMED, Arlington, MA, USA) (Guo et al., 2021). For ROS staining, slides were washed with cleaning reagent A and incubated with working reagent for 20 minutes at 37°C. To detect ROS in cells stimulated by hemin conditions, we diluted DCFH-DA (S0033, Beyotime, Shanghai, China) with PBS to 10 µM to acquire the working concentration. The cells were incubated with working reagent at 37°C for 20 minutes and washed with DMEM. ROS staining was visualized using a fluorescence microscope.
Measurement of MDA activity, SOD activity, and GSH/GSSG content
The malondialdehyde (MDA), superoxide dismutase (SOD), and reduced glutathione/oxidized glutathione disulfide (GSH/GSSG) levels in perihematomal brain tissues were evaluated using a lipid peroxidation MDA activity kit (S0131, Beyotime), SOD activity kit (S0088, Beyotime), and GSH/GSSG activity kit (S0053, Beyotime), respectively, in accordance with the manufacturer’s instructions.
Pro-inflammatory cytokines and cell viability detection
Cytokines, including interleukin-1β (IL-1β) and interleukin-18 (IL-18) in perihematomal brain tissues and cell supernatant (collected from cultured BV2 cells supernatant in anin vitro
experiment at 48 hours after the cultured BV2 cells reached 80% confluency), were evaluated using enzyme linked immunosorbent assay (ELISA) kits. The kits that were used are as follows: mouse IL-1β ELISA kit (MLB00C, R&D Systems, Minneapolis, MN, USA), mouse IL-18 ELISA kit (DY7625, R&D Systems), rat IL-1β ELISA kit (RA20020, Bio SWAMP, Wuhan, China), and rat IL-18 ELISA kit (RA20058, Bio SWAMP). The neuronal cell viability and death rates were estimated using a cell counting kit-8 (CCK8) kit (CK04, Dojindo, Tokyo, Japan) and lactate dehydrogenase (LDH) assay kit (KGT02448, KeyGen, Nanjing, China), as described previously (Hu et al., 2020). For CCK8 assays, 10 µL of CCK8 reaction solution was added to the co-cultured neuronal cells per well, and the cells were incubated at 37°C for 1 hour. Absorption in each well was estimated at 450 nm using a multifunction measuring instrument (Thermo Fisher Scientific). For LDH assays, each sample was incubated with the corresponding reaction solution in accordance with the manufacturer’s instructions, and the absorption of each well was estimated at 440 nm using a multifunction measuring instrument (Thermo Fisher Scientific).Reverse transcription quantitative polymerase chain reaction
For reverse transcription quantitative polymerase chain reaction (RT-qPCR), miRNAs were isolated from the exosomes using the miRcute miRNA isolation kit (TIANGEN, Beijing, China). TRIzol(Invitrogen, Waltham, MA, USA) was used for RNA extraction from tissues or cells in rat brain tissues. All primers were purchased from Sangon (Shanghai, China). Complementary DNA was synthesized using a reverse transcription kit, and amplified using the SYBR Green PCR kit (Takara Bio, Dalian, China) in a Lightcycler 96 instrument (Roche), using the following process: initial denaturation at 95°C for 30 seconds; 40 cycles at 95°C for 5 seconds, and 60°C for 20 seconds, followed by 65°C for 15 seconds. The primer sequences are described inAdditional Table 1
. The relative miRNA expression was normalized using U6, and the relative mRNA expression was normalized using β-actin.Western blot assay
Western blots were conducted in brain tissue and cells as described previously (Ding et al., 2015; Hu et al., 2020). The following primary antibodies were used: rabbit anti-NLRP3 (1:500; Cell Signaling Technology, Danvers, MA, USA, Cat# 13158; RRID: AB_2798134), rabbit anti-caspase-1 (1:1000, Proteintech Group, Wuhan, China, Cat# 22915-1-AP, RRID: AB_2876874), rabbit anti-Nterminal fragment of gasdermin D (GSDMD-N; 1:1000, Proteintech Group, Cat# 20770-1-AP, RRID: AB_10696319), rabbit anti-PTEN (1:500, Cell Signaling Technology, Cat# 9188, RRID: AB_2253290), rabbit anti-nuclear factor erythroid-2-related factor 2 (Nrf2; 1:500, Bioworld, Shanghai, China, Cat# BS1258, RRID: AB_1663675), mouse anti-heme oxygenase-1 (HO-1; 1:1000, Abcam, Cat# ab13248, RRID: AB_2118663), mouse anti-β-actin (1:1000, Sigma, Cat# A5441, RRID: AB_476744), and mouse anti-Lamin A (1:1000, Cell Signaling Technology, Cat# 86846, RRID: AB_2800093). Among them, NLRP3, caspase-1, and GSDMD-N were pyroptosis-related proteins. PTEN, Nrf2, and HO-1 were oxidative stress-related proteins. β-Actin and LaminA were internal controls. Primary antibodies were incubated with bands at 4°C overnight. The following secondary antibodies were used: horseradish peroxidase (HRP)-conjugated affinipure goat anti-rabbit IgG (H+L) (1:5000, Proteintech Group, Cat#SA00001-2, RRID: AB_2722564) and HRP-conjugated affinipure goat anti-mouse IgG(H+L) (1:5000; Proteintech Group, Cat# SA00001-1, RRID: AB_2722565). Secondary antibodies were applied to bands at 37°C for 1 hour. The immunoreactive signals were visualized using a gel imaging analysis system (Kodak, Tokyo, Japan).
Dual luciferase reporter assays
BV2 cells were cultured in 24-well plates and transfected when they reached 50‒60% confluence. Cells were co-transfected with either 0.1 µg pmirGLO-mut-PTEN or wild-type (wt)-PTEN (Genepharma, Suzhou, China) and 0.4 µg miR-NC or miR-23b mimics (Genepharma). The dual-luciferase assay system (E1910, Promega, Madison, WI, USA) was applied to evaluate the relative luciferase activity at 48 hours after transfection, in accordance with the protocol.
Co-immunoprecipitation analysis
For the co-immunoprecipitation (Co-IP) assay, the microglia BV2 were transfected with miR-NC or the miR-23b mimic. Whole-cell extracts were lysed 48 hours after transfection in immunoprecipitation buffer supplemented with a protease inhibitor mixture (Beyotime). The cell supernatants were collected after centrifugation for 10 minutes at 12,000 ×g
, and incubated overnight at 4°C with immunoprecipitation antibodies and Protein G Plus-Agarose Immunoprecipitation reagents (Beyotime). After they were washed and eluted, the immunoprecipitants were evaluated by Western blotting.Statistical analysis
GPower 3.1.9.2 software (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) (Faul et al., 2007) was used to determine the number of animal that were required. In thein vivo
experiments, no animals were lost, and the raters who performed the animals’ behavioral assessments were blinded to the group assignments. Data are presented as the mean ± standard deviation (SD). Behavioral data were analyzed using the Kruskal-Wallis test with Dunn’spost hoc
tests. The statistical significance between two groups was evaluated using the Student’st
-test. The statistical significance among multiple groups was analyzed using a one-way analysis of variance (ANOVA) with the Newman-Keulspost hoc
analysis. Statistical significance was set atP
< 0.05. GraphPad Prism 7.00 (GraphPad software, San Diego, CA, USA) was used to analyze the data.Results
Overexpression of miR-23b in BMSCs and identification of exosomes derived from BMSCs
MiR-23b was upregulated in the BMSC group transfected with lentivirus carrying miR-23b (LV-miR-23b) (Figure 2A
). The transmission electron microscope revealed that the exosomes derived from the BMSCs exhibited a round cup-shaped and complete structure (Figure 2B
). Western blot analysis further identified positive expression of the exosomal biomarkers CD63, TSG101, and CD81 in the exosomes derived from the BMSCs (Figure 2C
). As shown inFigure 2D
, we observed a higher miR-23b level in exosomes from BMSCs transfected with miR-23b (exo), suggesting that the overexpressed miR-23b could be delivered to BMSC-derived exosomes.BMSC-exosomal miR-23b improves behavioral functions and reduces brain injuries induced by ICH
To explore the effects of the BMSC-derived exosomes carrying miR-23b in ICH rats, the same volume of the exo, exo, or PBS was injected, and an increased miR-23b level was found in the perihematomal brain tissue in the exogroup (Figure 3A
). To observe the distribution of the BMSC exosomes, we labeled the exosomes with PKH67 green fluorescence and the microglia/macrophages with Iba1 staining. We found that the exosomes were delivered successfully into the microglia/macrophages in the perihematomal region (Figure 3B
). The neurological deficits were evaluated using the corner tests and limb placement assessments. ICH induction increased the frequency of right turns on days 1, 3, and 7 compared with that in the sham group, and the increase was mitigated by exoadministration on day 7 (Figure 3C
). Similar neurological function improvements were observed using limb placement tests (Figure 3D
). The brain water content of the ipsilateral hemisphere showed a significant increase after ICH induction, which was attenuated by exoor exoadministration (Figure 3E
). Brain tissue morphology in ICH rats was evaluated using H&E staining. Non-regularly arranged neuronal cells, deep staining, and a decreased number of nuclei with neutrophil infiltration were observed in the ICH group compared with those in the sham group. However, the large pathological deteriorations induced by ICH were attenuated in both exoand exogroups. The fewest histological impairments were observed in rats that received exoadministration (Figure 3F
).BMSC-exosomal miR-23b inhibits the amount of microglia/macrophages and cell apoptosis
Iba1 fluorescence staining for microglia/macrophages was performed to observe the inflammation in ICH rats. The amount of Iba1-positive cells was elevated after ICH induction but decreased by exoand exoinjection, whereas there was no significant difference between exoand exoinjections (Figure 4A
andB
). Cell apoptosis was assessed via the amount of TUNEL-positive cells. As depicted inFigure 4C
andD
, administration of exoor exoreduced cell death induced by ICH, and the exogroup further alleviated cell apoptosis compared with that in the exogroup.BMSC-exosomal miR-23b reduces oxidative stress in the ICH rat brain
ROS staining was used to estimate the oxidative stress level. The ROS level in the perihematomal brain tissue was increased after ICH induction compared with that in the sham group, but decreased after exoadministration compared with that in the ICH group (Figure 5A
andB
). We evaluated lipid peroxidation by estimating the MDA level, and we found that the MDA level was increased after ICH induction compared with that in the sham group, but it was decreased by exocompared with that in the ICH group (Figure 5C
). Major antioxidant enzyme SOD activity in brain tissue was elevated in the exogroup compared with that in the exo miR-NC or ICH group (Figure
5D
). GSH and GSSG maintain a dynamic balance, and a reduced GSH/GSSG ratio means that there is elevated oxidative stress. The ICH group exhibited a decreased GSH/GSSG ratio compared with that in the sham group. However, exoadministration significantly increased the ratio compared with that in the ICH group (Figure 5E
). Collectively, the results confirmed the antioxidant effect of exosomal miR-23b.BMSC-exosomal miR-23b attenuates pyroptosis via inhibiting NLRP3 inflammasome activation in ICH rats
The effect of exoon cell pyroptosis was evaluated using RT-qPCR and Western blot. Consistent with previous studies (Hu et al., 2020; Zheng and Kanneganti, 2020), our results showed that NLRP3 inflammasome and pyroptosis were induced by ICH (Figure 6A−D
). However, exoadministration repressed the increase in NLRP3, cleaved caspase-1, and GSDMD-N (Figure 6E
andF
). Mature IL-1β and IL-18, which are NLRP3 inflammasome effectors, were further decreased in the exogroup compared with those in the ICH group (Figure 6G
andH
). Collectively, the exosomal miR-23b hampered pyroptosis by inhibiting NLRP3 inflammasome activation induced by ICH.BMSC-exosomal miR-23b attenuates oxidative stress
in vitro
We stimulated microglia BV2 cells with 60 µM hemin for 24 hours to mimic ICH conditionsin vitro
, and exosomes were incubated separately with heminstimulated microglia BV2 cells; PBS incubation was set as the control. The results showed that the miR-23b level in the exogroup was higher than that in the other groups (Figure 7A
). Oxidative stress was evaluated using ROS, MDA, SOD, and GSH/GSSG levels. BV2 cells treated with 60 µM hemin induced the increase in intracellular ROS, which was reversed by the treatment with the antioxidant NAC, confirming that the increase in ROS was induced by hemin (Figure 7B
andC
). Additionally, exocould significantly decrease the ROS levels induced by hemin (Figure 7B
andC
). Similar results were also observed in MDA measurements (Figure 7D
). SOD levels were markedly decreased by hemin stimulation, and exoimproved the SOD levels compared with that in the hemin group (Figure 7E
). Furthermore, the decrease in the GSH/GSSG ratio induced by hemin was reversed by exoadministration (Figure 7F
).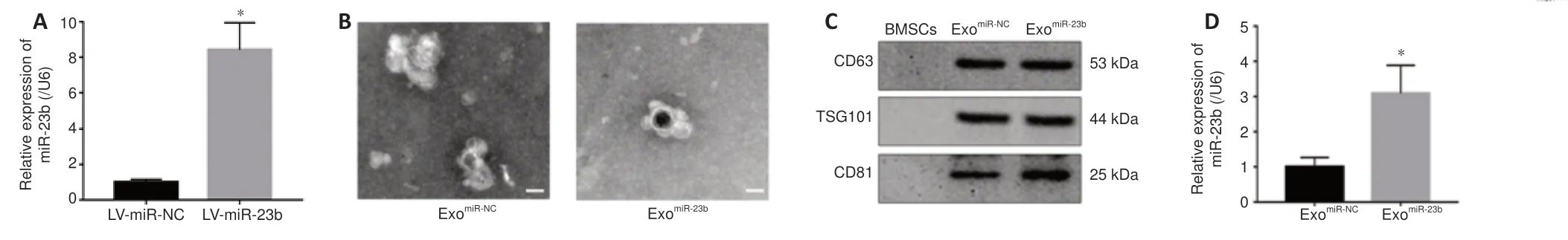
Figure 2 | Identification of exosomes derived from BMSCs overexpressing miR-23b. (A) MiR-23b expression in BMSCs detected by RT-qPCR after lentivirus transfection. (B) Transmission electron microscope scanning images of exosomes. The exosomes derived from the BMSCs showed a round cup-shaped and complete structure. Scale bars: 100 nm. (C) Western blot bands detecting exosome markers CD63, TSG101, and CD81. (D) MiR-23b expression levels in different exosome groups detected by RT-qPCR. Data are shown as the mean ± SD (n = 3). *P < 0.05 (Student’s t-test). BMSCs: Bone marrow mesenchymal stem cells; ExomiR-23b: exosomes derived from BMSCs transfected with miR-23b; ExomiR-NC: exosomes derived from BMSCs transfected with miR-NC; RT-qPCR: reverse transcription quantitative polymerase chain reaction.
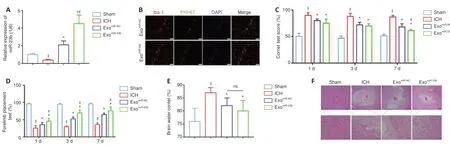
Figure 3 | BMSC-exosomal miR-23b improves behavioral functions and reduces ICH-induced brain injury. (A) miR-23b levels in different rat groups after exosomes administration. (B) Immunofluorescence staining showed that PKH67 dye staining (green, representing exosomes) was distributed in the Iba1-stained cells (red, stained by Alexa Fluor 594, representing microglia/macrophages) in both the exomiR-23b and exomiR-NC groups, indicating the successful transfer of exosomes into microglia/macrophages. Scale bar: 50 µm. (C) Behavioral functions were evaluated using the corner tests. ICH group showed an increased percentage of right turns compared with sham group, indicating severe neurological deficits after ICH induction; and the increase was mitigated by exomiR-23b administration on day 7, indicating the improvement of neurological function after exomiR-23b administration. (D) Behavioral functions were evaluated by forelimb placement assessments. The percentage of the trials in which the rats placed the appropriate forelimb responding to vibrissae stimulation was recorded. (E) Brain water content. (F) Representative images of H&E staining. There was a large pathological deterioration in the ICH group compared with that in the sham group, and the pathological deterioration was attenuated in the exomiR-23b and exomiR-NC groups compared with that in the ICH group. The exomiR-23b group showed the least pathological deterioration compared with that in the other groups after ICH induction. The bottom row is an enlargement of the box area in the top row. H represents the area containing the hematoma. Scale bars: 500 µm (upper), 100 µm (lower). Data are shown as the mean ± SD (n = 6). $P < 0.05, vs. sham group; *P < 0.05, vs. ICH model group; #P < 0.05, vs. exomiR-NC group (one-way analysis of variance with the Newman-Keuls post hoc analysis in A and E, Kruskal-Wallis tests followed by Dunn’s post hoc test in C and D). Sham group: Control; ICH group: PBS injection after ICH; exomiR-NC group: miR-NC transfected BMSC-exosome injection after ICH; exomiR-23b group: miR-23b transfected BMSC-exosome injection after ICH. BMSC: Bone marrow mesenchymal stem cell; DAPI: 4′,6-diamidino-2′-phenylindole; H&E: hematoxylin-eosin; Iba1: ionized calcium binding adapter molecule 1; ICH: intracerebral hemorrhage; ns: not significant; PBS: phosphate-buffered saline; TUNEL: terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling.

Figure 4 | BMSC-exosomal miR-23b inhibits the amount of microglia/macrophages and neuronal apoptosis in brain tissues.(A, B) Immunofluorescence and data analysis of microglia/macrophages with Iba1 staining (red, Alexa FluorTM 594) in the perihematomal brain region. There were more immunofluorescence staining showing Iba1-stained cells (microglia/macrophages) in the ICH group than that in the sham groups, and Iba1 staining decreased in the exomiR-23b and exomiR-NC groups compared with that in the ICH group. (C, D) TUNEL staining (green) and data analysis of neuronal apoptosis. There were more TUNEL-stained cells (apoptotic neuronal cells) in the ICH group compared with that in the sham group. TUNEL-stained cells decreased in the exomiR-23b and exomiR-NC groups compared that in the ICH group, and the exomiR-23b group showed less TUNEL staining than that in the exomiR-NC group. Scale bars: 50 µm. Data are shown as the mean ± SD (n = 6). $P< 0.05, $$P < 0.01, vs. sham group; *P < 0.05, **P < 0.01, vs. ICH group; #P < 0.05, vs. exomiR-NC group (one-way analysis of variance followed by Newman-Keuls post hoc analysis). Sham group: Control; ICH group: PBS injection after ICH; exomiR-NC group: miR-NC transfected BMSC-exosome injection after ICH; exomiR-23b group: miR-23b transfected BMSC-exosome injection after ICH. BMSC: Bone marrow mesenchymal stem cell; Iba1: ionized calcium binding adapter molecule 1; ICH: intracranial hemorrhage; ns: not significant; PBS: phosphate-buffered saline; TUNEL: terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling.
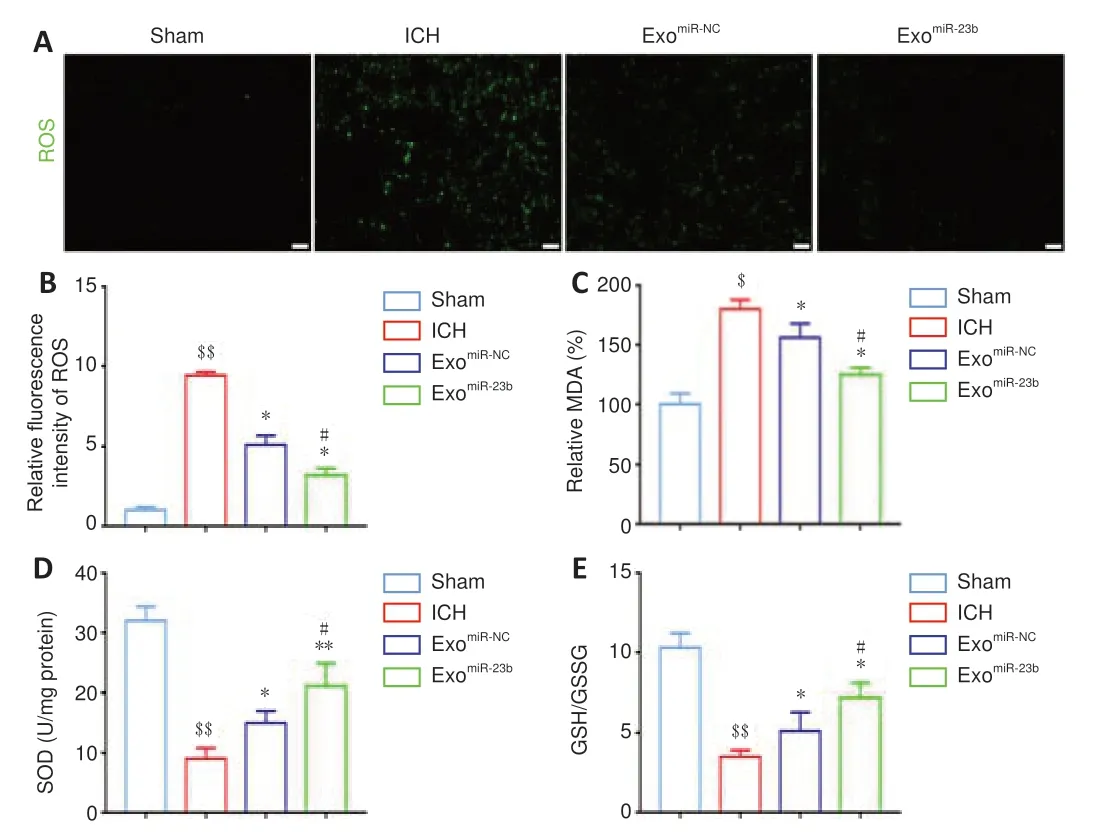
Figure 5 | BMSC-exosomal miR-23b inhibits oxidative stress in brain tissues. (A) ROS staining in each group. ROS showed green fluorescence. More ROS green staining was observed in the ICH group compared with that in the sham group. The ROS green staining was decreased in the exomiR-23b and exomiR-NC groups compared with that in the ICH group, and exomiR-23b group showed less ROS staining than that in the exomiR-NC group. Scale bars: 50 µm. (B) Relative fluorescence intensity of ROS. (C, D) MDA and SOD levels evaluated by economical kits . (E) GSH/GSSG ratios were calculated by the GSH and GSSG levels which evaluated by economical kits. Data are shown as the mean ± SD (n = 6). $P < 0.05, $$P < 0.01, vs. sham group; *P < 0.05, **P < 0.01, vs. ICH model group; #P < 0.05, vs. exomiR-NC group (one-way analysis of variance followed by Newman-Keuls post hoc analysis). Sham group: Control; ICH group: PBS injection after ICH; exomiR-NC group: miRNC transfected BMSC-exosome injection after ICH; exomiR-23b group: miR-23b transfected BMSC-exosome injection after ICH. BMSC: Bone marrow mesenchymal stem cell; GSH: reduced glutathione; GSSG: oxidized glutathione disulfide; ICH: intracranial hemorrhage; MDA: malondialdehyde; PBS: phosphate-buffered saline; ROS: reactive oxygen species; SOD: superoxide dismutase.
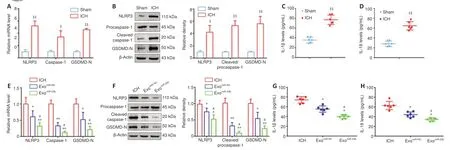
Figure 6 | BMSC-exosomal miR-23b attenuates NLRP3 inflammasome-induced pyroptosis in brain tissues.(A) mRNA expression of pyroptosis-related genes by RT-qPCR was significantly elevated after ICH induction. (B) Pyroptosis-related protein expression by western blotting was increased after ICH induction. (C, D) IL-1β (C) and IL-18 (D) levels evaluated by ELISA were increased after ICH induction. (E) mRNA levels of pyroptosis-related gene after exosomes administration. (F) Pyroptosis-related protein expression after exosome administration. (G, H) IL-1β (G) and IL-18 (H) levels were assessed by ELISA after exosome administration. Data are shown as the mean ± SD (n = 6). $P < 0.05, $$P < 0.01, vs. sham group; *P < 0.05, **P < 0.01, vs. ICH model group; #P < 0.05, vs. exomiR-NC group (Student’s t-test in A−D; oneway ANOVA with Newman-Keuls post hoc analysis in E−H). Sham group: Control; ICH group: PBS injection after ICH; exomiR-NC group: miR-NC transfected BMSC-exosome injection after ICH; exomiR-23b group: miR-23b transfected BMSC-exosome injection after ICH. BMSC: Bone marrow mesenchymal stem cell; ELISA: enzyme linked immunosorbent assay; GSDMD-N: N-terminal fragment of gasdermin D; ICH: intracerebral hemorrhage; IL-18: interleukin-18; IL-1β: interleukin-1β; NLRP3: NOD-like receptor family pyrin domain containing 3; PBS: phosphate-buffered saline; RT-qPCR: reverse transcriptase quantitative polymerase chain reaction.

Figure 7 | Exosomal miR-23b inhibits oxidative stress in microglia BV2 cells in vitro.(A) miR-23b levels in microglia BV2 cells after administration of different exosome groups under hemin stimulation. (B) Representative images of ROS staining. The hemin group showed more ROS staining than that in the PBS group, and antioxidant NAC administration further reduced the ROS staining compared with that in the hemin group. ROS levels were decreased in both exomiR-23b and exomiR-NC groups compared with those in the hemin group, and the exomiR-23b group showed less ROS staining than that in the exomiR-NC group. Scale bars: 50 µm. (C) Quantification of relative fluorescence intensity of ROS. (D) MDA levels were measured by economical kits. (E) SOD levels were measured by economical kits. (F) The GSH/GSSG ration was calculated on the basis the GSH and GSSG levels which evaluated by economical kits. Data are shown as the mean ± SD (n = 6). $P < 0.05, $$P < 0.01, vs. PBS group; *P < 0.05, **P < 0.01, vs. hemin model group; #P < 0.05, vs. exomiR-NC group (one-way analysis of variance followed by Newman-Keuls post hoc analysis). PBS: Control; PBS + NAC: treated with 1 mM N-acetylcysteine; Hemin: treated with 60 µM hemin for 24 hours; Hemin + NAC: treated with 1 mM N-acetylcysteine 2 hours after hemin stimulation; exomiR-NC group: treated with miR-NC transfected BMSCs-exosomes after hemin stimulation; exomiR-23b group: treated with miR-23b transfected BMSCs-exosomes after hemin stimulation. BMSC: Bone marrow mesenchymal stem cell; GSH: reduced glutathione; GSSG: oxidized glutathione disulfide; ICH: intracerebral hemorrhage; MDA: malondialdehyde; NAC: N-acetylcysteine; PBS: phosphate-buffered saline; ROS: reactive oxygen species; SOD: superoxide dismutase.
BMSC-exosomal miR-23b alleviates NLRP3 inflammasome-mediated pyroptosis of microglia BV2 cells and protects neuronal HT22 cells
Hemin stimulation markedly increased NLRP3, cleaved caspase-1, and GSDMD-N in microglia BV2 cells, whereas the increase was mitigated by exoor exoadministration. Exoadministration showed a stronger effect than that of exo(Figure 8A
andB
). IL-1β and IL-18 levels in microglia BV2 cells were higher after hemin stimulation compared with that in the PBS group, while exoadministration significantly reduced the IL-1β and IL-18 release compared with that in the hemin group (Figure 8C
andD
).The CCK8 test was performed to determine the viability of co-cultured hippocampal neuronal HT22 cells. Cell viability was decreased after hemin induction compared with that in the PBS group, while exoimproved the HT22 cell viability compared with that in the hemin group (Figure 8E
). Consistently, the neuronal HT22 cell death rates detected by the LDH assays showed stronger neuroprotective effects for exocompared with those of exo(Figure 8F
).BMSC-exosomal miR-23b attenuates oxidative stress via regulating PTEN/Nrf2 pathway
Nrf2 is crucial to maintain cellular redox homeostasis, and the evidence suggested that Nrf2 activation is influenced by nuclear export and PTEN/PI3K-mediated degradation (Ding et al., 2020). To explore whether PTEN/Nrf2 signaling is involved in the modulation of miR-23b in oxidative stress, we estimated the Nrf2 nuclear translocation and downstream antioxidant geneHO-1
. We found that Nrf2 nuclear translocation and the HO-1 level were increased by exoadministration compared with that in the hemin group (Figure 10A
andB
). PTEN was overexpressed by plasmid transfection. PTEN overexpression reduced Nrf2 nuclear translocation and the HO-1 level compared with that in the exogroup (Figure 10A
andB
) and inhibited the antioxidant effects of exosomal miR-23b compared with those in the exogroup (Figure 10C−E
).PTEN acts as a target of miR-23b
To investigate the mechanisms of exosomal miR-23b in exerting antioxidant and anti-inflammatory effects, we used Targetscan to investigate the target of miR-23b. The predicted binding between PTEN and miR-23b was observed (Figure 9A
). This notion was confirmed by the dual luciferase reporter assay. The luciferase activity of the wt-PTEN + miR-23b mimic group was reduced compared with that of the mut-PTEN+ miR-23b mimic group (Figure
9B
). The RT-qPCR and western blot results indicated consistent miR-23b overexpression with mimic downregulated PTEN expression (Figure 9C
andD
).BMSC-exosomal miR-23b alleviates NLRP3 inflammasome-mediated pyroptosis by regulating PTEN
The inhibition of NLRP3 inflammasome components and pyroptosis was reversed by PTEN overexpression (Figure 11A−D
). Moreover, exoadministration mitigated co-cultured neuronal cell death, which was increased by PTEN overexpression (Figure 11E
andF
). PTEN was suggested to facilitate the activation of NLRP3 inflammasome by binding with NLRP3 (Huang et al., 2020). We detected the binding of PTEN with NLRP3 after exoadministration using co-immunoprecipitation tests and found that overexpressing miR-23b could significantly reduce endogenous PTEN binding to NLRP3 compared with that in the hemin group (Figure 11G
).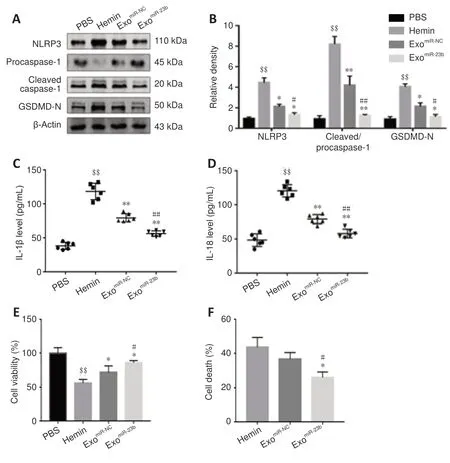
Figure 8 |Exosomal miR-23b alleviates NLRP3 inflammasome-mediated pyroptosis in microglia BV2 cells and protects neuronal cells in vitro.(A, B) Expression and data analysis of pyroptosis-related proteins in different groups of microglia BV2 cells. (C, D) IL-1β (C) and IL-18 (D) levels in microglia BV2 cells were evaluated by ELISA. (E) Cell viabilities of hippocampal neuronal HT22 cells by CCK8 tests. (F) Cell death rates of HT22 cells by LDH assays. Data are shown as the mean ± SD (n = 6). $$P < 0.01, vs. PBS group; *P < 0.05, **P < 0.01, vs. hemin group; #P < 0.05, ##P < 0.01, vs. exomiR-NC group (one-way analysis of variance followed by Newman-Keuls post hoc analysis). PBS group: Control; Hemin group: treated with 60 µM hemin for 24 hours; exomiR-NC group: treated with miR-NC transfected BMSCs-exosomes after hemin stimulation; exomiR-23b group: treated with miR-23b transfected BMSC-exosomes after hemin stimulation. BMSC: Bone marrow mesenchymal stem cell; CCK8: cell counting kit-8; ELISA: enzyme linked immunosorbent assay; GSDMD-N: N-terminal fragment of gasdermin D; IL-18: interleukin-18; IL-1β: interleukin-1β; LDH: lactate dehydrogenase; NLRP3: NOD-like receptor family pyrin domain containing 3; PBS: phosphate-buffered saline.
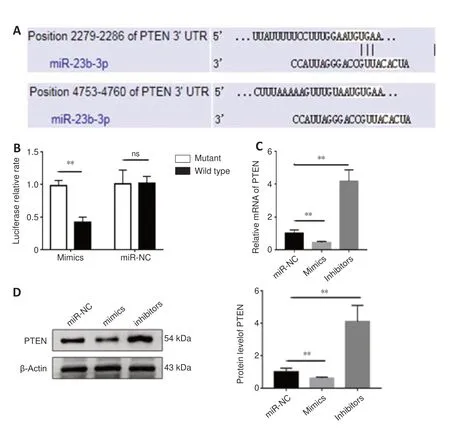
Figure 9 |miR-23b negatively regulated PTEN in BV2 cells. (A) Predicted binding between PTEN and miR-23b. (B) Luciferase report assays confirmed binding between miR-23b and PTEN. (C) Relative PTEN mRNA levels after transfection with the mimic and inhibitor. (D) PTEN protein expression after transfection with the mimic and inhibitor. Data are shown as the mean ± SD (n = 3). **P < 0.01 (Student’s t-test). Inhibitors: transfected with miR-23b inhibitors; mimics: transfected with miR-23b mimics; miR-NC: transfected with miR-NC; ns: not significant; PTEN: phosphatase and tensin homolog deleted on chromosome 10.
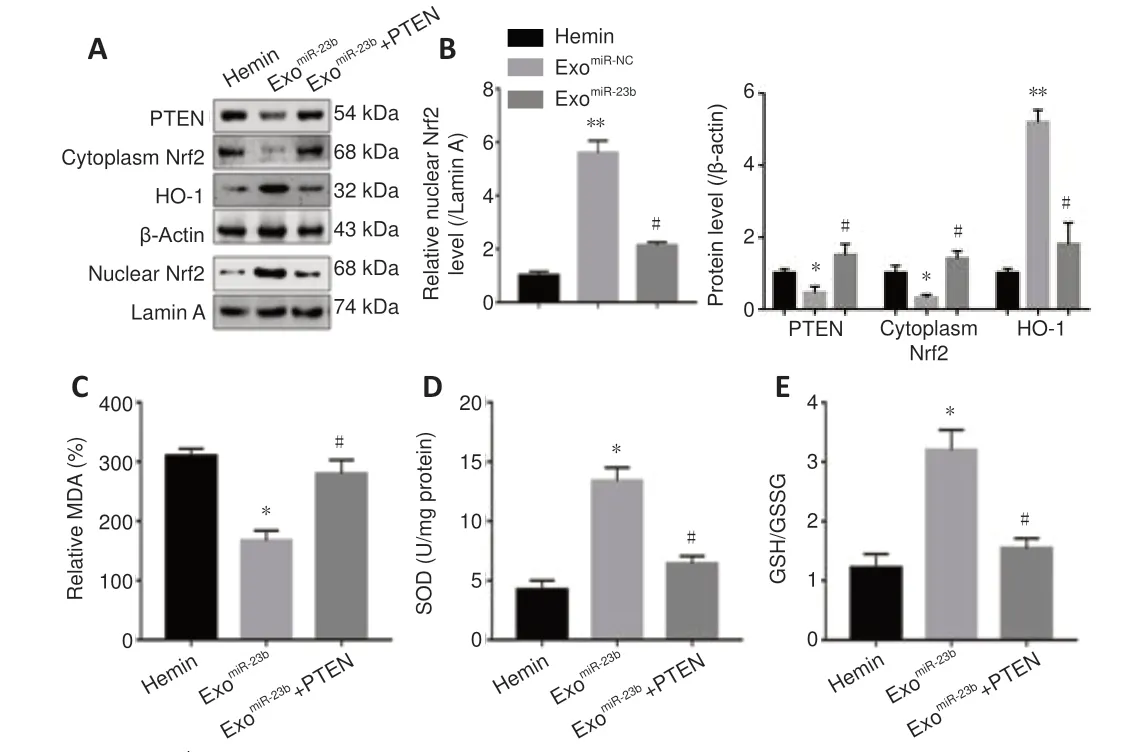
Figure 10 |BMSC-exosomal miR-23b attenuates oxidative stress by regulating the PTEN/Nrf2 pathway in microglia BV2 cells in vitro. (A) Western blot bands for PTEN, Nrf2 (cytoplasmic and nuclear), and HO-1 proteins in microglia BV2 cells from different groups. (B) Quantitative analysis of PTEN, Nrf2 (cytoplasmic and nuclear), and HO-1. (C) MDA levels were measured by economical kits. (D) SOD levels were measured by economical kits. (E) GSH/GSSG ratio was calculated on the basis of the GSH and GSSG levels which evaluated by economical kits. Data are shown as the mean ± SD (n = 6). *P < 0.05, **P < 0.01, vs. hemin group; #P < 0.05, vs. exomiR-23b group (one-way analysis of variance followed by Newman-Keuls post hoc analysis). Hemin: Treated with 60 µM hemin for 24 hours; exomiR-23b group: treated with miR-23b transfected BMSCs-exosomes after hemin stimulation; exomiR-23b + PTEN: treated with pcDNA3.1-PTEN plasmids and miR-23b transfected BMSCs-exosomes after hemin stimulation. BMSC: Bone marrow mesenchymal stem cell; GSH: reduced glutathione; GSSG: oxidized glutathione disulfide; HO-1: heme oxygenase-1; MDA: malondialdehyde; Nrf2: nuclear factor erythroid-2-related factor 2; PTEN: phosphatase and tensin homolog deleted on chromosome 10; SOD: superoxide dismutase.

Figure 11 |BMSC-exosomal miR-23b alleviates NLRP3 inflammasome-mediated pyroptosis by regulating PTEN in vitro. (A, B) Representative bands and data analysis for PTEN and pyroptosis-related proteins by western blotting in BV2 cells. (C) IL-1β and (D) IL-18 levels in the BV2 cellular supernatant were evaluated using ELISA. (E) Cell viability of hippocampal neuronal HT22 cells was assessed using the CCK8 test. (F) Cell death rates of HT22 cells by LDH assays. (G) Co-IP and data analysis of the interaction between endogenous PTEN and NLRP3 in BV2 cells. Data are shown as the mean ± SD (n = 6).*P < 0.05, **P < 0.01, vs. hemin group; #P < 0.05, ##P < 0.01, vs. exomiR-23b group (one-way analysis of variance with the Newman−Keuls post hoc analysis in A−F; Student’s t-test in G). Hemin: Treated with 60 µM hemin for 24 hours; exomiR-23b group: treated with miR-23b transfected BMSC-exosomes after hemin stimulation; exomiR-23b + PTEN: treated with pcDNA3.1-PTEN plasmids and miR-23b transfected BMSC-exosomes after hemin stimulation. BMSC: Bone marrow mesenchymal stem cell; CCK8: cell counting kit-8; Co-IP: co-immunoprecipitation; ELISA: enzyme linked immunosorbent assay; GSDMD-N: N-terminal fragment of gasdermin D; IL-18: interleukin-18; IL-1β: interleukin-1β; LDH: lactate dehydrogenase; NLRP3: NODlike receptor family pyrin domain containing 3; PTEN: phosphatase and tensin homolog deleted on chromosome 10.
Discussion
ICH is a serious type of stroke (Ren et al., 2020; Tschoe et al., 2020; Liu et al., 2021b), and one of its major pathophysiologic mechanisms is related to NLRP3 inflammasome-mediated pyroptosis, which induces inflammatory cascades and cell death (Luo et al., 2019; Xiao et al., 2020). Oxidative stress, caused by ROS accumulation, further aggravates the redox imbalance and acts as an intermediate in NLRP3 inflammasome activation (Chen et al., 2020a; Yao et al., 2021). Many clinical trials of exosomes for brain injuries have been conducted (clinicaltrials.gov). A recent prospective observational cohort study focused on using circulating exosomes to make an early diagnosis and to evaluate the prognosis of ICH patients, indicating the critical role that exosomes could play in translational medicine in the future. Thus, an in-depth exploration of the therapeutic mechanisms of exosomes to provide a solid theoretical base for further clinical translation is urgently needed. Our study reported that BMSC-exosomes carrying miR-23b inhibited oxidative stress and pyroptosis and alleviated brain inflammation and edema, thereby promoting behavioral recovery in rats with ICH. We also demonstrated that exosomal miR-23b may regulate the Nrf2 signaling pathway to exert antioxidant effects and inhibit cell pyroptosis by suppressing NLRP3 inflammasome activation via targeting PTEN. Collectively, our research suggests that exosomal miR-23b derived from BMSCs plays a neuroprotective role in ICH.
Because free biomedical nanoparticle levels may be reduced by nonspecific intra- and extra-cellular interaction in tissues afterin vivo
delivery (González-Nieto et al., 2020), carriers that can target injured organs, such as BMSCs or exosomes, seem to be more efficient. Compared with BMSCs, exosomes harvested from the naive BMSCs showed therapeutic effects that were consistent with those from BMSCs with a lower risk of embolism formation and tumorigenicity (Wang et al., 2012; Xin et al., 2013). Thus, we administered BMSC-exosomes to ICH rats using tail vein injections. On the basis of our previous data, which showed that miR-23b was downregulated in patients with ICH (Zhu et al., 2015; Wang et al., 2016) and that it had an anti-inflammatory function in many diseases including ICH (Zhu et al., 2012; Zhang et al., 2018; Hu et al., 2019), we selected miR-23b as a target for our study using tailored exosomes with modified miRNA content to improve functional recovery in ICH. Ourin vivo
studies demonstrated that tailored BMSC-exosomes containing elevated miR-23b could be internalized with the brain host cells, which led to miR-23b elevation in the tissue surrounding the hematoma. Additionally, BMSC-derived exosomes containing miR-23b further attenuated neurological deficits compared with those of naive exosomes. Because brain edema is an independent risk factor for mortality in ICH (Wu et al., 2017), we observed that exosomal miR-23b improved the functional recovery of ICH with reduced hematoma and edema volumes. These further confirmed the neuroprotective role of BMSC-exosomes with miR-23b in ICH.When ICH occurs, the hemoglobin-heme-iron metabolic axis is triggered and contributes to ROS overproduction (Zhu et al., 2021). ROS overproduction is known to aggravate ICH-induced neuroinflammation. Pyroptosis requires inflammasome activation, such as in the NLRP3 inflammasome, converts precursor caspase-1 into cleaved caspase-1, and then cleaves the precursor of IL-1β and IL-18 into mature pro-inflammatory factors. The cleaved caspase-1 cleaves the N-terminal domain of GSDMD to form cellular membrane pores, which subsequently release pro-inflammatory intracellular contents that amplify the inflammatory response (Shi et al., 2017; McKenzie et al., 2020). In our study, the ROS and oxidative stress marker levels were increased after ICH induction. We then evaluated pyroptosis and neuroinflammation in ICH rats and found that the pyroptosis markers (such as NLRP3, cleaved caspase-1, GSDMD) and pro-inflammatory factors (IL-1β, IL-18) were elevated in the ICH group compared with that in the sham group. A universal components of neuroinflammation is an increase in the number and polarization dynamics of microglia/macrophages (Park et al., 2011; Hu et al., 2012), which were further increased following ICH induction. Previous studies showed similar outcomes (Yao et al., 2017; Xie et al., 2020; Zhu et al., 2021). An increasing amount of evidence has shown that ROS scavenger administration decreases NLRP3 and its downstream pyroptosis protein levels in a ROS-dependent manner, thereby alleviating neuroinflammation and secondary brain injury following ICH (Zeng et al., 2017; Chen et al., 2020b, 2022; Zhu et al., 2021). Results from this study showed that exosomal miR-23b derived from BMSCs reduced the intracellular ROS formation and oxidative stress levels, which was accompanied by further suppression of the NLRP3 activation-mediated inflammatory cascade and pyroptosis.
Consistent with the effects on oxidative stress and pyroptosis, our study found that co-cultured neuronal cell death was reduced by exosomal miR-23b, as shown by the TUNEL staining, CCK8 test, and LDH detection. In addition to oxidative stress-induced neuronal cell death, two studies have suggested that neuronal death could be influenced by inflammation, indicating that the deleterious proinflammatory cytokines (e.g., IL-1β) have a neurotoxic effect on neurons or exacerbate neurodegeneration, which causes neuronal death (Codolo et al., 2013; Wan et al., 2020). IL-1β is secreted in a mature form after NLRP3 inflammasome activation and in conjunction with pyroptosis induction (Shi et al., 2017; McKenzie et al., 2020). Therefore, we hypothesized that neuronal death could be mitigated by suppressing microglia pyroptosis and oxidative stress using exosomal miR-23b.
To investigate these mechanisms, we used online prediction tools and dual luciferase reporter assays to verify that PTEN was a target gene of miR-23b. PTEN is an onco-suppressive factor in various cancers (Lee et al., 2018), and a large amount of evidence also implicates PTEN in oxidative stress and inflammation. Li et al. (2021) reported that suppressing PTEN could activate the downstream PI3K/AKT pathway to attenuate HO-induced oxidative stress, while the mechanism of PTEN in modulating oxidative stress in ICH is not fully understood. Nrf2 serves as an important transcription factor that maintains the redox balance. Upon stimulating oxidative stress, Nrf2 translocates to the nucleus and binds with the antioxidant response element, and subsequently regulates antioxidant enzymes (Ryoo and Kwak, 2018; Wang et al., 2019). A previous study revealed that PTEN leads to GSK-3-mediated NRF2 phosphorylation, which prevents Nrf2 signaling activation (Rojo et al., 2014). Here, our study demonstrated that exosomal miR-23b reduced ROS production and oxidative stress by promoting Nrf2 nuclear translocation, while the antioxidant capacity was eliminated by PTEN overexpression. These results suggested that exosomal miR-23b increases the antioxidant capacity to mitigate ICH-induced oxidative stress by regulating the PTEN/Nrf2 axis.
Recent studies have reported that inhibiting PTEN suppressed the proinflammatory responses in ICH injury (Zhou et al., 2017; Cheng et al., 2020). PTEN was suggested to play a crucial role in NLRP3 inflammasome activation (Huang et al., 2020; Li et al., 2020; Liu et al., 2021a). Mechanistically, PTEN could interact directly with and dephosphorylate NLRP3 to enable the NLRP3-ASC interaction, thereby promoting inflammasome activation in myeloid tumors (Huang et al., 2020). Ourin vitro
results clarified that miR-23b overexpression suppressed PTEN expression and hampered the interaction between PTEN and NLRP3, thus mitigating NLRP3 inflammasome-mediated pyroptosis. PTEN overexpression could further reverse the inhibition of pyroptosis by miR-23b. Thus, exosomal miR-23b may ameliorate cell pyroptosis and neuroinflammation in ICH by inhibiting NLRP3 inflammasome activation by targeting PTEN.However, our study had some limitations. Our study investigated whether exosomal miR-23b alleviates the oxidative stress and pyroptosis in ICH by targeting PTEN, but other mechanisms by which exosomal miR-23b exerts its protective effects remain unclear. Evidence shows that mitochondrial dysfunction can promote excessive ROS generation to increase total cellular oxidative stress (Cacialli et al., 2021). Because this study mainly focused on exosomal miR-23b for total intracellular ROS, further investigation of the involvement of mitochondrial stress with exosomal miR-23b is warranted in the future study. Furthermore, our research clarified the mechanisms by which exosomal miR-23b regulates NLRP3/caspase-1-dependent pyroptosis, but whether other activated caspase (caspase-4/5/11) activation is involved in pyroptosis remains unknown and needs to be investigated in the future.
In conclusion, this study clarified that BMSC-exosomal miR-23b attenuated oxidative stress and pyroptosis, thereby alleviating neuroinflammation and exerting neuroprotection in ICH. PTEN may serve as a target gene to mediate the antioxidant activity and the anti-inflammatory effect of miR-23b via regulating the Nrf2 signaling pathway and NLRP3 inflammasome activation. Our study demonstrated that ICH may be treated with exosomes with contents that are engineered to enhance functional recovery, thereby providing a novel therapeutic strategy for ICH.
Author contributions:
LTH, YHF, ZYH designed the study. LTH performed the research, analyzed the data and wrote the paper. BYW assisted some of the experiments. YHF analyzed the data and revised the manuscript. ZYH and WXZ revised the manuscript. All authors have read and approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflict of interests.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Table 1: The sequences of primers in this study.
- 中国神经再生研究(英文版)的其它文章
- Inflammation and retinal degenerative diseases
- Synaptic alterations as a common phase in neurological and neurodevelopmental diseases: JNK is a key mediator in synaptic changes
- Brain-derived neurotrophic factor in main neurodegenerative diseases
- The best of both worlds: mastering nerve regeneration combining biological and nanotechnological tools
- Chlorogenic acid alleviates hypoxic-ischemic brain injury in neonatal mice
- DUSP2 deletion with CRISPR/Cas9 promotes Mauthner cell axonal regeneration at the early stage of zebrafish

