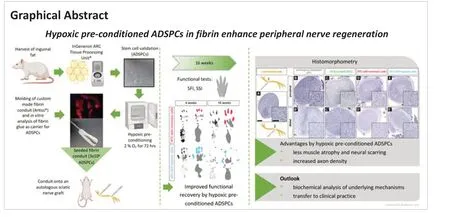
Hypoxic pre-conditioned adipose-derived stem/progenitor cells embedded in fibrin conduits promote peripheral nerve regeneration in a sciatic nerve graft model
Julius M. Mayer , Christian Krug , Maximilian M. Saller Annette Feuchtinger, Riccardo E. Giunta, Elias Volkmer , Thomas Holzbach
Abstract Recent results emphasize the supportive effects of adipose-derived multipotent stem/progenitor cells (ADSPCs) in peripheral nerve recovery. Cultivation under hypoxia is considered to enhance the release of the regenerative potential of ADSPCs. This study aimed to examine whether peripheral nerve regeneration in a rat model of autologous sciatic nerve graft benefits from an additional custom-made fibrin conduit seeded with hypoxic pre-conditioned (2% oxygen for 72 hours) autologous ADSPCs (n = 9). This treatment mode was compared with three others: fibrin conduit seeded with ADSPCs cultivated under normoxic conditions (n = 9); non-cell-carrying conduit (n = 9); and nerve autograft only (n = 9). A 16-week follow-up included functional testing (sciatic functional index and static sciatic index) as well as postmortem muscle mass analyses and morphometric nerve evaluations (histology, g-ratio, axon density, and diameter). At 8 weeks, the hypoxic pre-conditioned group achieved significantly higher sciatic functional index/static sciatic index scores than the other three groups, indicating faster functional regeneration. Furthermore, histologic evaluation showed significantly increased axon outgrowth/branching, axon density, remyelination, and a reduced relative connective tissue area. Hypoxic pre-conditioned ADSPCs seeded in fibrin conduits are a promising adjunct to current nerve autografts. Further studies are needed to understand the underlying cellular mechanism and to investigate a potential application in clinical practice.
Key Words: adipose-derived progenitor cells; adipose-derived multipotent stem/progenitor cell; autologous nerve graft; fibrin conduit; hypoxia; hypoxic preconditioning; nerve defect; nerve tissue engineering; peripheral nerve regeneration; regenerative medicine
Introduction 652 Methods 653 Results 654 Discussion 655
Introduction
Severe injuries, especially to patients’ extremities, are often associated with acute peripheral nerve trauma. Incomplete axonal regeneration can greatly compromise function and quality of life and is associated with a high financial burden on the healthcare system (Raza et al., 2020). Whenever feasible, an appropriate tension-free end-to-end coaptation of the stumps is recommended (Diao and Vannuyen, 2000). In peripheral nerve defects of a critical size, autografting is still considered the clinical gold standard, but donor site morbidity remains a significant drawback (Kuffler and Foy, 2020). Nerve conduits (e.g., of bioresorbable materials or autologous vessel grafts) as guiding structures for axonal outgrowth and targeting have been evaluated and found to be a satisfactory treatment option for defects < 20 mm. Current research in peripheral nerve regeneration focuses on cell-based therapies to further axonal regeneration (Brooks et al., 2012; Daly et al., 2012; Braga Silva et al., 2017; Bingham et al., 2019; Midha and Grochmal, 2019). Recent results from experimental models emphasize the supportive effects of easily harvested adipose-derived multipotent stem/progenitor cells (ADSPCs) in peripheral nerve recovery and regeneration (Reichenberger et al., 2016; Sowa et al., 2016; Masgutov et al., 2019).
The therapeutic potential of mesenchymal stromal cells, such as ADSPCs, can be modulated by various techniques of cellular pre conditioning (Ferreira et al., 2018). These include treatment of stem cells with pharmacologic agents, growth factors or hormones, or incubation in a 3-dimensional culture and cultivation under hypoxic conditions (Wei et al., 2017; Ferreira et al., 2018). There is evidence that hypoxic cultivation of mesenchymal stem cells can lead to upregulation of the transcription factor hypoxia-inducible factor 1 alpha, which subsequently results in enhanced protection against oxidative stress and increases vascularization by elevated concentrations of vascular endothelial growth factor and angiotensin, both supportive factors in the regenerative process (Ahluwalia and Tarnawski, 2012; Kiani et al., 2013). Furthermore, hypoxic pre-conditioning was shown to increase the stemness of hMSC by upregulating important pluripotency genes, includingKLF4
,NANOG
, andOCT4
(Wei et al., 2017).To release the potential of a cell-based regenerative approach at the site of injury, a scaffold matrix is required, as direct injection of cells often results in poor engraftment. The ideal scaffold must meet a long list of requirements, including biocompatibility and biodegradability, promotion of cell adhesion and proliferation, and adequate cell nutrition. As a scaffold carrier for ADSPCs, the clinically approved natural biomaterial fibrin shows promisingin vitro
characteristics (Krug et al., 2019), such as homogeneous cell distribution within the fibrin matrix, high biocompatibility, high degradability, and proven cell viability within the matrix over the long term, as well as metabolic, migrating and remodeling cell activities. Additionally, fibrin conduits themselves are capable of guiding axon regrowth and reducing neuroma formation, consequently benefitting nerve recovery (Isaacs, 2013).In thisin vivo
animal study, we examined whether nerve regeneration after surgical repair of a critically sized nerve defect with an autologous nerve graft can significantly be improved by the addition of a fibrin conduit seeded with undifferentiated hypoxic pre-conditioned autologous ADSPCs.Methods
Study design
The rats were handled and housed according to the federal and institutional guidelines for the care and use of laboratory animals approved by the government of Upper Bavaria on May 23, 2014 (approval No. 55.2-1-54-2532-177-13), as follows: controlled temperature of 20‒22°C, 12-hour light/dark cycle, feedingad libitum
, ventilated cages with an effective area of 1500 cm(maximum four rats per cage). A statistical expert opinion is mandatory and was conducted before the approval was accepted. For comparability reasons, a total of 36 four-month-old female Sprague-Dawley rats (RjHan:SD, Janvier Labs, Le Genest-Saint-Isle, France) with a mean weight of 350 g were used.The animals were randomly assigned to four groups (n
= 9). Three different therapeutic regimens were compared with the current clinical gold standard, which served as a control (group AT):• ATCH group: Nerve autograft plus fibrin conduit seeded with hypoxic preconditioned autologous ADSPCs;
• ATCN group: Nerve autograft plus fibrin conduit seeded with autologous ADSPCs cultivated under normoxic conditions;
• ATC group: Nerve autograft plus fibrin conduit ;
• AT group: Nerve autograft.
A 16-week postoperative follow-up of lower extremity functional improvement was conducted before sacrificing the animals for histologic assessment. Primary endpoints were the sciatic functional index (SFI) and static sciatic index (SSI), which evaluate functional recovery. Secondary endpoints were muscle mass atrophy and histomorphologic findings.Surgical intervention
The autologous nerve graft model, a well-established peripheral nerve defect model in rats, has been shown to be reliablein vivo
(Saller et al., 2018). All surgical procedures were performed under sterile conditions. Pre-operative anesthesia was initiated by intramuscular injection of 0.02 mg/kg fentanyl (Janssen, Neuss, Germany), 1.0 mg/kg midozilam (ratiopharm GmbH, Ulm, Germany) and 0.2 mg/kg medetomidin (Orion, Espoo, Finnland). Anesthesia was post operatively antagonized with 0.03 mg/kg naloxone (Bristol myers Squibb, New York, NY, USA), 0.1 mg/kg flumazenile (Roche, Basel, Switzerland) and 1.0 mg/kg atipamezole (cp-pharma, Burgdorf, Germany). The animals were positioned on a 37°C heating plate (Tempcontrol 37 ‒ 2 digital, PeCon GmbH, Erbach, Germany) to prevent hypothermia.Three days before the operation, inguinal fat pads were resected bilaterally to harvest ADSPCs. Wounds were closed with interrupted absorbable sutures (Vicryl 4-0, Ethicon, Norderstedt, Germany).
To induce an artificial nerve defect of critical length (20 mm or longer in rats), we followed a previously published surgical protocol (Saller et al., 2018). In brief, animals were placed on a warming device and the right hind limb was shaved and sterilized. The skin was incised and, after a blunt intermuscular split of the superficial gluteal and biceps femoris muscles, the sciatic nerve was gently exposed. Between its proximal origin close to the spine and its division into its branches (common peroneal nerve and tibial nerve), a 20-mm-long segment was resected and reverse-positioned into the neural gap to imitate the mismatch when bridging a critically sized nerve defect by an autologous nerve transplant. Proximal and distal microsurgical epineural coaptations were carried out with non-absorbable thread (Ethilon 9-0, Ethicon, Norderstedt, Germany). After the nerve coaptation, the cell-seeded or non-cell-seeded fibrin conduit (see below) was placed around the nerve transplant to cover the entire 20-mm segment and both coaptation sites completely (ATC, ATCN, and ATCH groups). Finally, the superficial gluteal and biceps femoris muscles were carefully readapted and the cutis incision was closed with interrupted absorbable sutures (Vicryl 4-0, Ethicon). Postoperative care was provided with 0.05 mg/kg buprenorphine (Temgesic®, Essex Pharma, Munich, Germany) subcutaneously daily for 3 days, 1.5 mg/kg fluphenazine decanoate (Dapotum® D 12.5 mg/0.5 mL solution, Sanofi-Aventis Germany GmbH, Frankfurt, Germany) intramuscularly weekly, and metamizol (Novaminsulfon-ratiopharm® 500 mg/mL, ratiopharm GmbH, Ulm, Germany) in the drinking water (ad libitum
) within the first postoperative weeks to prevent postoperative and neuropathic pain and avoid auto-mutilation of the limb (Carr et al., 1992).Autologous ADSPC isolation and culture
After surgical harvest, the inguinal fat tissue was washed with phosphatebuffered saline (PBS), minced, and enzymatically (Matrase, InGeneron, Houston, TX, USA) isolated with a clinically approved and commercially available centrifuge (ARC-Processing Unit, InGeneron) according to the manufacturer’s protocol (Krug et al., 2016). Isolated cells were resuspended in standard culture medium (89% DMEM, 10% heat inactivated fetal calf serum, 1% penicillin/streptomycin, Life Technology, Carlsbad, CA, USA), placed onto T-225 cell culture flasks (Nuclon, Thermo Fisher Scientific, Waltham, MA, USA), and incubated under normal conditions (21% O, 5% CO) or hypoxic conditions of the study’s protocol (2% O, 5% CO) (MCO-5M, Sanyo, Osaka, Japan). Non-adhered cells were removed by repeated rinsing with PBS. The culture medium was changed once on day 1 during the expansion phase of 3 days. The stem cell characteristics of obtained cells were determined as described previously (Saller et al., 2018; Krug et al., 2019). In brief, cell surface markers CD90, CD29, CD45 and CD11b/c (CD90 and CD29 as stem cell markers and CD45 and CD11b/c as leukozyte markers in the sense of negative control) were analyzed by FACS. Furthermore, the multilineage differentiation potential was assayed by osteogenic and adipogenic differentiation of the ADSPCs. All antibodies and appropriate isotype controls were obtained from BioLegend, San Diego, CA, USA.
Fibrin conduit preparation and cell seeding
To create cell seeded fibrin conduits (25 mm length, 2 mm wall thickness, 2 mm inner diameter), we used a clinically approved and commercially available two-component fibrin sealant (ARTISS, Baxter, Deerfield, IL, USA) as previously described (Saller et al., 2018). In brief, cells were trypsinized (0.5% trypsin, Merck, Darmstadt, Germany) and counted, and 3 × 10were resuspended in standard culture medium and mixed in a 1:10 ratio with thrombin. The conduit was prepared in sterile syringes with a metal wire in the middle. After a polymerization period of 30 minutes at 37°C in an incubator, the cell seeded conduits were applied during surgery as described above.Functional evaluation of recovery
To determine lower extremity functional recovery, a walking track analysis of the sciatic function index (SFI) and a footprint image analysis of the static sciatic index (SSI) were conducted preoperatively and on postoperative weeks 4, 8, 12, and 16. Both indices are reliable methods to evaluate functional peripheral nerve recovery after injury and repair to the sciatic nerve of rats (de Medinaceli et al., 1982; Bervar, 2000; Smit et al., 2004). The hind feet of the animals were marked with dark ink, and the animals had to pass through a 110-cm-long tunnel. A cover at the end of the tunnel served as a shelter, thus motivating them to traverse the walking track determinedly to produce accurate footprints. To document the SSI plantar view, photos of the hind feet were taken at right-angles from below while having the rodents sit in a box of acrylic glass with a transparent floor plate. The parameters for the indices were measured manually with the open source software ImageJ (version1.51d; NIH, Bethesda, MD, USA; Schneider et al., 2012).
The three variables required to calculate the SFI are the print length, the toe spread between the first and fifth toes, and the intermediate toe spread between the second and fourth toes. The SSI is defined by the toe spread and the intermediate toe spread (de Medinaceli et al., 1982; Bervar, 2000). The print length is not relevant to this non-dynamic index. For both indices, scores of nearly 100 display complete impairment of the sciatic nerve, while scores around 0 indicate normal function.
Muscle mass analysis
Peripheral nerve injuries can affect the motor innervation of target muscles. As denervation results in muscular atrophy, nerve regeneration can be evaluated by the analysis of the degree of atrophy, resulting in an inverse correlation of muscle atrophy and muscle mass values. Bilateral gastrocnemius muscles that are innervated by the tibial nerve, which branches from the sciatic nerve, were harvested after animals were sacrificed by COchamber euthanasia (100% CO, fill rate of 50‒70% COof the chamber volume per minute) at 16 weeks. The wet masses of the muscles of the impaired and contralateral limbs were determined.
Histological assessment
Sciatic nerve segments of about 30 mm in length, including the autograft zone and 5-mm segments proximal and distal to both coaptation sites, were harvested for histologic evaluation of axon recovery. The contralateral sciatic nerve was collected as a control. Samples were fixed overnight in 4% paraformaldehyde in PBS (Carl Roth, Karlsruhe, Germany) at 4°C, embedded in paraffin (Paraplast Plus, Leica Biosystems, Nussloch, Germany) and then cut into 4-µm semi thin cross-sections with a sliding microtome (Leica SM2000R, Leica Biosystems, Nussloch Germany). After deparaffinization with xylol (Merck) and rehydration in a decreasing ethanol row, sections were stained with 1% toluidine blue (Merck) solution for 2 minutes, washed in distilled water, and mounted. Fully automated microscopic (400× magnification; AxioObserver, Zeiss, Jena, Germany) images were taken, covering the complete cross section of the contralateral sciatic nerve and the proximal and distal segments of the autograft side. The images were processed to detect all axons by shape and color features and evaluated for axon variations with a defined algorithm of the commercially available image analysis software Definiens Developer XD2 (Definiens AG, Munich, Germany) (Feuchtinger et al., 2015; Helmbrecht et al., 2015). We performed a thorough histomorphologic analysis of various nerve and axon parameters. For each cross section image, the axon density (axons/region of interest), the percentage of connective tissue, the mean axon diameter, and the g-ratio (axon myelination)—i.e., the ratio of axon diameter to myelinated fiber diameter--were determined. As scar formation in the regeneration site affects axon regeneration in the central nervous system (Anderson et al., 2016) and similar mechanisms might be involved in the regeneration of peripheral nerves, we quantified the relative connective tissue area in the proximal and distal parts of the defect. As the electrophysiologic properties of regenerating axons strongly depend on remyelination (Gillespie and Stein, 1983) and axon diameter, both were quantified in an unbiased and automatic manner.
Statistical analysis
All analyses were performed by investigators who were not blinded to experimental condition. For all statistical comparisons, a Kruskal Wallis test was calculated with GraphPad Prism (version 8, GraphPad Software Inc., La Jolla, CA, USA). AP
-value of ≤ 0.05 was considered significant. All data are represented as box plots (median, 25%/75% quartiles, and range).Results
All 36 animals were included in the analysis, all without pathologic findings regarding their general condition for the entire study period.
Conduits with hypoxic pre-treated cells lead to a faster functional recovery
Four weeks after surgery, animals of all groups showed no significant intergroup difference in gait recovery (SFI;Figure 1A
). Applying a fibrin conduit only and a fibrin conduit seeded with cells resulted in a significantly improved SSI compared with that in the AT group (P
< 0.01 andP
< 0.001;Figure 1A’
). At 8 weeks, animals in the ATCH group had a significantly increased SFI values compared with those of the ATCN (P
< 0.05) and ATC groups (P
< 0.01;Figure 1B
). In addition, the SSI values in the ATCH group at 8 weeks were significantly improved over the other groups (P
< 0.05 orP
< 0.001) (Figure 1B’
). The SSI values in the ATCN group were the same as the ATC group.At 12 weeks, the SFI values in the ATCN group were significantly improved over those in the ATC group (P
< 0.05;Figure 1C
andD
). Although not statistically significant, the trend in the ATCH group was toward a better performance than in the other groups (Figure 1C
,C’
,D
, andD’
andAdditional Figure 1
).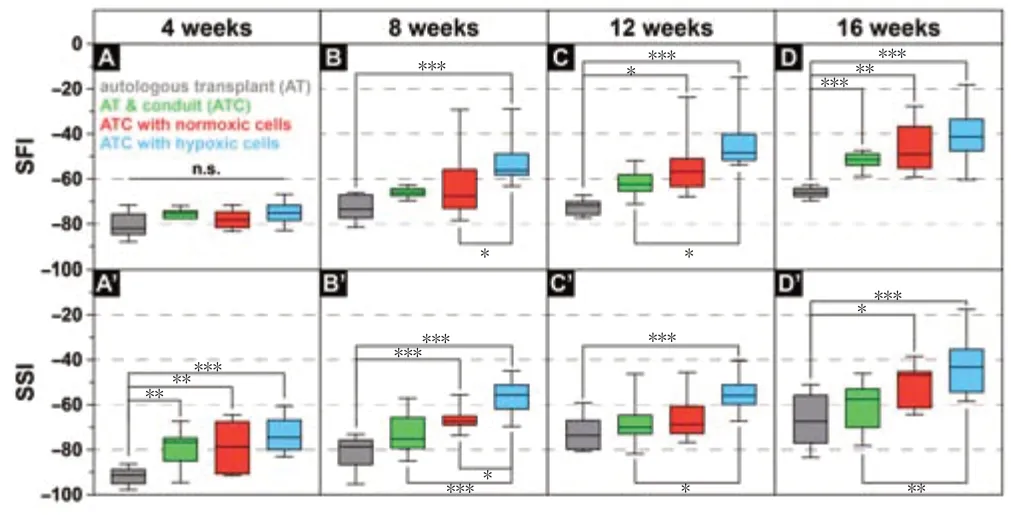
Figure 1 |Hypoxia pretreated ADSPC loaded fibrin conduits lead to a faster functional peripheral nerve regeneration. The sciatic functional index (SFI) and static sciatic index (SSI) on postoperative weeks 4, 8, 12, and 16. All values are presented as box plots (median, 25%/75% quartiles, and range). Nine rats per group. *P < 0.05, **P < 0.01, ***P < 0.001. The experiments were repeated three times for every time point and animal. AT: Autologous transplant; ATC: autologous transplant plus conduit.
Reduced scar formation and increased axon density in rats with cell seeded conduits
In all groups, no coaptation failure or neuroma formation was found macroscopically when the nerve segments were harvested at 16 weeks.
Descriptive evaluation of toluidine blue-stained nerve cross-sections showed that the number of axons, axon size and myelinization appeared to be reduced in animals in the AT (Figure 2B
) and ATC groups (Figure 2C
) compared with animals in the ATCN (Figure 2C
) and ATCH groups (Figure 2E
). Connective tissue distribution appeared less in the ATCH group than that in the other three groups. However, the nerve morphology in all pathologic specimens (Figure 2B’–E’
) was clearly different compared with the healthy contralateral side (Figure 2A
).The gastrocnemius muscle (GM) of the operated side was significantly lighter than that of the contralateral side in all experimental groups (Figure 3A
). The GM of animals in the ATCH group showed the least muscle atrophy (a decrease of 28.5 ± 7.5%) in comparison with the ATCN, ATC, and AT groups. However, the differences were not significant, and therefore can only be seen as a trend.Unbiased automatic quantification of the cross-sections indicated that the animals in the ATC and AT (control) groups showed no significant increase of relative connective tissue area in the proximal part compared with the contralateral side (Figure 3B
). However, animals in the ATCN or ATCH groups had a significantly larger proximal relative connective tissue area in comparison with the contralateral side (P
< 0.05 andP
< 0.01;Figure 3B
). In the ATC and AT groups, a significant increase in the relative connective tissue area in the distal end of the nerve defect (Figure 3B
) was seen compared with the contralateral side (P
< 0.01 andP
< 0.001) or ATCH group (P
< 0.05). In contrast, in the distal region, no significant difference of the relative connective tissue area in the ATCN and ATCH groups was detected compared with the contralateral side.Evaluation of the axon density in the proximal and distal defect regions revealed significantly more axons in the sciatic nerve of animals treated with cell-seeded conduits cultivated under normoxic or hypoxic conditions compared with the contralateral side (P
< 0.001;Figure 3C
). Axon density was 37 ± 4.3% higher in the distal region than in the proximal region of the defect, independent of the intervention.At 16 weeks, the application of fibrin conduits only (ATC group) led to a significantly smaller axon diameter (P
< 0.01;Figure 3D
) and reduced g-ratio (P
< 0.05;Figure 3E
), independent of the defect region, compared with the contralateral side. Treatment regimens in the ATCH and ATCN groups did not lead to a larger axon diameter in the distal end (Figure 3D
), but to a reduction in the loss of myelin in the distal defect end compared with the findings in the ATC and AT groups (Figure 3E
). In addition, plotting the axon diameterversus
the g-ratio indicated that, compared with a healthy sciatic nerve, the application of cell seeded fibrin conduits (ATCH and ATCN groups) led to an axon diameter/g-ratio that was closer to healthy than in the ATC and AT groups (Figure 3F
).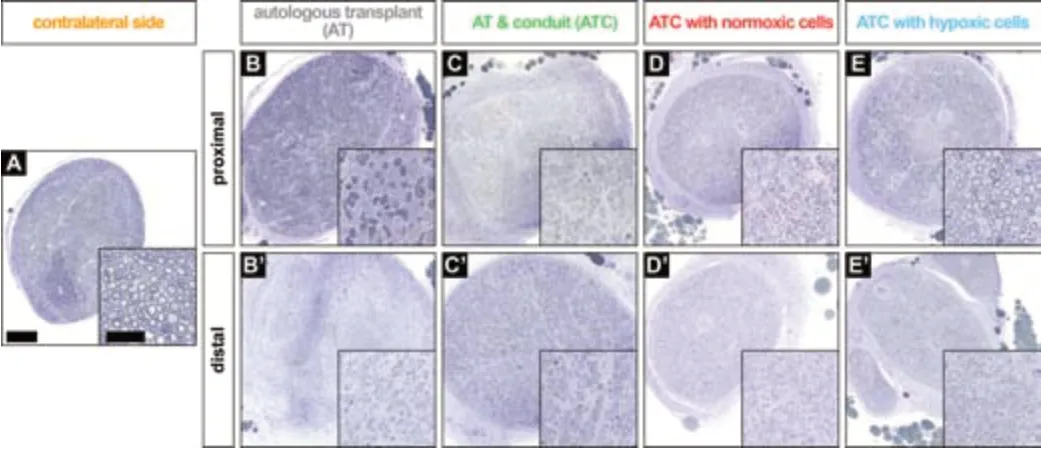
Figure 2 |Descriptive nerve morphology. Examples of toluidine blue stained semi-thin nerve sections, which reveal that the number of axons, axon size and myelination seem to be increased in adipose derived multipotent stem/progenitor cells treated groups and proximal sections of animals treated with hypoxic cultured adipose derived multipotent stem/progenitor cells seem more homogenous. Boxes represent the magnifications in A‒E and B’‒E’ (scale bars: 200 µm; inserts: 50 µm). AT: Autologous transplant; ATC: autologous transplant plus conduit; CS: contralateral side (native sciatic nerve).

Figure 3 |The adjunct of adipose derived multipotent stem/progenitor cells leads to slightly less muscle atrophy, reduced scar tissue formation in the distal portion, and increased axon density and myelination in comparison to sole autologous transplants in rats after sciatic nerve injury. (A) Gastrocnemius muscle mass. (B) Relative connective tissue area. (C) Axon density. (D) Mean axon diameter. (E) Mean g-ratio. (F) Plot mean g-ratio/axon diameter. The values in A‒E are presented by a box plot. *P < 0.05, **P < 0.01, ***P < 0.001; nine animals per group. The boxes in F present the 95% CI. The experiments were repeated three times for every time point and animal. AT: Autologous transplant; ATC: autologous transplant plus conduit; CS: contralateral side (native sciatic nerve); ECM: extracellular matrix; ROI: region of interest.
In summary, slightly less muscle atrophy was seen when hypoxic pre treated cells seeded in a fibrin conduit were applied. In the distal defect region, the relative connective tissue area was significantly decreased in the ATCH group compared with the AT and ATC groups. An increase in axon outgrowth/branching was found in the ATCH group.
Discussion
Minor nerve defects can successfully be repaired with allografts or artificial nerve tubes. As an alternative to autografts, these approaches eliminate donor site morbidity (Brooks et al., 2012). However, neural tubes cannot bridge peripheral nerve gaps of more than 20 mm. In these cases, nerve autografts remain the treatment of choice. Thus, successful recovery of motor and sensory function after peripheral nerve repair of discontinuities of a critical size predominantly depends on adequate and timely microsurgical coaptation. However, additives to the coaptation site have been shown to promote the complex processes of nerve regeneration (Diao and Vannuyen, 2000; Jiang et al., 2017). The model used in this study is a true critical-size defect model, which means that the defect created in the sciatic nerve would not heal on its own. In consequence, anything applied into the gap to heal the defect is the actual cause of any resulting recovery of function. In the animal model used here, the criterion of a critical nerve gap (20 mm or longer) is met. This is a crucial methodologic/technical prerequisite because rats are endowed with an inherent capability to regenerate short nerve gaps (Daly et al., 2012). A special feature of the animal model is that, whenever a nerve autograft is needed, the excised sciatic nerve itself is used. To mimic better the oft-encountered clinical problem of size mismatch of nerve grafts, the excised nerve was flipped 180° before coaptation. In the present study, we explored possibilities to improve nerve regeneration after autologous nerve grafting (Jiang et al., 2017; Kuffler and Foy, 2020).
Adding a sole fibrin conduit to the autograft yields a faster and better functional recovery. This is in line with earlier findings, which indicate that fibrin conduits themselves are capable of guiding axon regrowth and reducing neuroma formation—consequently benefitting nerve recovery (Isaacs, 2013). Our novel fibrin conduit design and fabrication provide stable and reproducible architecture with convenient cell integration and homogeneous cell distribution, which was shown in an earlier study (Krug et al., 2019). Furthermore, the interaction (e.g., degradation rate of fibrin, ADSPCs-release rate) of the fibrin glue and differently cultivated ADSPCs was investigatedin vitro
before thisin vivo
study. The creation of the conduit itself is not very time-consuming, which may be of economic relevance for a potential clinical application.Recentin vitro
experiments on ADSPCs embedded in a fibrin matrix revealed that the metabolic activity of ADSPCs is significantly higher in a hypoxic setting than in a normoxic setting (Krug et al., 2019). In our study, hypoxic pre conditioning of transplanted cells significantly accelerated the recovery of the rats’ physical performance when compared with that in rats treated with a fibrin conduit or a conduit loaded with normoxic cultivated cells. Muscle atrophy was the least in the hypoxia group; however, the differences were not significant and can therefore only be seen as a trend.The functional findings also indicate a significant advantage of hypoxic pre conditioning over normoxic cultivation during the initial phase of peripheral nerve regeneration (see SFI/SSI at 8 weeks). The quicker the autograft is revascularized, the earlier the neural recovery begins (Kuffler and Foy, 2020). The sooner and quicker the axons extend distally, the fewer Schwann cells of the distal nerve stump are affected by senescence from denervation (“Wallerian degeneration”); thus, less of their capacity to proliferate and release neurotrophic factors is lost (Kuffler and Foy, 2020). These more favorable findings of the hypoxic pre-conditioned group could be explained by increased metabolic activity, cell survival, protection against metabolic and cellular stresses, and the idea that hypoxia enhances the proliferation of ADSPCs and stimulates the regenerative potential of ADSPCs by the upregulation of growth factors (Chung et al., 2009; Kiani et al., 2013). The capacity to adopt a proangiogenic phenotype by hypoxia (Thangarajah et al., 2009) may have the potential to enhance the ability of ADSPCs to promote neovascularization, which is proven to support regenerative processes. Fu et al. (2016) publishedin vitro
data on the induction of ADSPCs into Schwannlike cells and Schwann-like cell proliferation. While (trans-)differentiation of ADSPCs towards neural tissue types has been speculated to explain the effect of ADSPCs, there is some evidence that paracrine secretion of neuroprotective, regenerative and angiogenic factors (such as brain-derived neurotrophic factor, nerve growth factor, neurotrophin-3, glial cell linederived neurotrophic factor and vascular endothelial growth factor) is of higher relevance to peripheral nerve regeneration (Kingham et al., 2007; Erba et al., 2010; Sowa et al., 2012; Faroni et al., 2013). Interestingly, however, other authors have conjectured active contribution of ADSPCs in regenerative processes by detection of vital ADSPCs at the nerve coaptation site (Reichenberger et al., 2016). Further trials are crucial to evaluate the exactin vivo
behavior and mechanisms of ADSPCs at the site of nerve regeneration.Scar formation is considered an inhibiting factor for nerve regeneration (Tannemaat et al., 2009). We found a reduced relative connective tissue portion, which can be considered as less scarring, in the distal nerve stumps of hypoxic-cell-treated animals. The higher the connective tissue percentage, the worse the regeneration and the more likely neuroma formation. Reduced scarring seems to correlate with better functional recovery (Burnett and Zager, 2004). The more axons grow through the distal stump of the nerve, the more favorable the target re-innervation and subsequent neurological restitution. In our work, unbiased and automatic quantification of histological cross-sections revealed trends of increased axon outgrowth in cell loaded groups. The g-ratio specifies the degree of myelinization of axons. The diameter/g-ratio values of cell loaded groups were least different from those of the contralateral native sciatic nerves, which may indicate acceleration of remyelination of regenerating axons by the application of ADSPCs. These quantitative histological results may be considered in line with the findings on functional outcomes, indicating the beneficial effects of ADSPCs for nerve regeneration in the framework of this study.
Our data show a modest but consistent improvement in axonal growth and regeneration in the hypoxic pre-conditioned ADSPCs group. However, although statistically significant at 8 weeks, these differences were not significant at 16 weeks. Hypoxic pre-conditioning might enable ADSPCs to promote a faster axonal regeneration, when compared with normal ADSPCs, but clarification may require further investigation. Severe peripheral nerve injuries can lead to a dramatic increase in reactive oxide species and oxidative stress status in the nerve microenvironment. In this regard, thein vitro
hypoxic treatment may fail to exert a sustainable effect on ADSPCs. Thein vivo
performance and fate of ADSPCs and whether ADSPCs can maintain their hypoxic state are as yet unclear. These are key considerations regarding hypoxic preconditioning and whether it is worth the effort. It may explain why only a modest difference was detected between the ATCH and ATCN groups.In vitro
data regarding these aspects is already available. Additional monitoring of thein vivo
processes inside the conduit, such as its biodegradation, as well as an understanding of the cellular and molecular behaviour of the ADSPCs in the fibrin matrixin vivo
, would have added value to the study and will be included in future investigations.Regarding possible transfer of our findings to clinical application, a closer look at the aspect of hypoxia is imperative. Hypoxia in the context of cell culture is defined as oxygen concentration from 0% to 10% (Eliasson and Jonsson, 2010; Ejtehadifar et al., 2015). It is relevant to bear in mind that oxygen concentrations of this range represent “in situ
normoxia” for most human tissues, including fat tissue (Ivanovic, 2009). Normal oxygen concentration (atmospheric pressure of 21% O), as routinely applied for standard cell culture, is higher than the oxygen concentration in fat tissue given in physiologicalin vivo
conditions. Time-consuming pretreatment and expansion (even outside of the operating room) may not be necessary in the clinical application of this therapy.In accordance with current literature (Mazini et al., 2019), our observations are encouraging. ADSPCs in custom made fibrin scaffolds appear to be a promising strategy for enhancement of peripheral nerve recovery. Another noteworthy aspect for clinical application is that ADSPCs provide a sufficient cell population in humans and are easy to harvest and process without the limitation of extensive donor site morbidity. The focus of this study was the use of pre-conditioned ADSPCs. However, attention must be paid to the latest findings on the role of exosomes in peripheral nerve regeneration (Ching and Kingham, 2015; Liu et al., 2020). Recently published studies indicate that the use of ADSPC-derived exosomes, engineered to encapsulate NT-3 for example, are a promising approach to enhance peripheral nerve regeneration (Li et al., 2018; Yang et al., 2021).
Further investigations comparing the effects of an exosome based therapy and hypoxic pre-conditioned ADSPCs, or even a combination, might be of interest.
Nevertheless, ethical restrictions in engineering such exosomes or the processing of ADSPCs before implantation have to be considered critically, as in most countries “modifications such as centrifuging and accumulating stem cells do require the permission of an ethical committee and/or regulatory agencies” (Saller et al., 2018). Another very considerable aspect regarding clinical transfer of our approach is patient safety with regard to drug compatibility. The fibrin glue used is clinically approved, biocompatible, preserves high cell viability, and is already utilized in a broad range of clinical applications (Krug et al., 2019).
One limitation of the study is that only endpoint histomorphological data are provided. Moreover, immunofluorescence staining of axons and myelin for 200-kDa neurofilament protein and myelin basic protein and detection of the related indicators of nerve regeneration from the transcriptional or translational level would have added further value and should be integrated into future studies.
In summary, adding hypoxic pre-conditioned ADSPCs embedded in a custommade fibrin conduit to nerve autografting in a sciatic nerve defect of critical size was revealed to be beneficial for remyelination, axon outgrowth/branching, avoidance of muscle atrophy, and motor function. However, physiological function and histology were not entirely restored within 16 weeks’ follow-up. To ensure a proper transfer of the benefits to clinical practice, further studies will have to focus on the underlying mechanisms of how ADSPCs in a fibrin matrix promote peripheral nerve regeneration after autologous nerve grafting. Until the approval of hypoxic pre-conditioned ADSPCs, clinically approved fibrin glue may already be used to improve nerve regeneration after autologous nerve grafting.
Author contributions:
Concepts, design, definition of intellectual content, literature search, experimental studies, data acquisition and analysis, manuscript editing and review, guarantor: CK, TH. Concepts, design, literature
search, experimental studies, data acquisition and analysis, manuscript preparation, editing and review, guarantor: JMM. Concepts, design, literature search, experimental studies, data acquisition and analysis, manuscript editing and review: MMS. Concepts, design, literature search, data acquisition and analysis, manuscript review: AF. Design, data analysis, manuscript review: REG. Concepts, design, literature search, data analysis, manuscript editing and review: EV. All authors approved the final version of the paper.
Author statement:
The study was presented at 6 Munich Symposium for Experimental Orthopedics, Trauma Surgery and Research Munich, Germany, July 2016; 93 Annual Meeting of the Association of Bavarian Surgeons Munich, Germany, July 2016; 47 Annual Meeting of the German Society of Plastic, Reconstructive and Aesthetic Surgeons, Kassel, Germany, September 2016; 4 International Symposium of Peripheral Nerve Regeneration, Barcelona, Spain, July 2017; 56 Congress of Swiss Plastic Surgery & 8 Congress of Swiss Aesthetic Surgery Lausanne, Switzerland, September 2020.
Conflicts of interest:
The authors declare no competing or financial interests.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Examples of footprints of rats in the ATCN and ATCH groups on postoperative weeks 4 and 16.
- 中国神经再生研究(英文版)的其它文章
- Inflammation and retinal degenerative diseases
- Synaptic alterations as a common phase in neurological and neurodevelopmental diseases: JNK is a key mediator in synaptic changes
- Brain-derived neurotrophic factor in main neurodegenerative diseases
- The best of both worlds: mastering nerve regeneration combining biological and nanotechnological tools
- Exosomal miR-23b from bone marrow mesenchymal stem cells alleviates oxidative stress and pyroptosis after intracerebral hemorrhage
- Chlorogenic acid alleviates hypoxic-ischemic brain injury in neonatal mice

